Copyright
©The Author(s) 2020.
World J Gastroenterol. Oct 28, 2020; 26(40): 6111-6140
Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6111
Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6111
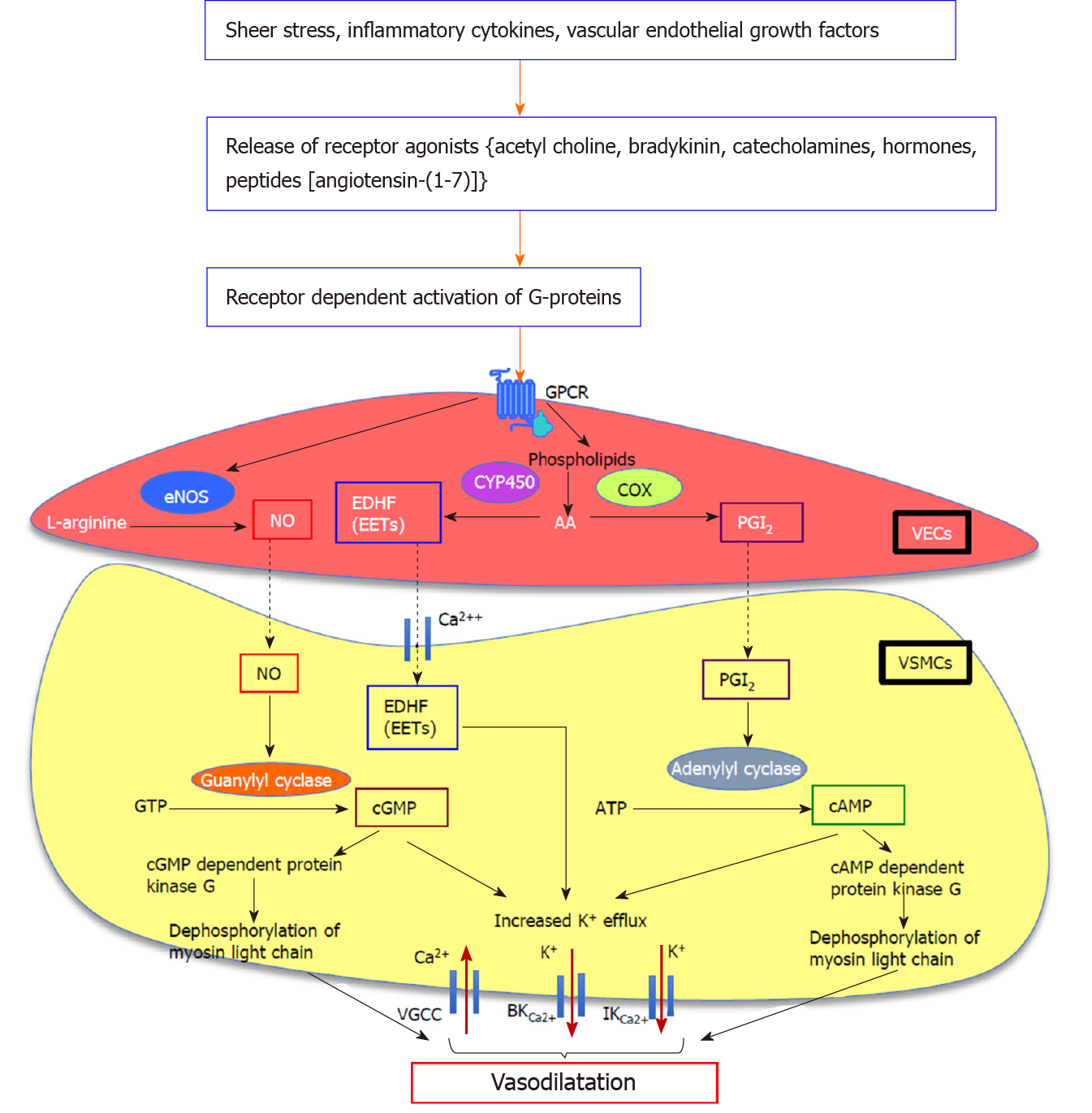
Figure 1 Proposed downstream signalling pathways of endothelium-dependent vasodilatation in splanchnic vasculature.
Nitric oxide (NO) is a key mediator of mesenteric vasodilatation in cirrhosis. G protein-coupled receptor-mediated activation of G-proteins augments the production and activation of a number of vasodilatory pathways including endothelial nitric oxide synthase/nitric oxide (eNOS/NO), vasodilating prostacyclins, and metabolites of arachidonic acid (AA) metabolism which act as endothelium-derived hyperpolarizing factors such as epoxyeicosatrienoic acids (EETs) in vascular endothelial cells. The eNOS converts L-arginine to produce NO which diffuses into the vascular smooth muscle cells (VSMCs). In VSMCs, NO which activates membrane-bound guanylyl cyclase enzyme to release cyclic guanosine monophosphate from guanosine triphosphate, mediates vasodilatation by increasing K+ efflux via large-conductance and intermediate-conductance calcium-activated potassium channels and by decreasing Ca2+ influx via voltage-gated calcium channels, and by dephosphorylation of myosin light chain. In addition to NO, vasodilators such as prostaglandin I2, derived from AA by the action of cyclooxygenase enzyme which activates membrane-bound adenylyl cyclase enzyme to release cyclic adenosine monophosphate from adenosine triphosphate, causes vasodilatation by increasing K+ efflux and dephosphorylation of myosin light chain. The EETs deriving from AA by the action of endothelial epoxygenases such as cytochrome P450 directly act on Ca2+ and K+ channels, causing hyperpolarization of VSMC membrane and subsequent vasodilatation. NO: Nitric oxide; GPCR: G protein-coupled receptor; eNOS/NO: Endothelial nitric oxide synthase/nitric oxide; AA: Arachidonic acid; EDHFs: Endothelium-derived hyperpolarizing factors; EETs: Epoxyeicosatrienoic acids; VECs: Vascular endothelial cells; VSMCs: Vascular smooth muscle cells; cGMP: Cyclic guanosine monophosphate; GTP: Guanosine triphosphate; BKCa2+: Large-conductance; IKCa2+: Intermediate-conductance; VGCCs: Voltage-gated calcium channels; PGI2: Prostaglandin I2; COX: Cyclooxygenase; cAMP: Cyclic adenosine monophosphate; ATP: Adenosine triphosphate; CYP450: Cytochrome P450.
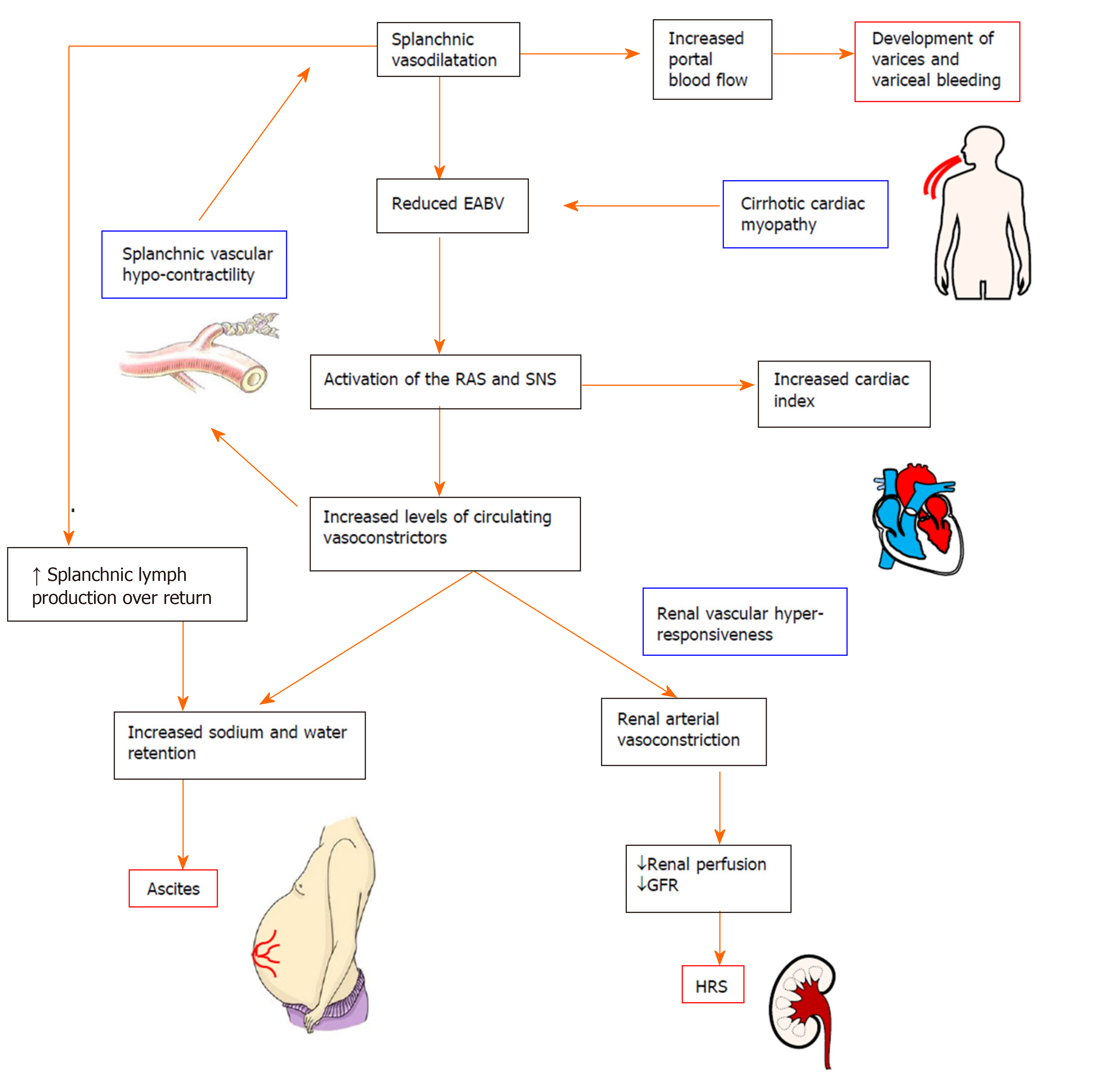
Figure 2 Pathophysiology of the complications in decompensated cirrhosis.
Splanchnic vasodilatation and subsequent reduction of effective arterial blood volume (EABV) triggers the activation of homeostatic mechanisms such as the renin angiotensin system and sympathetic nervous system to promote sodium and water retention and vasoconstriction. Increased hydrostatic pressure and increased capillary permeability in splanchnic vessels cause leaking of excess fluid into peritoneal cavity and the onset of ascites. The renal vasculature is hyper-responsive to the activated circulating vasoconstrictor systems, creating a deficit in renal perfusion and glomerular filtration rate which in turn leads to the development of hepatorenal syndrome. In decompensated cirrhosis, the compensatory mechanisms to restore EABV are ineffective due to the intrinsic splanchnic vascular hypocontractility and cardiomyopathy. EABV: Effective arterial blood volume; RAS: Renin angiotensin system; SNS: Sympathetic nervous system; GFR: Glomerular filtration rate; HRS: Hepatorenal syndrome.
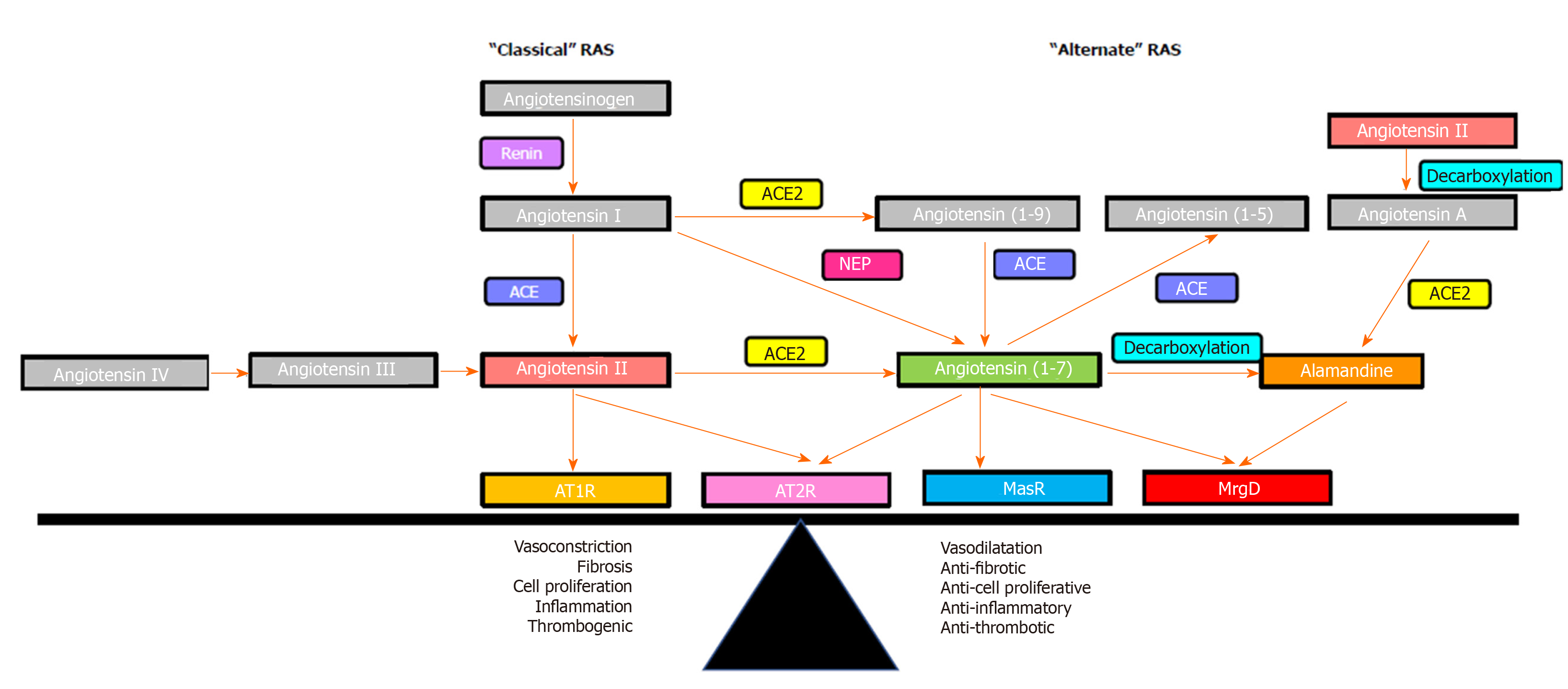
Figure 3 Overview of the renin angiotensin system.
The effects of the renin angiotensin system (RAS) are determined by the balance between its “classical axis” and the “alternate axis”. Classical axis comprises of angiotensin converting enzyme, angiotensin II, angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R), which mediate vasoconstrictive (AT1R) or vasodilatory (AT2R) functions, proinflammatory and profibrogenic pathways. The alternate axis comprises of angiotensin converting enzyme 2, angiotensin-(1-7) and the Mas receptor opposes the effects of the classical RAS[53]. Recent studies identified that the vasodilatory action of angiotensin-(1-7) is also mediated via the Mas-related G protein-coupled receptor-type D, which also mediates the action of alamandine. RAS: Renin angiotensin system; ACE: Angiotensin converting enzyme; ACE2: Angiotensin converting enzyme 2; AT1R: Angiotensin II type 1 receptor; AT2R: Angiotensin II type 2 receptor; MasR: Mas receptor; MrgD: Mas-related G protein-coupled receptor-type D.
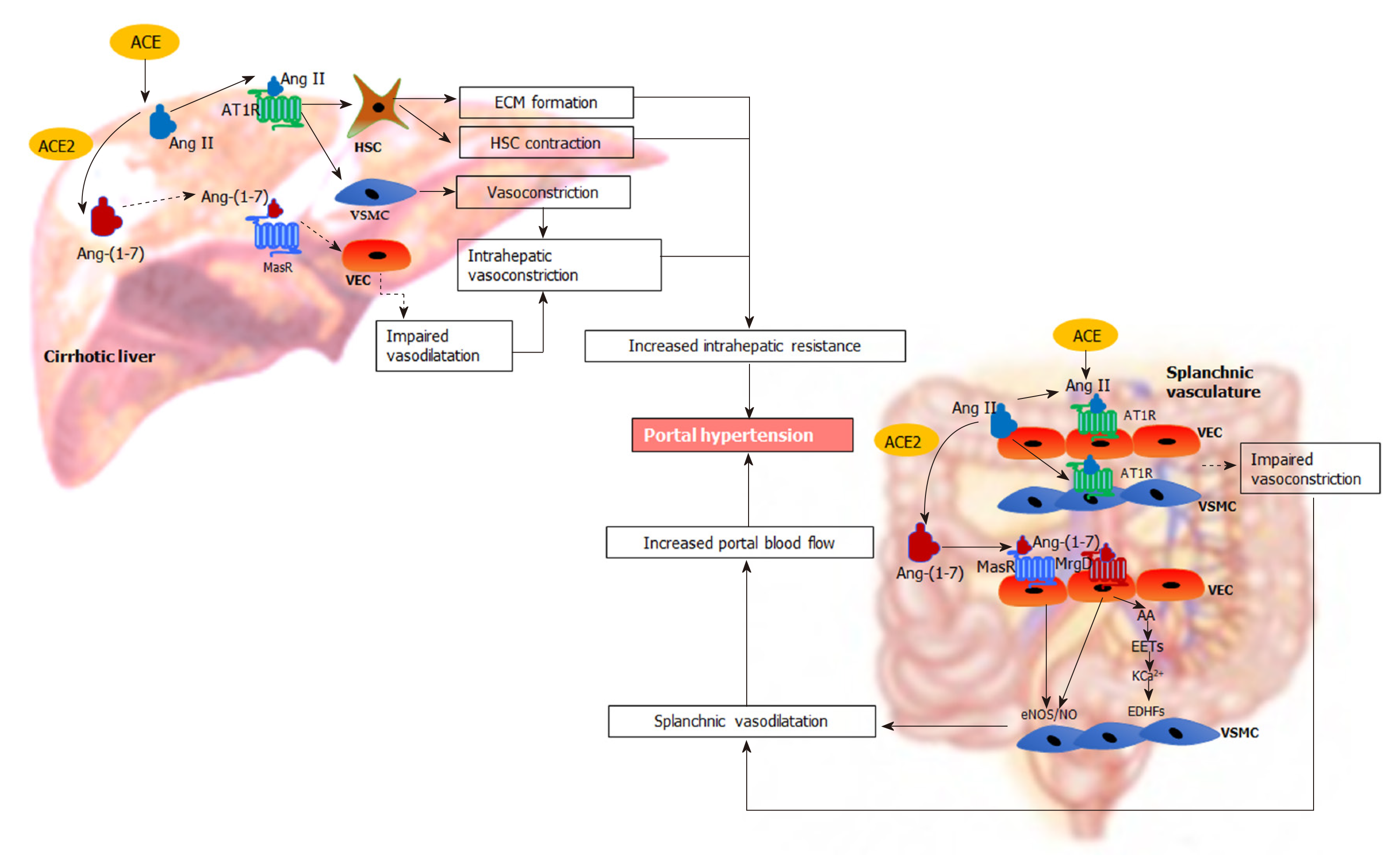
Figure 4 Renin angiotensin system-mediated pathophysiological changes in portal hypertension.
In the cirrhotic liver, the “classical axis” of the renin angiotensin system (RAS) predominates. The vasoconstrictor octapeptide angiotensin II (Ang II), via the Ang II type 1 receptor in hepatic stellate cells (HSC) increases the deposition of extracellular matrix proteins, creating a fixed barrier to portal blood flow within the cirrhotic liver. In addition, Ang II also increases intrahepatic vascular tone by stimulating contraction of activated HSCs and vascular smooth muscle cells (VSMCs). Reduced release of vasodilating intracellular signalling molecules such as nitric oxide (NO) from vascular endothelial cells (VECs) by the action of endothelial NO synthase further impairs intrahepatic vasodilatation. In contrast, the “alternate axis” of the RAS predominates in the cirrhotic splanchnic vascular bed. The vasodilator heptapeptide angiotensin-(1-7), via its Mas receptor and, the Mas related G protein coupled receptor type-D, increases the release of NO from VECs to promote the relaxation of VSMCs causing splanchnic vasodilatation. This condition may be exacerbated by intrinsic vascular hypocontractility of mesenteric arteries to vasoconstrictors such as Ang II. Other than NO activity, release of other potent endothelium-derived hyperpolarizing factors such as epoxyeicosatrienoic acids derived from arachidonic acid of membrane phospholipids may play an important role in splanchnic vasodilatation by increasing K+ efflux through Ca2+-activated K+ channels in VSMCs. These lead to an increased portal blood flow, resulting in increased portal pressure. ACE: Angiotensin converting enzyme; ACE2: Angiotensin converting enzyme 2; Ang II: Angiotensin II; AT1R: Angiotensin II type 1 receptor; Ang-(1-7): Angiotensin-(1-7); MasR: Mas receptor; MrgD: Mas-related G protein-coupled receptor-type D; HSC: Hepatic stellate cells; VSMCs: Vascular smooth muscle cells; VECs: Vascular endothelial cells; ECM: Extracellular matrix; eNOS/NO: Endothelial nitric oxide synthase/nitric oxide; EDHFs: Endothelium-derived hyperpolarizing factors; EETs: Epoxyeicosatrienoic acids; AA: Arachidonic acid; KCa2+: Ca2+-activated K+ channels.
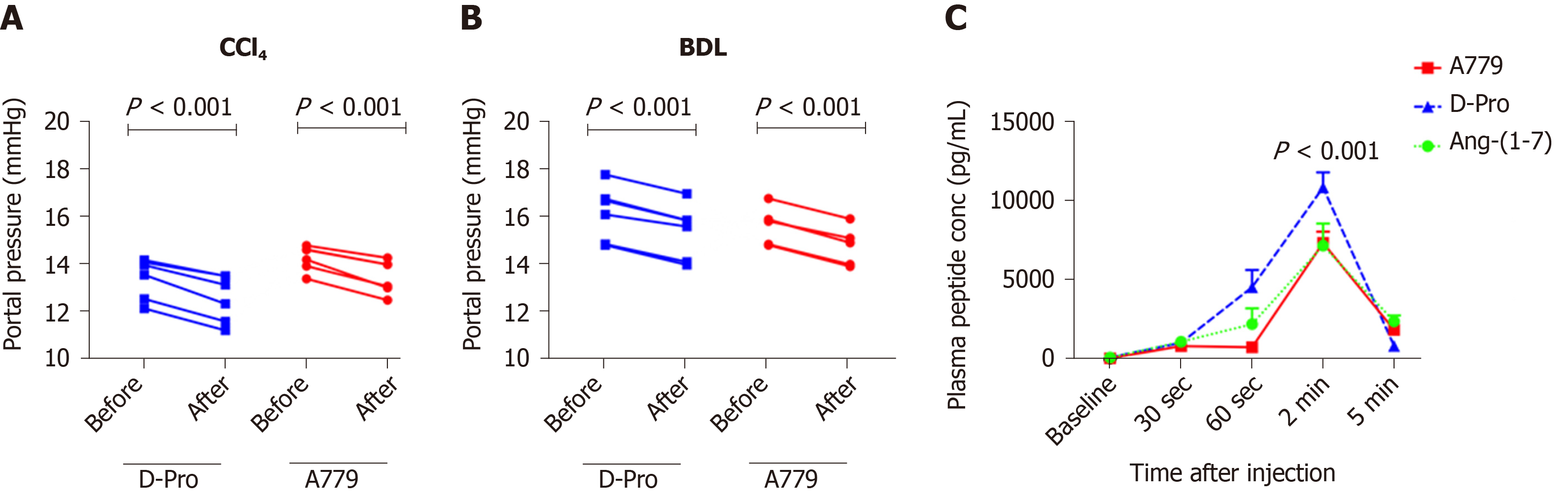
Figure 5 Portal pressure responses with Mas receptor and Mas-related G protein-coupled receptor type-D blockade and plasma angiotensin-(1-7) peptide (or blocker) concentrations.
A and B: Portal pressure responses after systemic administration of a bolus dose of Mas receptor (MasR) blocker D-Ala7-Ang-(1-7) (A779) (10 mg/kg) or Mas-related G protein-coupled receptor type-D (MrgD) blocker D-Pro7-Ang-(1-7) (D-Pro) (10 mg/kg) in cirrhotic carbon tetrachloride-induced (A) and bile duct ligated (B) rats. Both MrgD and MasR blockade significantly reduced portal pressure likely by blocking angiotensin-(1-7) [Ang-(1-7)] mediated splanchnic vasodilatation; C: Plasma concentrations of Ang-(1-7) peptide and blockers measured before and after a bolus intra-jugular injection of Ang-(1-7) peptide (3.5 mg), MasR blocker A779 (3.5 mg) or MrgD blocker D-Pro (3.5 mg). The peptide and the blockers were injected into healthy rats and plasma levels of Ang-(1-7) peptide were measured by a radioimmunoassay using an antibody directed at middle amino acids of the peptide. Each time point represents the mean ± SEM profile from 4 rats per treatment group. P < 0.001, baseline vs 2-min post-injection and 2-min vs 5-min post-injection. Adapted from reference[23]. CCl4: Carbon tetrachloride-induced; BDL: Bile duct ligated; A779: D-Ala7-Ang-(1-7); D-Pro: D-Pro7-Ang-(1-7); Ang-(1-7): Angiotensin-(1-7).
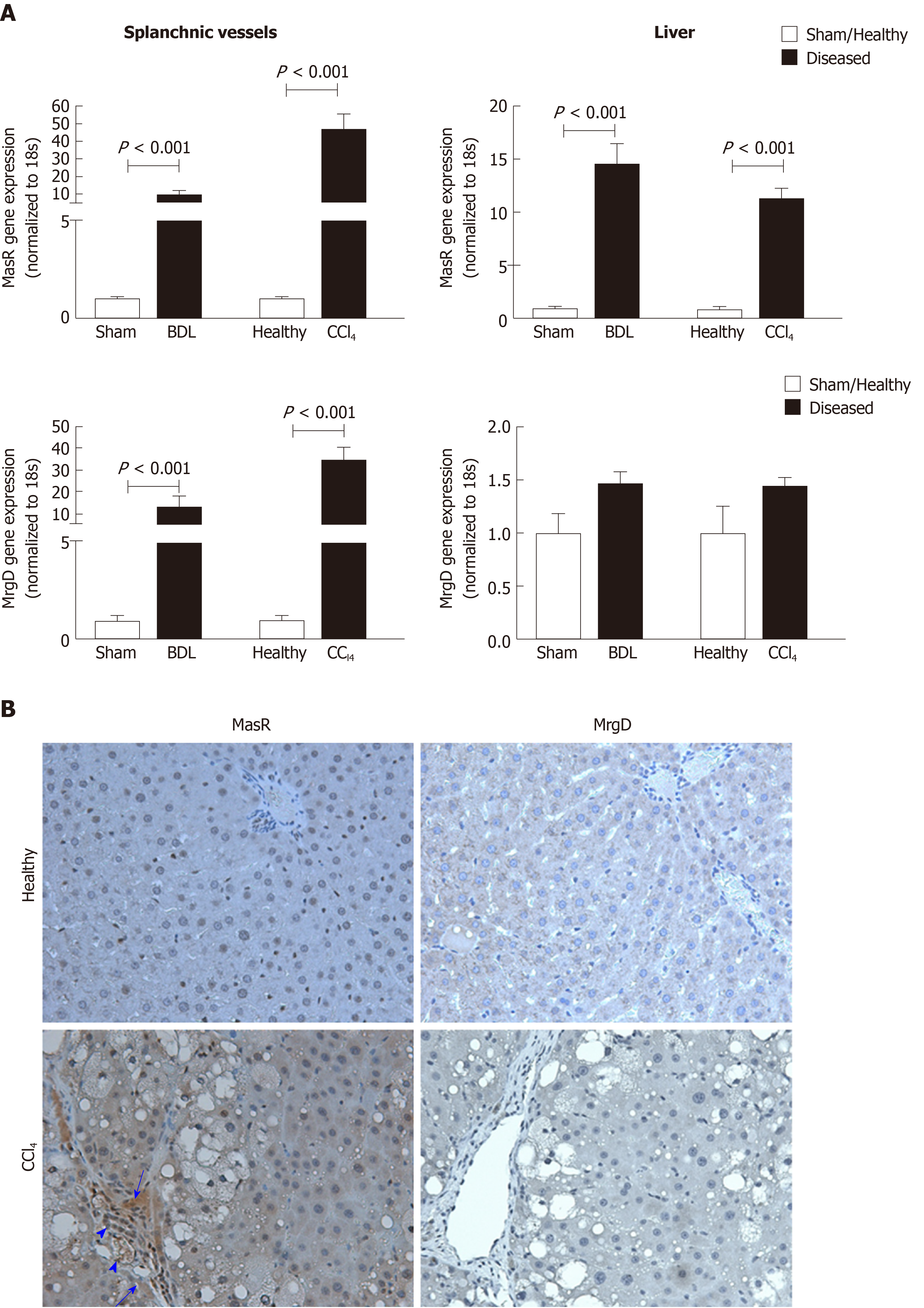
Figure 6 Expression of the receptors of the alternate renin angiotensin system in the splanchnic and hepatic vasculatures in cirrhosis.
A: Gene expression of Mas receptor (MasR) and Mas-related G protein-coupled receptor type-D (MrgD) in cirrhotic mesenteric arterial vessels and livers of carbon tetrachloride-injected (CCl4) and bile duct ligated rats compared with sham-operated and healthy controls. Each bar represents the mean ± SEM profile from 6-7 rats per group. Gene expression of both MasR and MrgD are upregulated in the splanchnic vascular bed of both cirrhotic models, suggesting that both receptors are likely involved in angiotensin-(1-7)-mediated splanchnic vasodilatation in cirrhosis. In marked contrast, MasR, but not MrgD, is upregulated in the cirrhotic livers, suggesting that MasR, but not MrgD of the alternate renin angiotensin system, likely contributes to the regulation of hepatic vascular resistance in cirrhosis; B: Shows immunohistochemical localization of MasR and MrgD in the livers of CCl4 rats and healthy controls. Consistent with gene expression analysis, strong positive staining for MasR is shown in liver sinusoids (arrow), bile duct epithelial cells (arrowhead-large) and hepatic arterioles (arrowhead-small) of the cirrhotic liver. Consistent with gene expression analysis, there was no positive staining for MrgD in the cirrhotic liver. Adapted from reference[23]. MasR: Mas receptor; MrgD: Mas-related G protein-coupled receptor-type D; CCl4: Carbon tetrachloride-injected; BDL: Bile duct ligated.
- Citation: Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol 2020; 26(40): 6111-6140
- URL: https://www.wjgnet.com/1007-9327/full/v26/i40/6111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i40.6111