Copyright
©The Author(s) 2018.
World J Gastroenterol. Sep 21, 2018; 24(35): 4036-4053
Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4036
Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4036
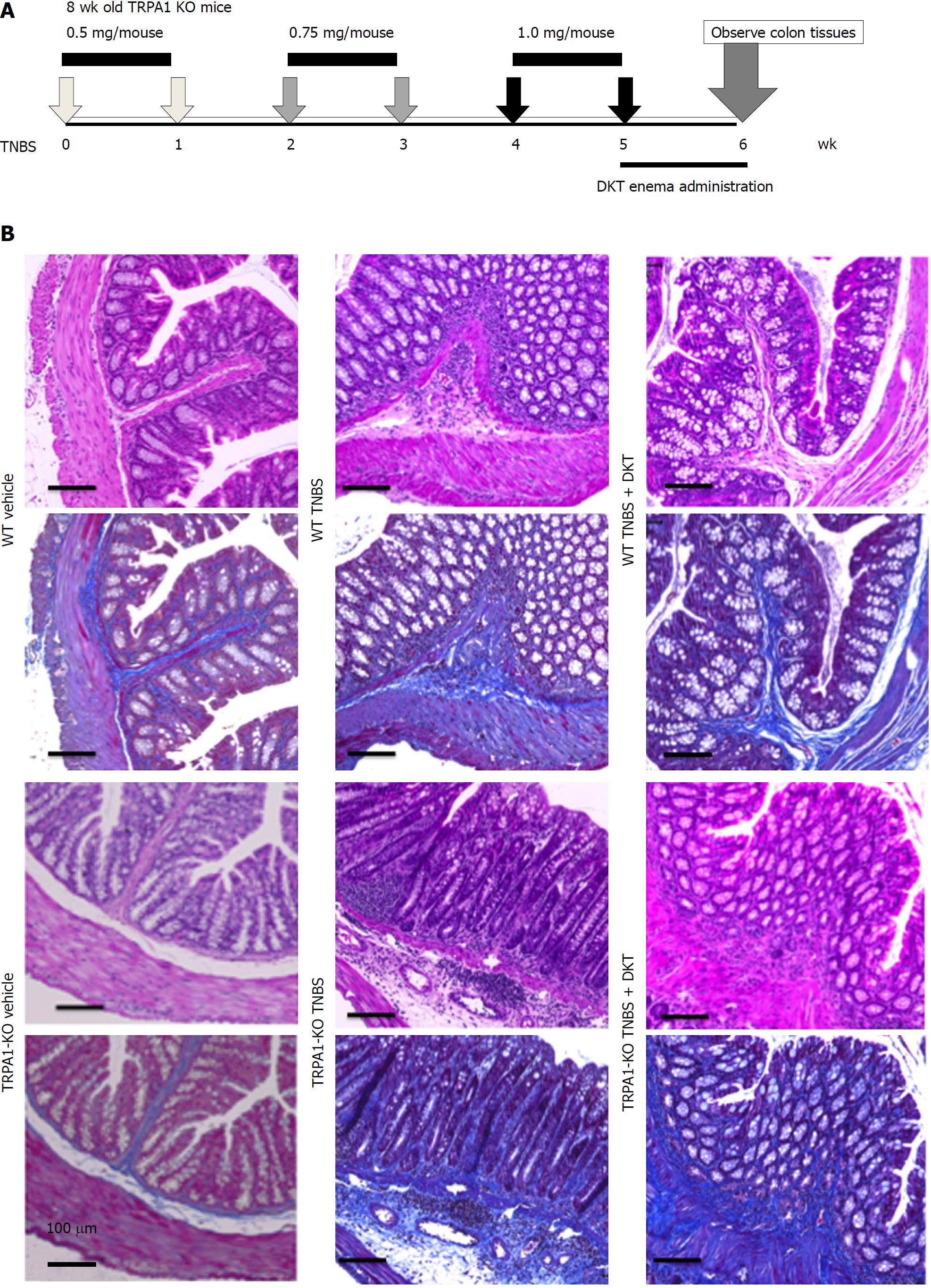
Figure 1 Chronic 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis models of wild-type and transient receptor potential ankyrin 1-knockout mice.
Chronic colitis was induced by weekly intrarectal injection of TNBS for six weeks. Immediately after the final TNBS treatment, a DKT enema was administered daily for one week. A: schematic presentation of TNBS injection and DKT enema; B: Hematoxylin–eosin (HE)- and Masson's trichrome (MT)-stained tissues from the vehicle control, TNBS and TNBS + DKT groups of WT and TRPA1-/- mice. TNBS: 2, 4, 6-trinitrobenzenesulfonic acid; WT: Wild-type; TRPA1: Transient receptor potential ankyrin 1; TRPA1-/-: Transient receptor potential ankyrin 1-knockout; DKT: Daikenchuto; HE: Hematoxylin-eosin; MT: Masson's trichrome.
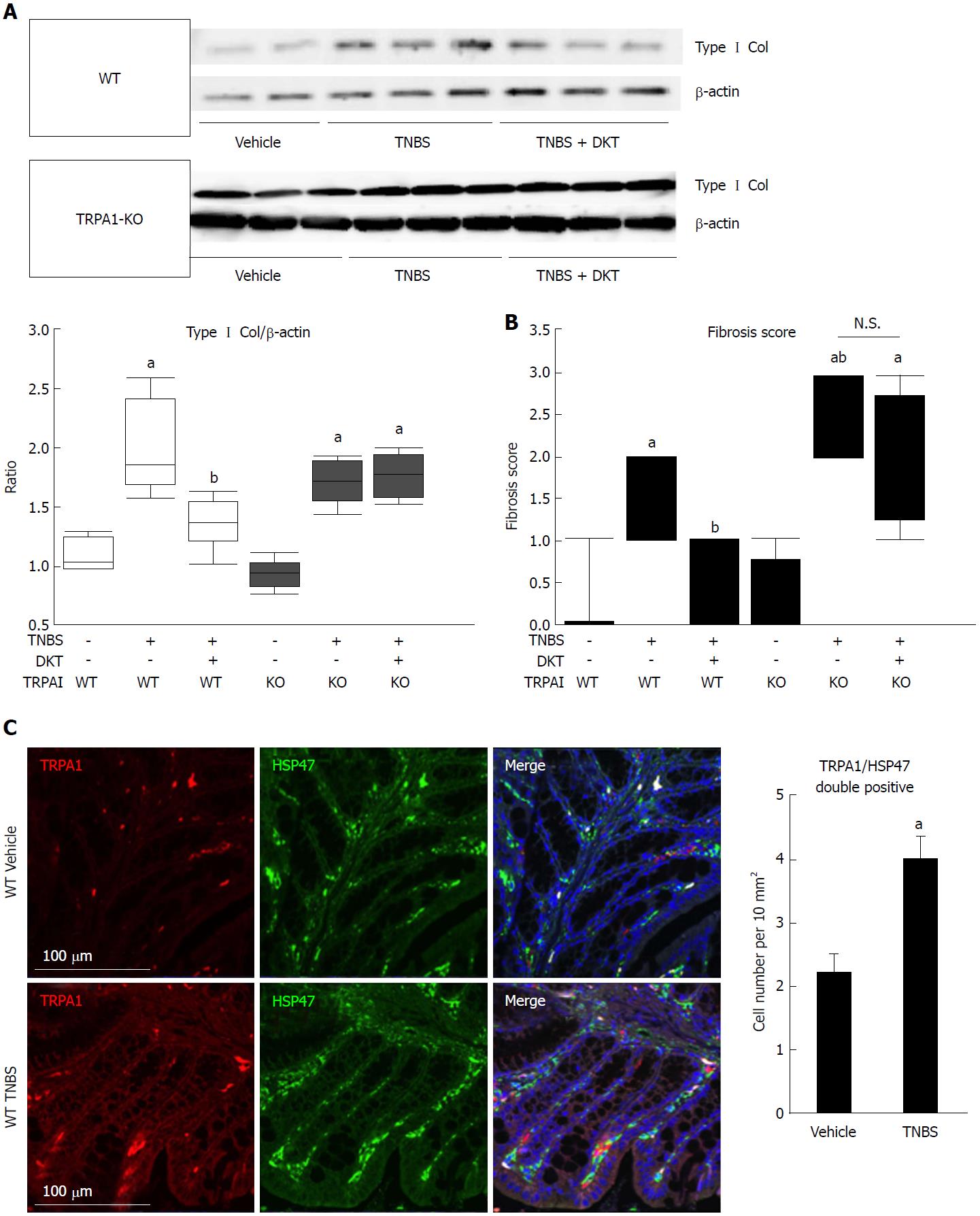
Figure 2 Fibrotic changes in wild-type and transient receptor potential ankyrin 1-knockout CRISPR mice treated with 2, 4, 6-trinitrobenzenesulfonic acid and/or Daikenchuto.
A: Type I collagen expression was examined by western blotting. The ratio of changes in each experimental condition is shown in the middle lower-left graph. aP < 0.01 vs vehicle control, bP < 0.01 vs TNBS (n = 6); B: Fibrosis scores based on histological examination ranging from 0 (no fibrosis) to 3 (severe fibrosis) are shown as the dot-plot graph. The leftmost circles (n = 8) show absolutely zero values. Values are the means ± SD; C: Co-localization of TRPA1 and HSP47 proteins in the mouse intestine. WT vehicle control, WT TNBS: Immunostaining images of mouse intestine stained with anti-TRPA1 (red) antibody, anti-HSP47 (green), and DAPI (blue). Cell number per 10 mm2 is summarized in right graph, aP < 0.05 vs vehicle control (n = 6). TNBS: 2, 4, 6-trinitrobenzenesulfonic acid; WT: Wild-type; HSP47: Heat shock protein 47; TRPA1: Transient receptor potential ankyrin 1; DAPI: 4',6-diamidino-2-phenylindole.
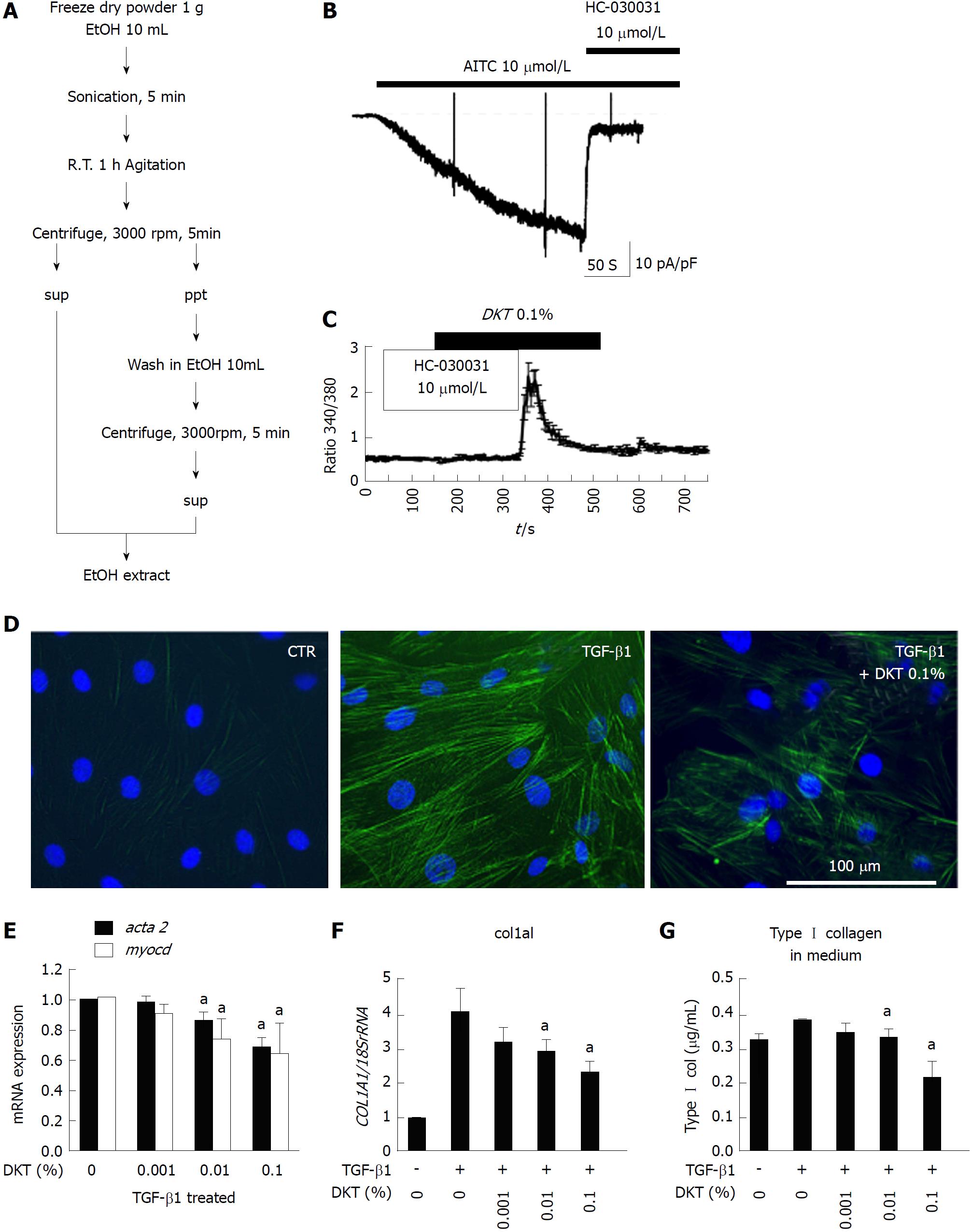
Figure 3 Daikenchuto-induced Ca2+ influx via transient receptor potential ankyrin 1 and anti-fibrosis effects in intestinal myofibroblasts.
A: Method for ethanol-extraction of DKT and its components, i.e., ginger, Japanese pepper, and ginseng; B: Representative traces of whole-cell currents recorded from InMyoFibs cells at a holding potential of -60 mV. TRPA1 nonselective cation currents are activated by AITC (10 μmol/L) and inhibited by HC-030031 (10 μmol/L); C: Representative [Ca2+]i response in InMyoFibs cells. Cells were exposed to DKT extracts (0.1%), which evoked prominent increases in [Ca2+]i. The TRPA1 antagonist HC-030031 (10 μmol/L) was added to examine whether the [Ca2+]i response involved the activation of TRPA1 channels. Respective data points represent means ± SEM from > 30 cells; D: Immunostaining images of InMyoFibs with anti-α-SMA (green) antibody and DAPI (blue) in untreated controls, 24 h TGF-β1 (5 ng/mL) treatment, or 24 h TGF-β1 (5 ng/mL) + DKT extracts (0.1%) treatment; E and F: DKT extracts (0.001%, 0.01%, and 0.1%) were co-administered with TGF-β1, and acta2 (α-SMA), myocd (myocardin), and col1a1 mRNA expression levels were quantified by real-time PCR; G: Concentrations of Type I collagen in the culture medium were measured by ELISA from the control and TGF-β1-treated cells. aP < 0.05 vs TGF-β1-treated cells (n = 4). DKT extracts (0.001%, 0.01%, and 0.1%) were co-administered with TGF-β1. DKT: Daikenchuto; InMyoFibs: Intestinal myofibroblasts; TRPA1: Transient receptor potential ankyrin 1; AITC: Allyl isothiocyanate; α-SMA: α-Smooth muscle actin; DAPI: 4',6-diamidino-2-phenylindole; TGF-β1: Transforming growth factor-β1.
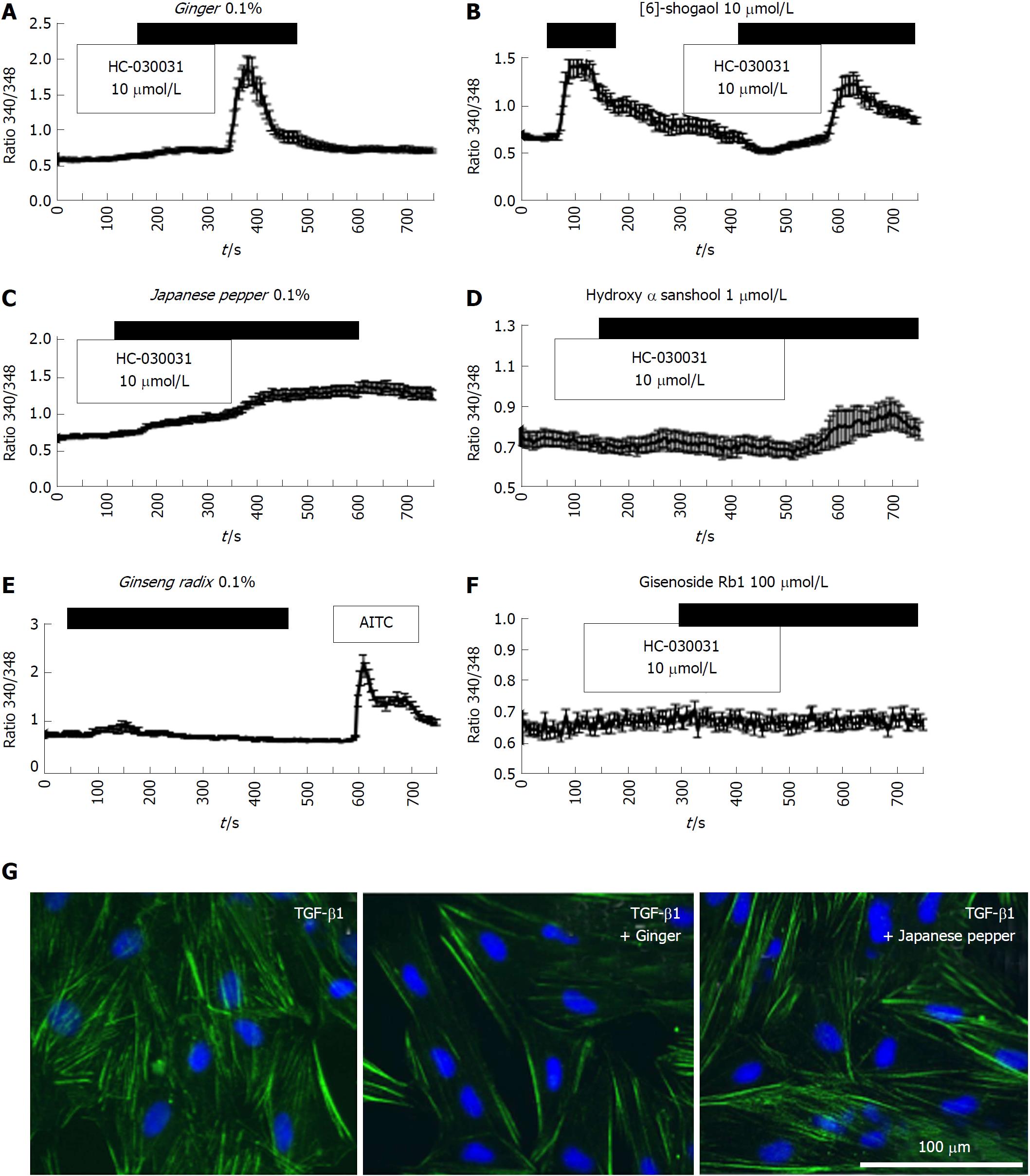
Figure 4 Crude components/ingredient of Daikenchuto induce Ca2+ responses via the transient receptor potential ankyrin 1 channel with anti-fibrotic effects in intestinal myofibroblasts.
A-D: Representative [Ca2+]i responses in InMyoFibs cells. Cells were exposed to ginger (0.1%), Japanese pepper (0.1%), 6-shogaol (10 μmol/L), or hydroxy-α-sanshool (1 μmol/L), which evoked measurable increases in [Ca2+]i; E and F: Cells were exposed to ginseng radix (0.1%), ginsenoside Rb1 (100 μmol/L), or AITC (10 μmol/L). Only AITC evoked a measurable increase in [Ca2+]i in InMyoFib. The vertical axis indicates the 340/380 fluorescence ratio. Respective data points represent means ± SEM from > 30 cells; G: Ginger (0.1%) or Japanese pepper (0.1%) suppressed TGF-β1-induced α-SMA expression in InMyoFibs. Immunostaining images of InMyoFibs with anti-α-SMA (green) antibody and DAPI (blue), and no treatment or 24 h TGF-β1 (5 ng/mL) treatment. AITC: Allyl isothiocyanate; InMyoFib: Intestinal myofibroblast; α-SMA: α-Smooth muscle actin; TGF-β1: Transforming growth factor-β1.
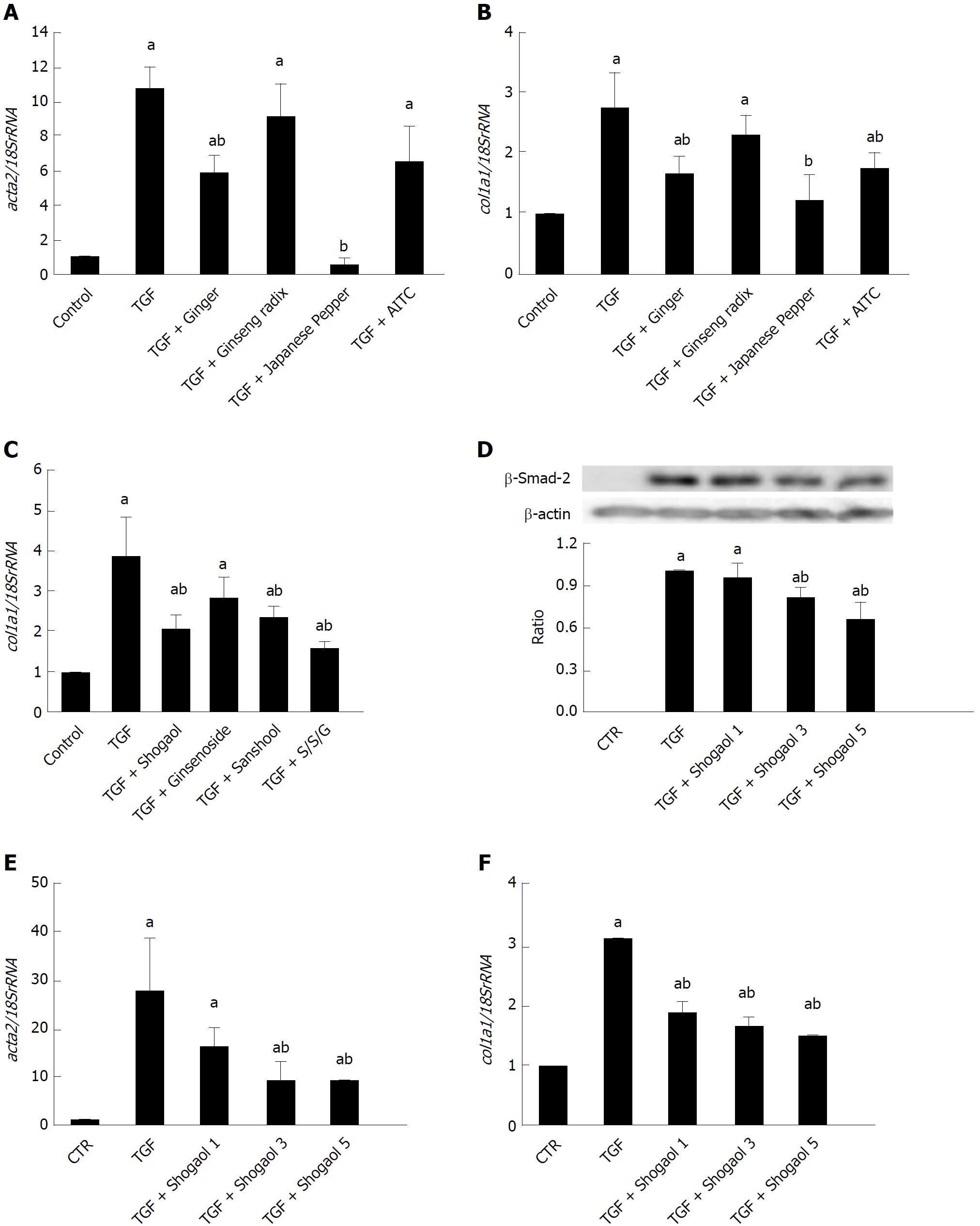
Figure 5 Daikenchuto components/ingredients suppress increased expression of α-smooth muscle actin and collagen by transforming growth factor-β1.
ACTA2 and COL1A1 mRNAs were quantified by real-time PCR in control and TGF-β1-treated (5 ng/mL, 24 h) InMyoFibs. A-C: DKT components Ginger (0.1%), Japanese pepper (0.1%) and Ginseng radix (0.1%) (A, B), and DKT ingredients 6-shogaol (10 μmol/L), hydroxy-α-sanshool (1 μmol/L) and ginsenoside Rb1 (100 μmol/L) (C) were co-administered with TGF-β1. AITC (10 μmol/L) was used as the reference of full TRPA1 activation; D: Phosphorylation of Smad-2 was measured by western blot analysis. 1, 3, or 5 μmol/L 6-shogaol was co-administered with TGF-β1. Relative expression of Smad-2 protein (normalized to β-actin) in TGF-β1-treated cells is shown as the ratio of the two in the histogram; E and F: COL1A1 and ACTA2 mRNAs were quantified by RT-PCR in TGF-β1 treated InMyoFibs and normalized to 18S rRNA. 1, 3 or 5 μmol/L 6-shogaol was co-administered with TGF-β1. aP < 0.05 vs control cells; bP < 0.05 vs TGF-β1-treated cells (n = 4). TGF-β1: Transforming growth factor-β1; InMyoFibs: Intestinal myofibroblasts; DKT: Daikenchuto; AITC: Allyl isothiocyanate; TRPA1: Transient receptor potential ankyrin 1.
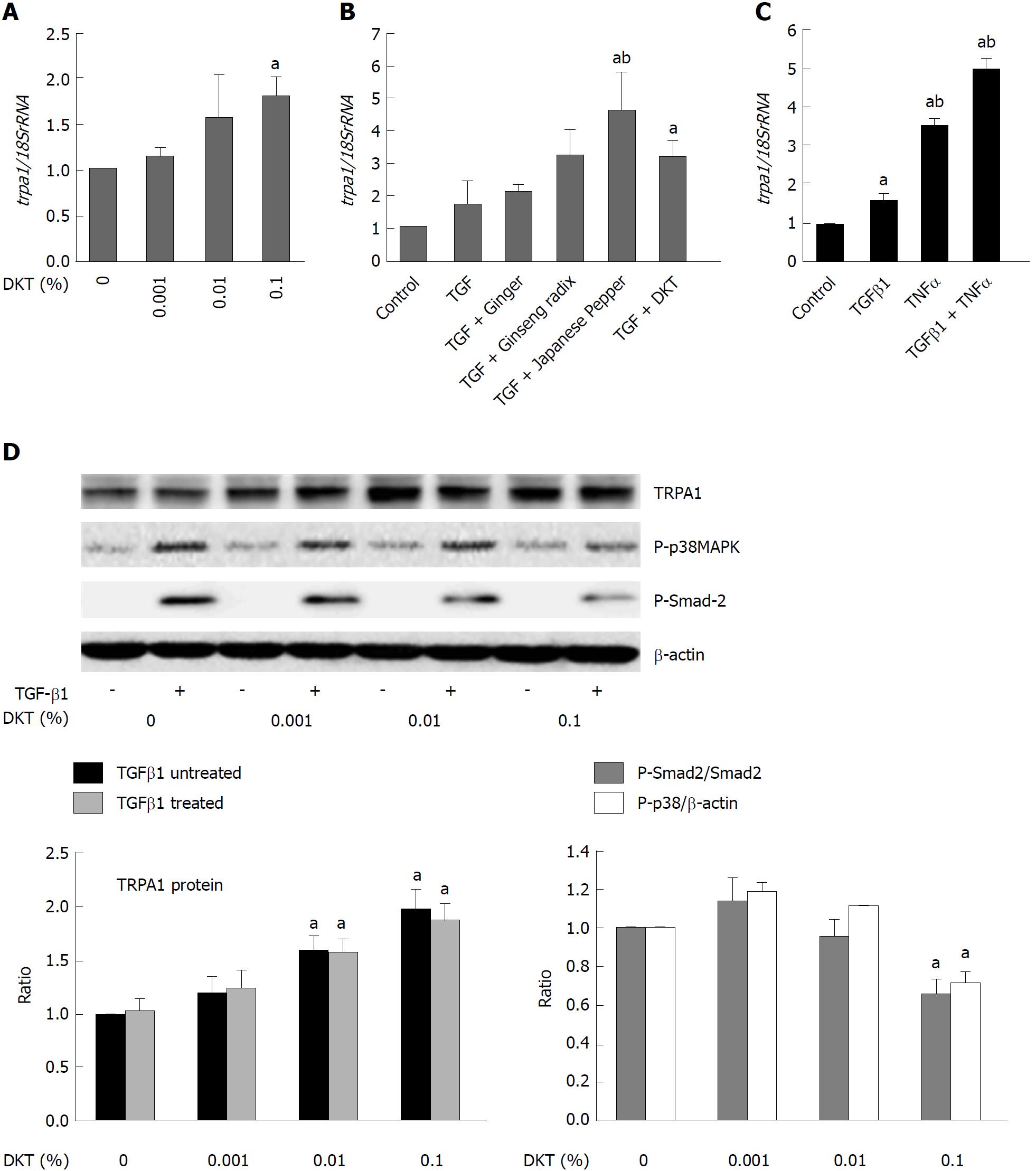
Figure 6 Daikenchuto upregulates transient receptor potential ankyrin 1 expression and suppresses phosphorylation events downstream of transforming growth factor-β1 signaling.
A: trpa1 mRNA expression quantified by real-time PCR in control and TGF-β1-treated (5 ng/mL, 24 h) InMyoFibs (normalized to 18S rRNA). DKT extracts (0.001%, 0.01%, 0.1%) were applied for 24 h; B: Relative mRNA expression of trpa1 with ginger (0.1%), ginseng radix (0.1%), Japanese pepper (0.1%), or DKT (0.1%) plus co-administered TGF-β1; C: trpa1 mRNA expression was upregulated by TGF-β1, TNF-α, or both; D: TRPA1 protein expression and phosphorylation of Smad-2 or p38-MAPK with and without TGF-β1 treatment at various concentrations of DKT extracts (0.001%, 0.01%, and 0.1%). The upper panel shows actual immunoblots. Graphs show relative expression level of TRPA1 (normalized to β-actin) and the relative ratios of phosphorylated/unphosphorylated Smad-2 and p38-MAPK (normalized to β-actin) after 1 h TGF-β1 treatment at various DKT concentrations. aP < 0.05 vs control cells, bP < 0.05 vs TGF-β1-treated cells (n = 4). TGF-β1: Transforming growth factor-β1; InMyoFibs: Intestinal myofibroblasts; DKT: Daikenchuto; TRPA1: Transient receptor potential ankyrin 1; p38-MAPK: p38-mitogen-activated protein kinase.
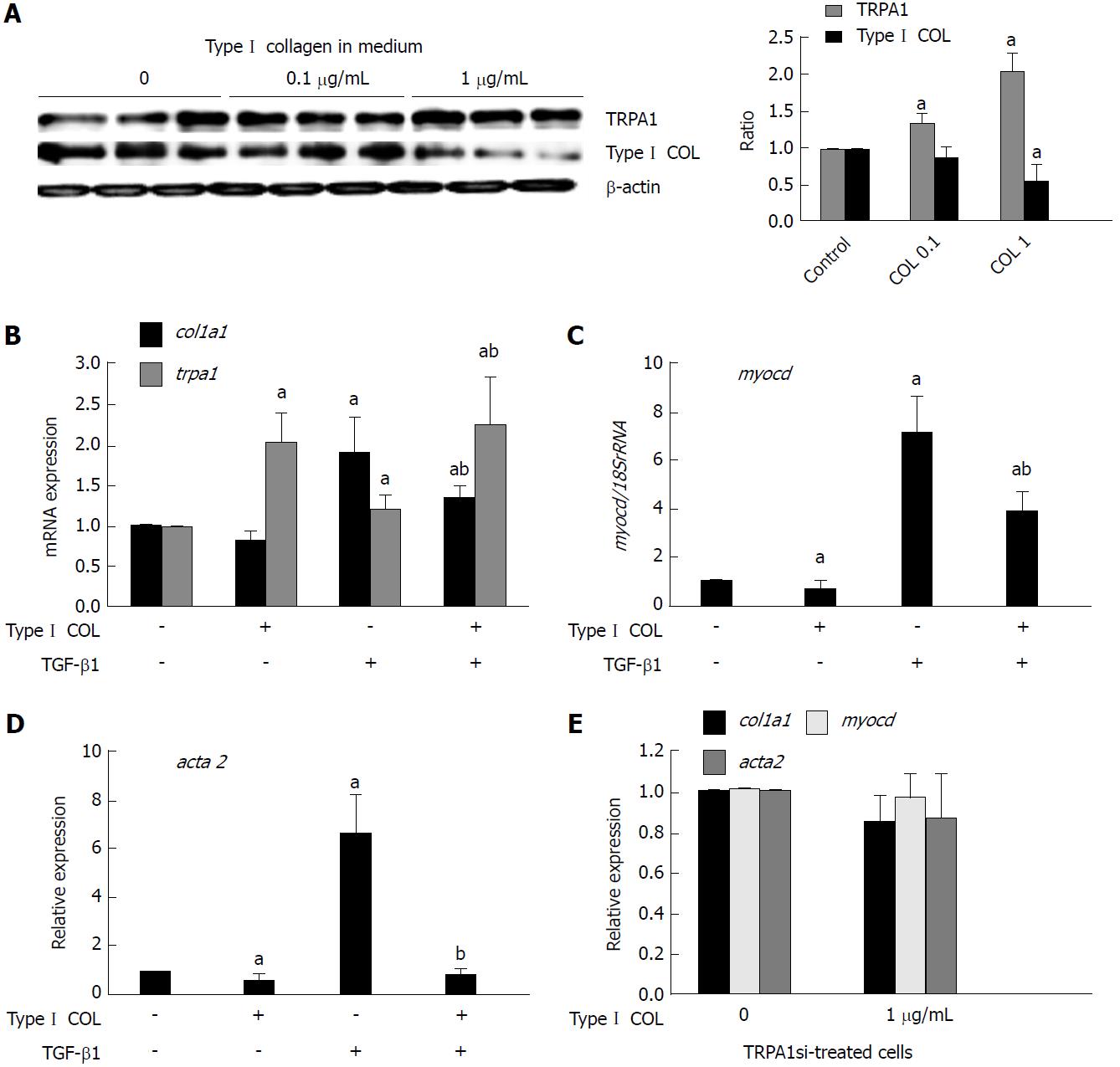
Figure 7 Extracellular collagen upregulates transient receptor potential ankyrin 1 expression to exert anti-fibrotic effects.
A: Type I collagen solution (0, 0.1, or 1 μg/mL) was added into the culture medium, and immunoblotting was performed to detect TRPA1 and Type I collagen expression in InMyoFibs. The rightmost graph shows the summary (n = 4) B-E: Relative expression levels (shown as the post/pre ratio) of col1a1, trpa1, myocd, and acta2 mRNAs quantified by RT-PCR in Type Icollagen- or TGF-β1-treated InMyoFibs. In E, InMyoFibs were pre-treated with TRPA1 siRNA. aP < 0.05 vs control cells; bP < 0.05 vs TGF-β1-treated cells (n = 4). TRPA1: Transient receptor potential ankyrin 1; InMyoFibs: Intestinal myofibroblasts; TGF-β1: Transforming growth factor-β1.
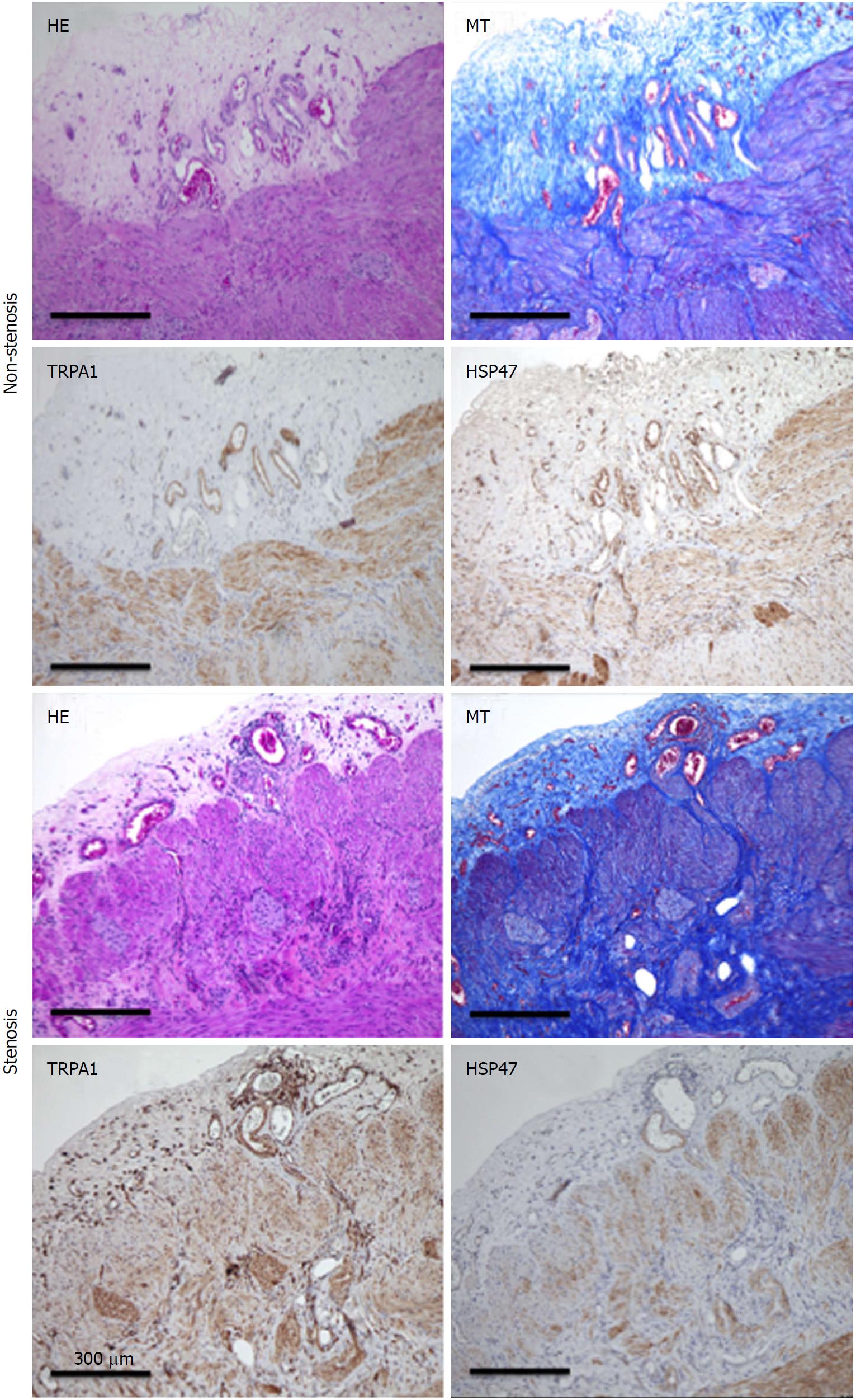
Figure 8 Hematoxylin-eosin, Masson’s trichrome staining.
TRPA1-DAB, and HSP47-DAB-stained non-stenosis/stenosis surgical tissue from the transverse colon of a CD patient. TRPA1: Transient receptor potential ankyrin 1; DAB: Diaminobenzidine; HSP47: Heat shock protein 47; CD: Crohn’s disease.
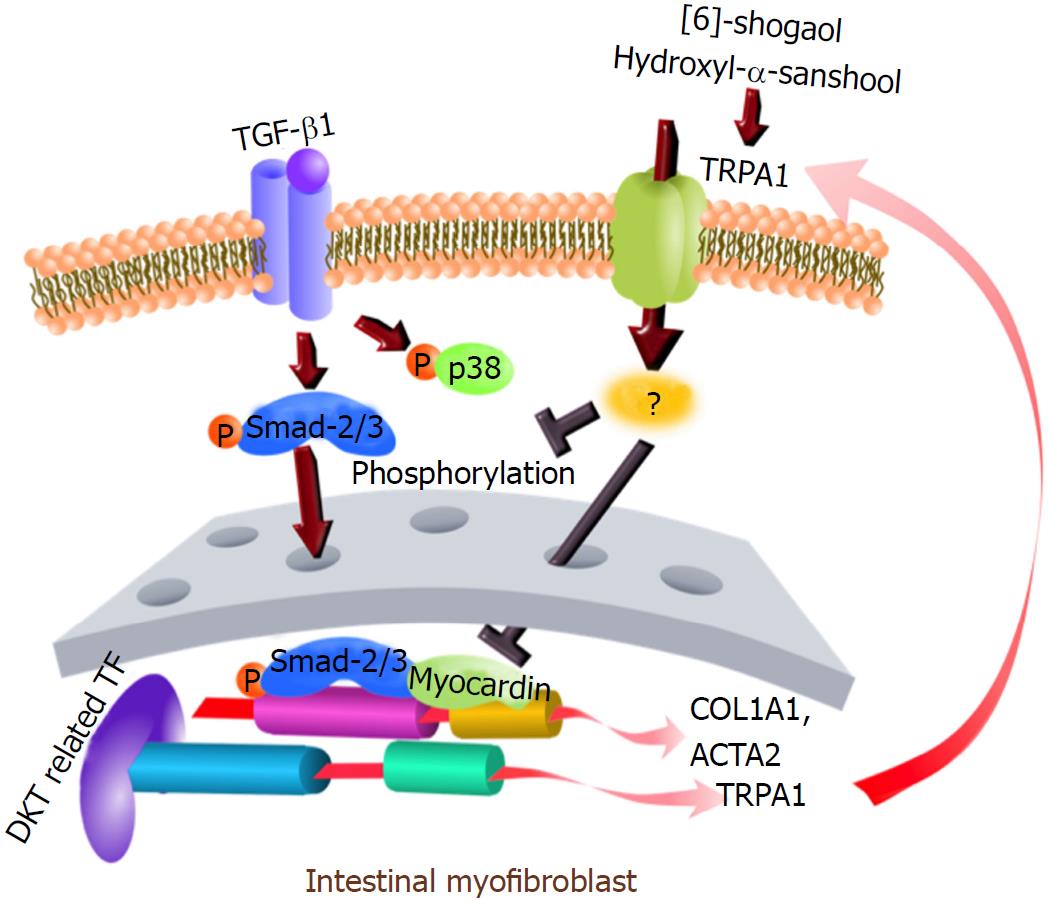
Figure 9 Graphical abstract.
- Citation: Hiraishi K, Kurahara LH, Sumiyoshi M, Hu YP, Koga K, Onitsuka M, Kojima D, Yue L, Takedatsu H, Jian YW, Inoue R. Daikenchuto (Da-Jian-Zhong-Tang) ameliorates intestinal fibrosis by activating myofibroblast transient receptor potential ankyrin 1 channel. World J Gastroenterol 2018; 24(35): 4036-4053
- URL: https://www.wjgnet.com/1007-9327/full/v24/i35/4036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i35.4036