Copyright
©The Author(s) 2018.
World J Gastroenterol. Jul 14, 2018; 24(26): 2867-2877
Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2867
Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2867
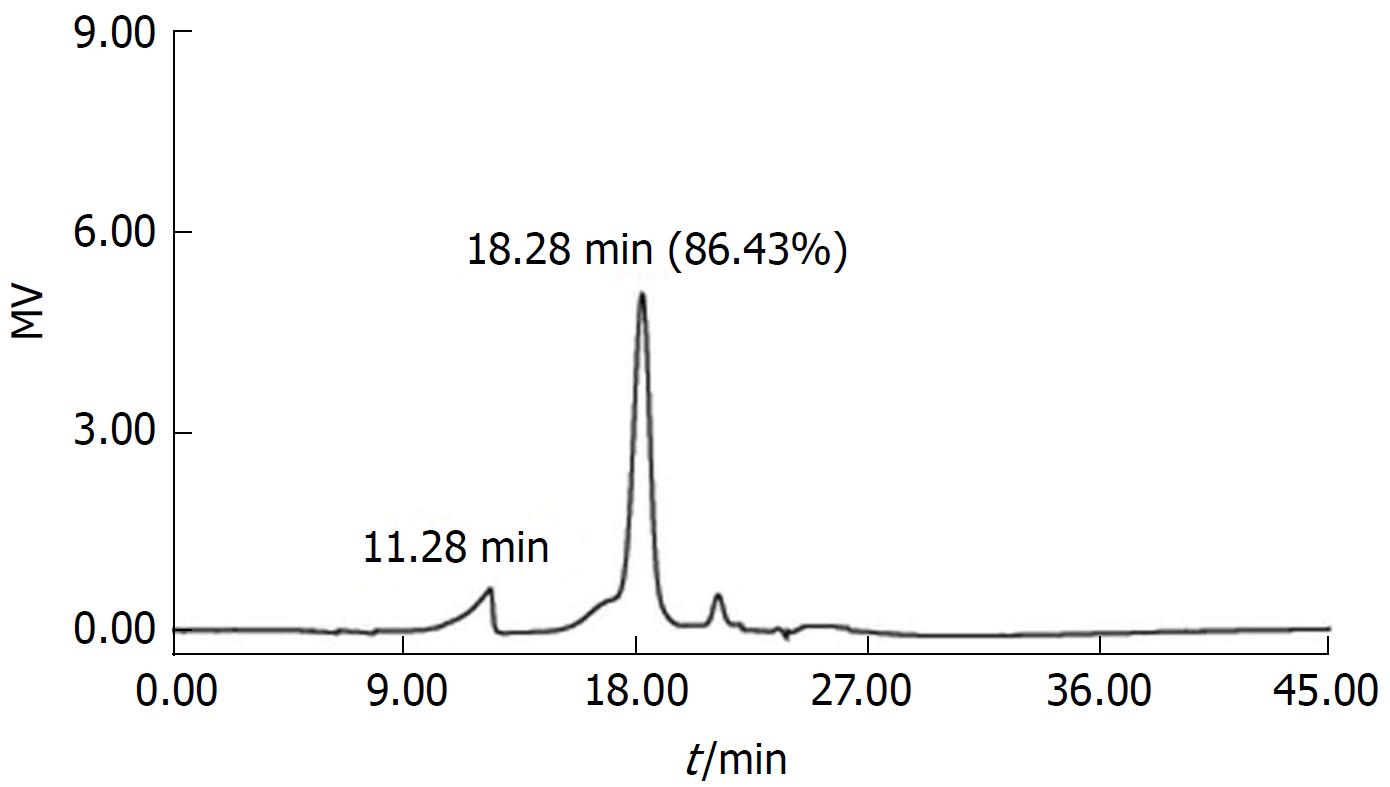
Figure 1 Gel permeation chromatography of total polysaccharides of the Sijunzi decoction.
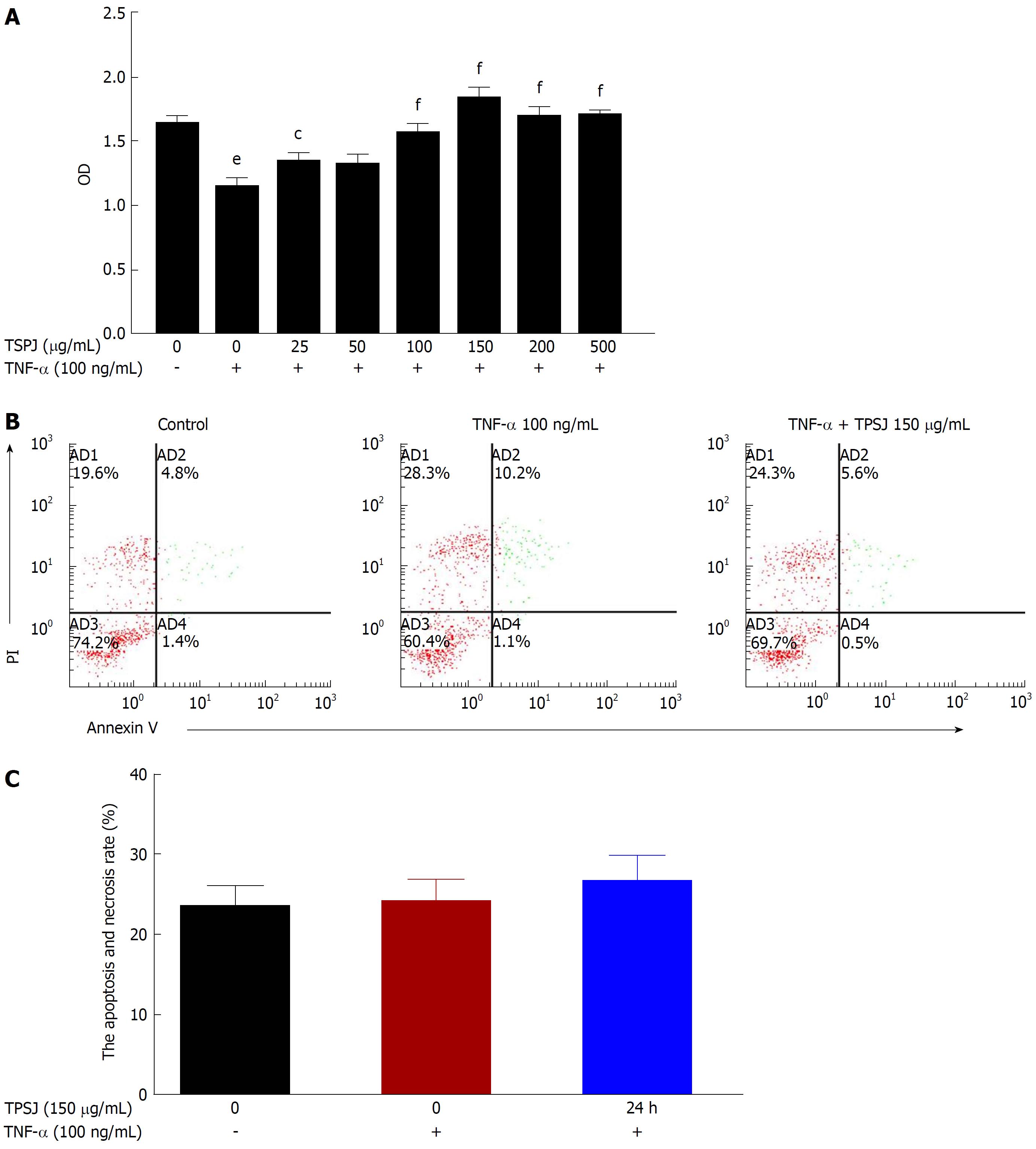
Figure 2 Total polysaccharides of the Sijunzi decoction promoted the proliferation of tumor necrosis factor-α-damaged Caco-2 cells.
A: Caco-2 cells were treated with various concentrations of TPSJ (25-500 μg/mL) or control DMEM for 24 h. The effect of TPSJ on cell viability was measured using the MTT assay; B: TNF-α-damaged Caco-2 cells were incubated with 150 μg/mL TPSJ or control DMEM for 24 h. The induction of apoptosis was determined using an Annexin V-FITC/propidium iodide staining assay; C: Quantification of the numbers of apoptotic and necrotic cells. Data are shown as the means ± standard errors of the means of at least three independent experiments (eP < 0.001 vs control Caco-2 cells; cP < 0.05, fP < 0.001 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; TNF: Tumor necrosis factor; DMEM: Dulbecco’s modified Eagle’s medium; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Figure 3 Total polysaccharides of the Sijunzi decoction attenuated intestinal epithelial barrier dysfunction induced by tumor necrosis factor-α.
A: Caco-2 monolayers were incubated with or without 100 ng/mL TNF-α in the absence or presence of TPSJ for 24 h. TPSJ significantly increased the transepithelial electrical resistance of Caco-2 cell monolayers damaged by TNF-α; B: TPSJ markedly attenuated phenolsulfonphthalein flux across the epithelial monolayers. Data are shown as the means ± standard errors of the means of at least three independent experiments (bP < 0.01 vs control Caco-2 cells; cP < 0.05 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; TNF: Tumor necrosis factor.
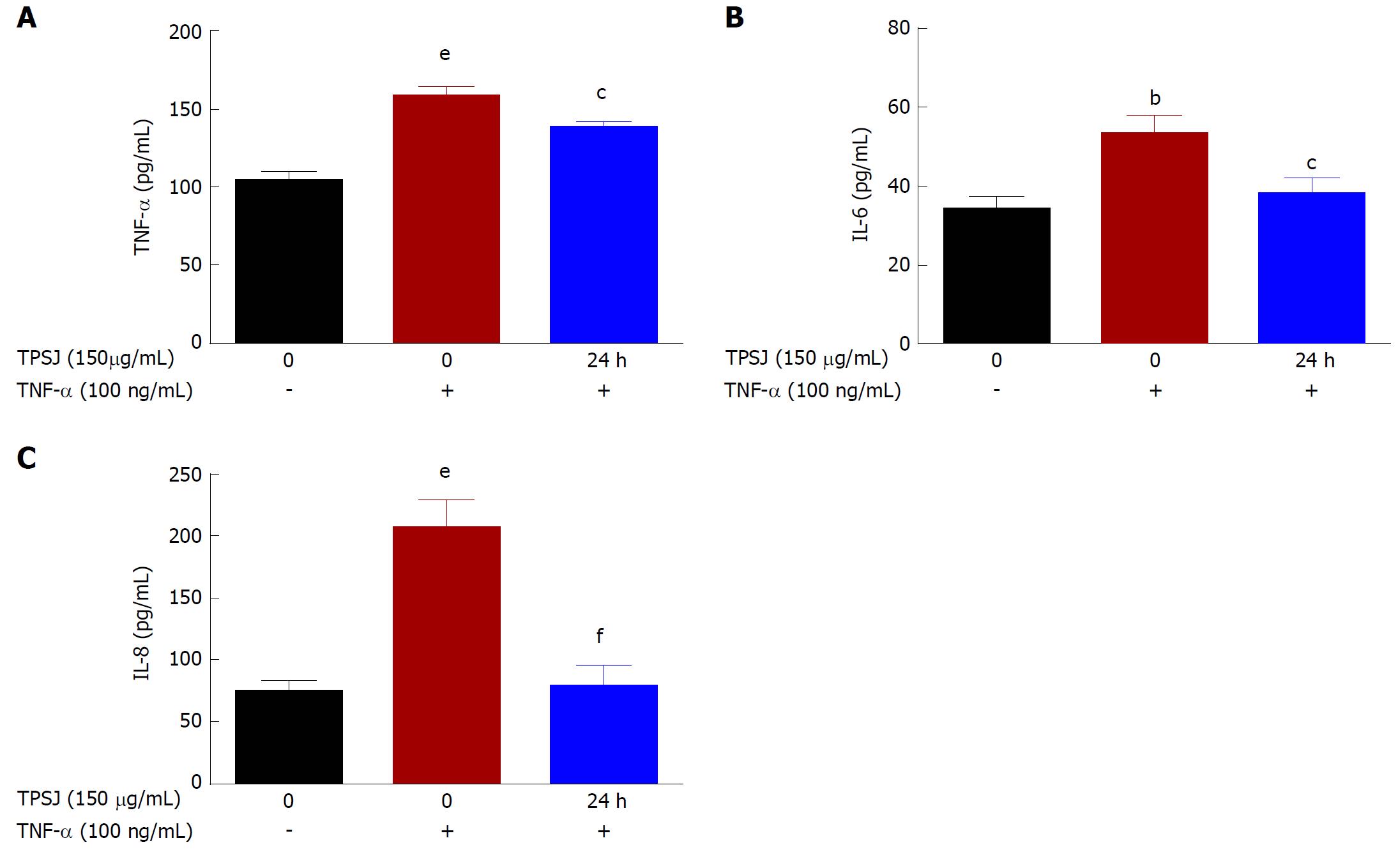
Figure 4 Total polysaccharides of the Sijunzi decoction regulated the secretion of inflammatory cytokines by Caco-2 cells.
Caco-2 cells were incubated with or without 100 ng/mL TNF-α in the absence or presence of TPSJ for 24 h. Cell culture media were collected, and the concentrations of TNF-α (A), IL-6 (B), and IL-8 (C) were detected by ELISA (bP < 0.01, eP < 0.001 vs control Caco-2 cells; cP < 0.05, fP < 0.001 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; TNF: Tumor necrosis factor.
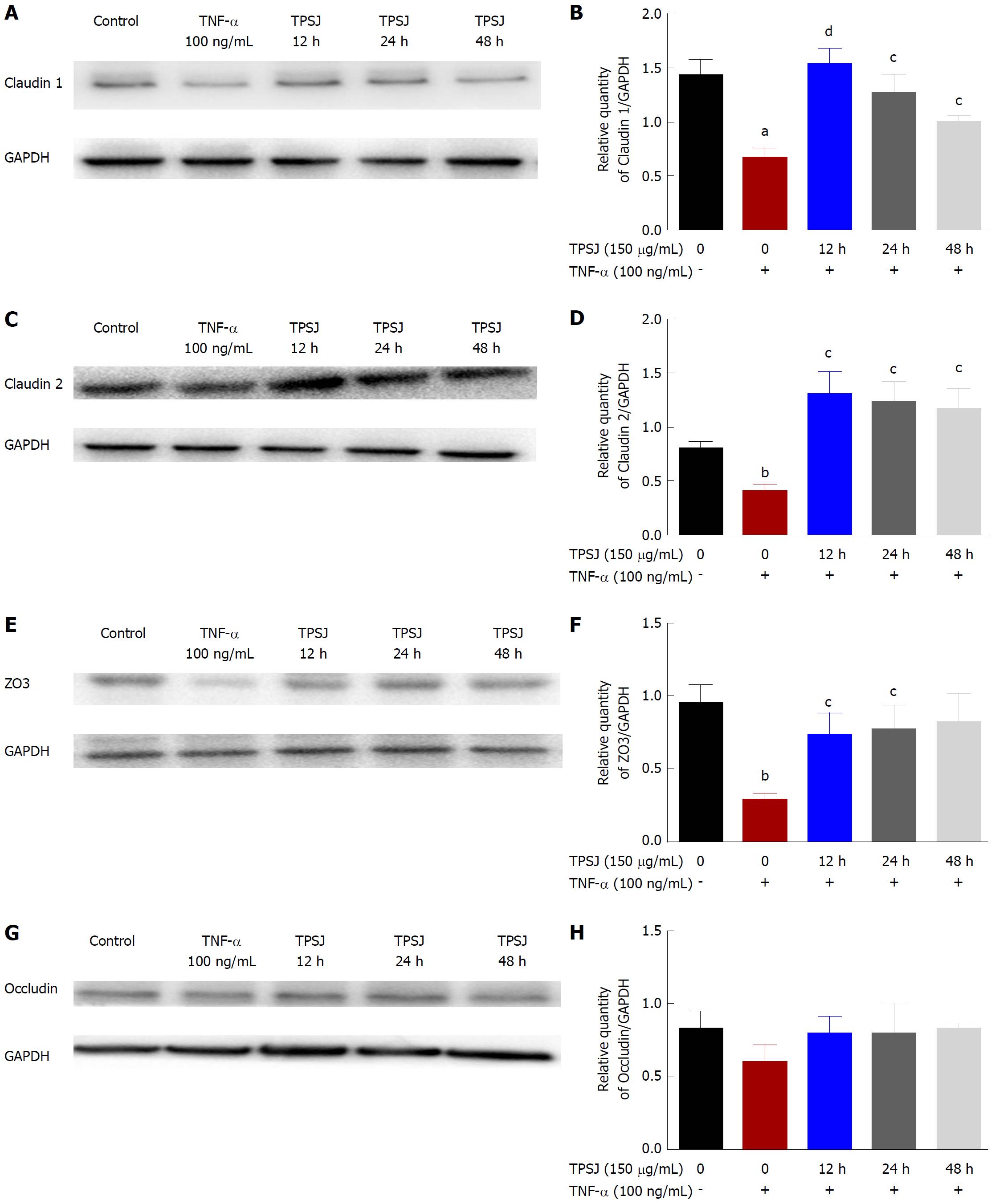
Figure 5 Total polysaccharides of the Sijunzi decoction modified the expression of claudin 1, claudin 2, zo3, and occludin.
A: A representative western blot of claudin 1 expression in TNF-α-damaged Caco2 cells treated with TPSJ for 12 h, 24 h, and 48 h; B: Quantification of the amounts of claudin 2 relative to GAPDH at different time points; C: A representative western blot of claudin 2 in TNF-α-damaged Caco-2 cells treated with TPSJ for 12 h, 24 h, and 48 h; D: Quantification of the amounts of claudin 2 relative to GAPDH at different time points; E: A representative western blot of zo3 in TNF-α-damaged Caco-2 cells treated with TPSJ for 12 h, 24 h, and 48 h; F: Quantification of the amounts of zo3 relative to GAPDH at different time points; G: A representative western blot of occludin in TNF-α-damaged Caco-2 cells treated with TPSJ for 12 h, 24 h, and 48 h. H: Quantification of the amounts of occludin relative to GAPDH at different time points. Data are shown as the means ± standard errors of the means of at least three independent experiments (aP < 0.05, bP < 0.01 vs control Caco-2 cells; cP <0.05, dP < 0.01 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; TNF: Tumor necrosis factor.
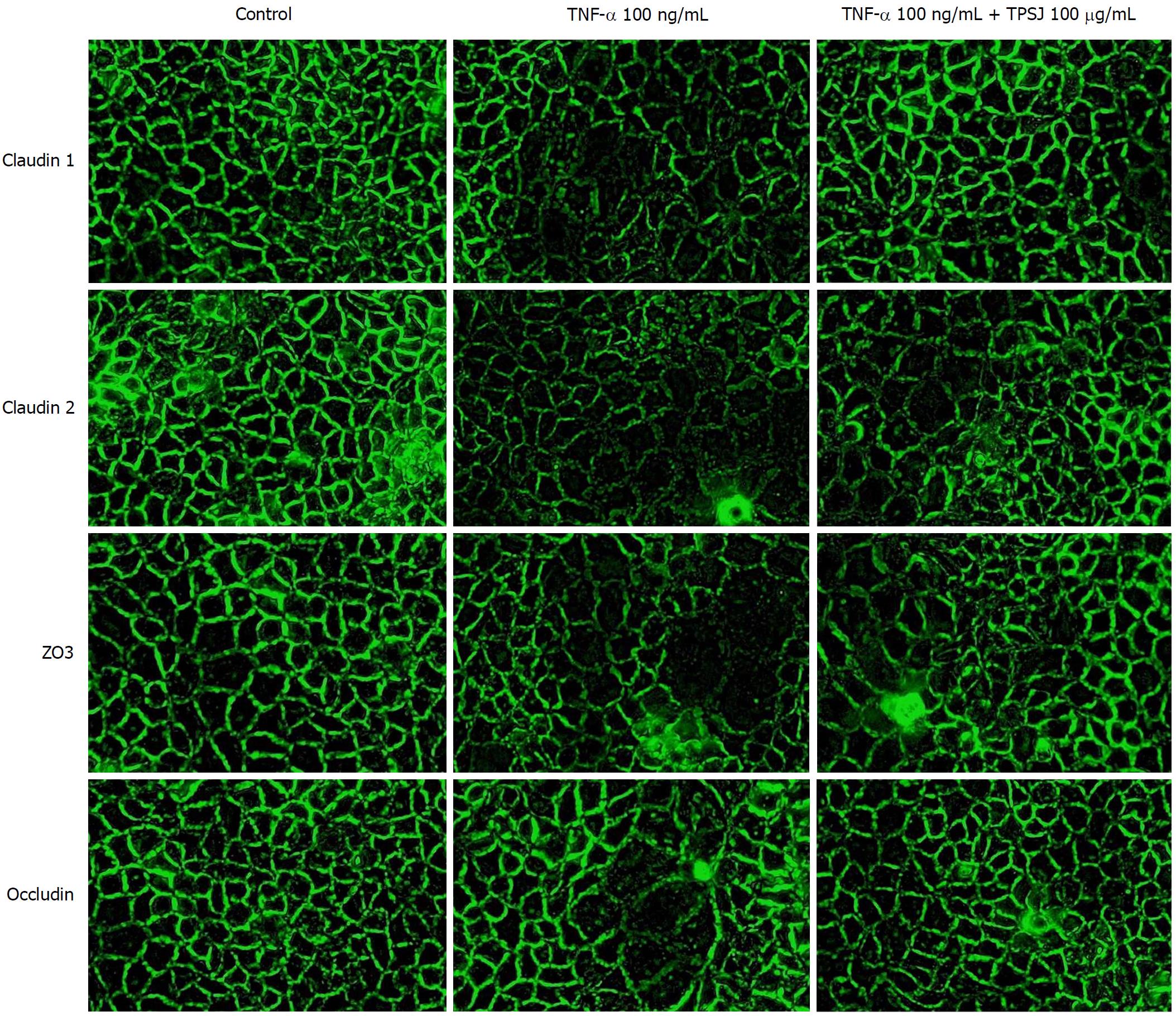
Figure 6 Immunofluorescence analysis of the effects of total polysaccharides of the Sijunzi decoction on the tight junction proteins claudin 1, claudin 2, zo3, and occludin in tumor necrosis factor-α-damaged Caco-2 cells.
Results are representative of three independent experiments. Original magnification = 400 ×.
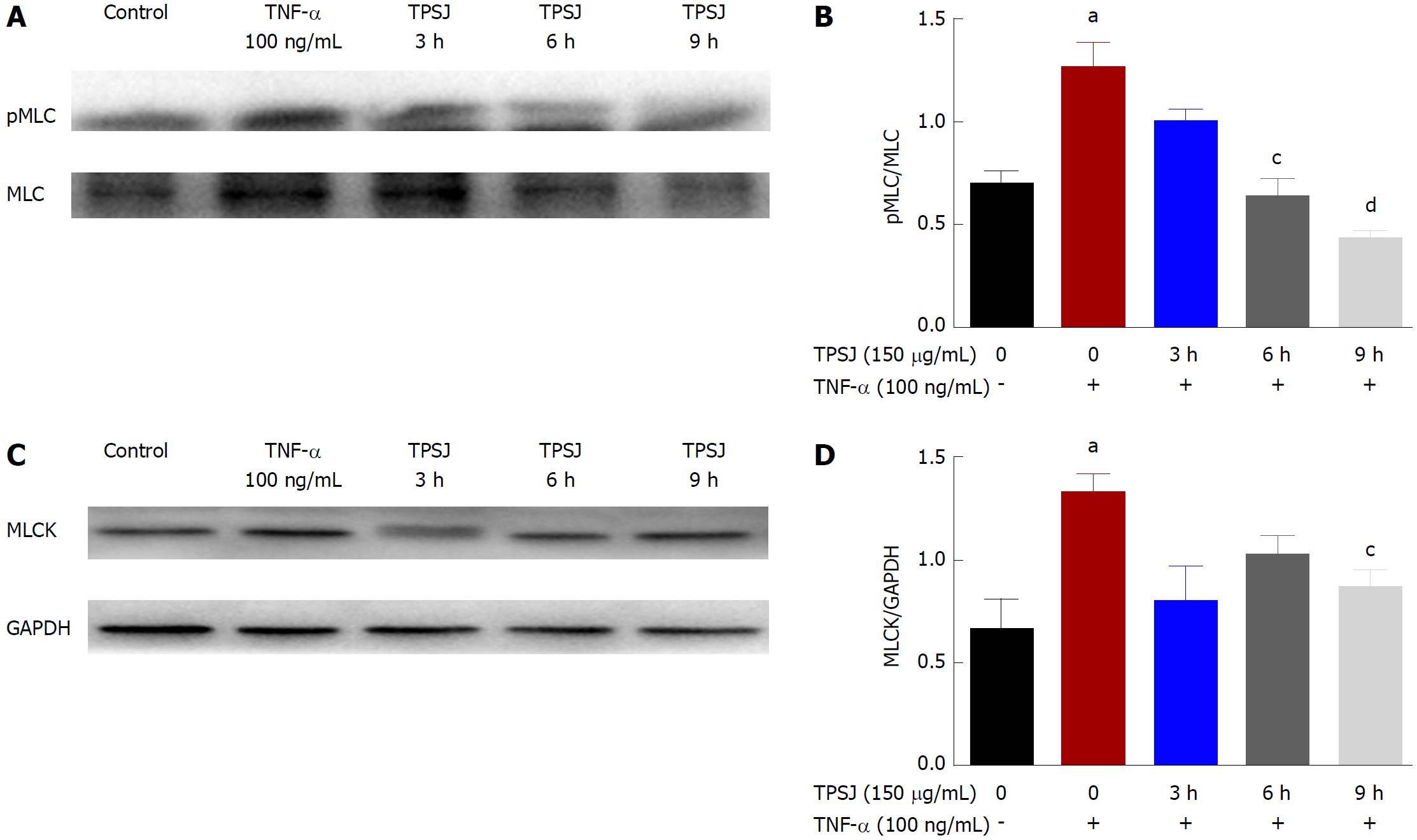
Figure 7 Total polysaccharides of the Sijunzi decoction inhibited tumor necrosis factor-α-induced increases in myosin light chain phosphorylation and myosin light chain kinase protein expression.
A: A representative western blot of pMLC and MLC in TNF-α-damaged Caco-2 cells treated with TPSJ; B: Quantification of the amounts of pMLC relative to MLC; C: A representative western blot of MLCK in TNF-α-damaged Caco-2 cells treated with TPSJ; D: Quantification of the amounts of MLCK relative to GAPDH. Data are shown as the means ± standard errors of the means of at least three independent experiments (aP < 0.05 vs control Caco-2 cells; cP < 0.05, dP < 0.01 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; MLC: Myosin light chain; MLCK: MLC kinase; TNF: Tumor necrosis factor.
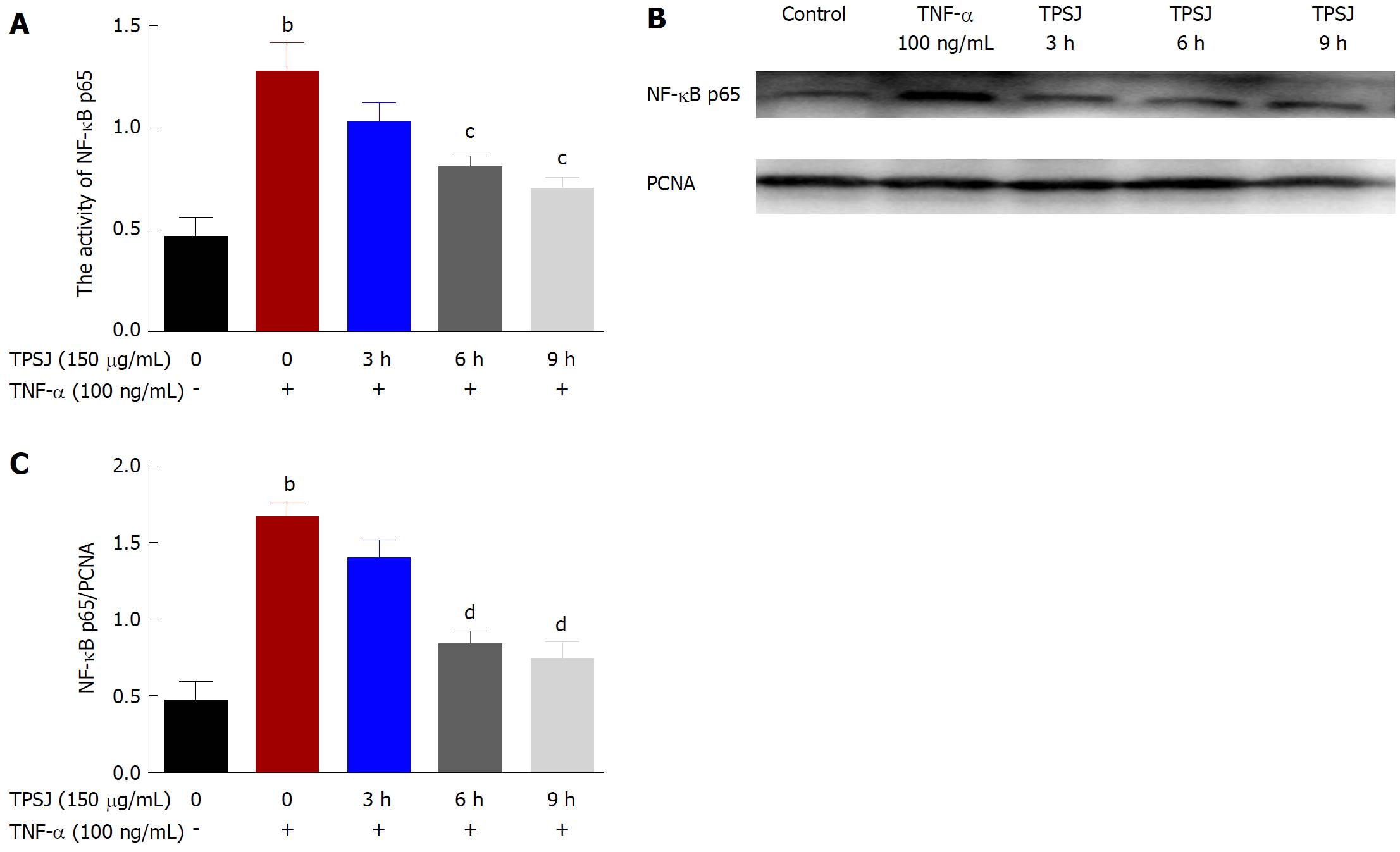
Figure 8 Effects of total polysaccharides of the Sijunzi decoction on the nuclear factor-κB signaling pathway.
A: TPSJ upregulated the activity of the NF-κB transcription factor p65; B: A representative western blot of NF-κB p65 in TNF-α-damaged Caco-2 cells treated with TPSJ; C: Quantification of the amounts of p65 relative to PCNA. Data are shown as the means ± standard errors of at least three independent experiments bP < 0.01 vs control Caco-2 cells; cP < 0.05, dP < 0.01 vs TNF-α-damaged Caco-2 cells). TPSJ: Total polysaccharides of the Sijunzi decoction; NF: Nuclear factor; TNF: Tumor necrosis factor.
- Citation: Lu Y, Li L, Zhang JW, Zhong XQ, Wei JA, Han L. Total polysaccharides of the Sijunzi decoction attenuate tumor necrosis factor-α-induced damage to the barrier function of a Caco-2 cell monolayer via the nuclear factor-κB-myosin light chain kinase-myosin light chain pathway. World J Gastroenterol 2018; 24(26): 2867-2877
- URL: https://www.wjgnet.com/1007-9327/full/v24/i26/2867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i26.2867