Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Jul 14, 2014; 20(26): 8471-8481
Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8471
Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8471
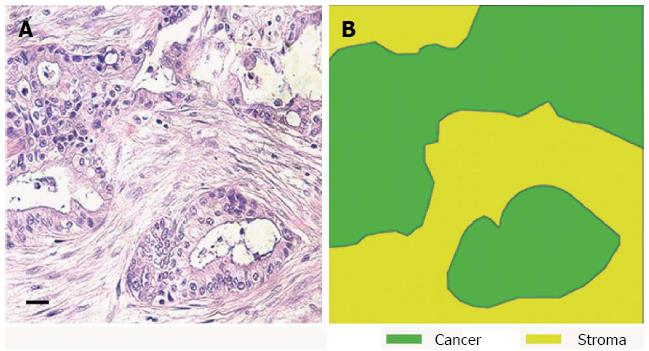
Figure 1 Human pancreatic ductal adenocarcinoma has a dense desmoplastic stromal component.
A: HE of human pancreatic cancer shows an area of invasive tumour; B: Stromal and epithelial components of the tumour are highlighted from figure A (scale bar 100 μm).
Figure 2 Submerged organotypic culture models used to investigate pancreatic ductal adenocarcinoma.
A: Cancer cells were embedded in the extracellular matrix mixture before it was allowed to polymerise. These cells were then fed with culture media placed on top of the gel; B: Representative configuration of cells within the gel after 7 d of culture. The cancer cells forming duct-like structures within the gel. This model mimics the behaviour of invading cancer cells; C, D: Show the same model with both pancreatic stellate cells and cancer cells embedded within the extracellular matrix gel. Using this model the interaction between stellate cells and invading cancer cells can be examined; E: Stellate cells embedded in the gel prior to polymerisation, with cancer cells seeded on top of the gel 24 h later. In this model cancer cell invasion can be analysed in the presence of pancreatic stellate cells (F). Representatice HE of these organotypic models are reviewed in Froeling et al[26].
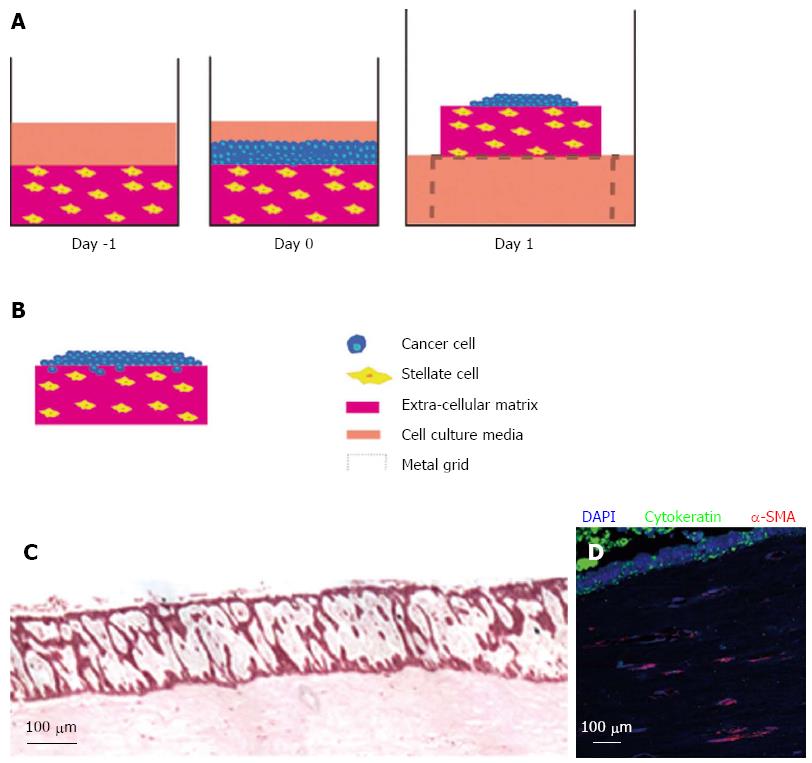
Figure 3 Raised organotypic model of pancreatic ductal adenocarcinoma with embedded pancreatic stellate cells.
A: Extracellular matrix gels containing stellate cells were polymerised in 24-well plates before cancer cells were seeded on top and allowed to attach. These gels were then raised onto metal grids and fed from below creating a chemotactic gradient. Cells were cultured for up to 14 d; B: Illustration of cancer cells in a raised model containing stellate cells showing proliferation and invasion into the gel; C: HE section of a raised gel containing stellate cells with cancer cells seeded on top; D: Immunofluorscence in the same gel showing strong cytokeratin expression in the cancer cells and α-smooth muscle actin (α-SMA) expression in the embedded stellate cells.
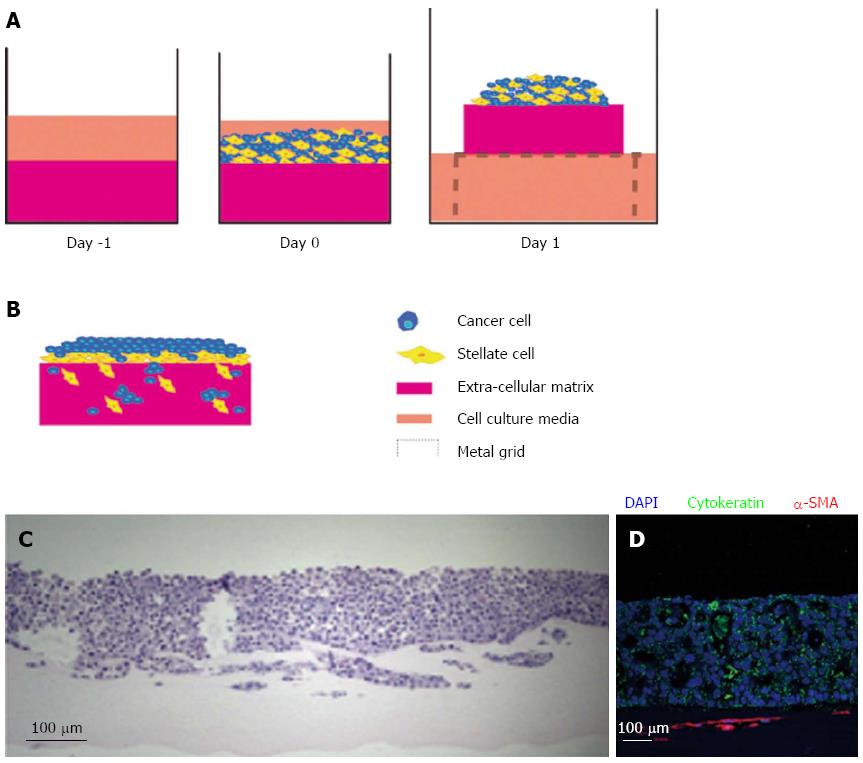
Figure 4 Raised organotypic model of pancreatic ductal adenocarcinoma with cancer cells and pancreatic stellate cell interaction.
A: Extracellular matrix gels containing were polymerised in 24-well plates before cancer stellate cells were seeded on top and allowed to interact and attach. These gels are then raised onto metal grids and fed from below creating a chemotactic gradient. Organotypic models were cultured for up to 14 d; B: Illustration of cancer cells and stellate cells in a raised model showing increased proliferation and invasion of both stellate and cancer cells into the gel; C: HE section of a raised gel with cancer cells and pancreatic stellate cells seeded on top; D: Immunofluorescence in the same gel showing strong cytokeratin expression in the cancer cells and α-smooth muscle actin (α-SMA) expression in the stellate cells which form a layer below the cancer cells.
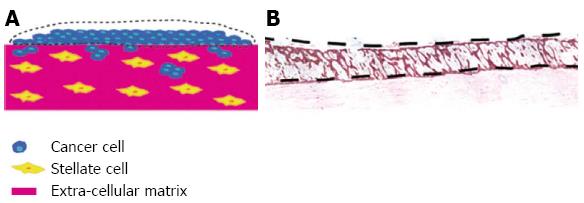
Figure 5 Use of organotypic model to isolate cell types grown together by laser microdisection.
By using the raised organotypic model with pancreatic stellate cells embedded within the gel (Figure 4B) the sellate and cancer cells are kept separate. A, B: Laser microdissection of the cancer cells can then be performed to allow analysis of cancer cells grown in the presence of pancreatic stellate cells. Scale bar 100 μm.
Figure 6 Mini-organotypic model of pancreatic ductal adenocarcinoma.
Extra cellular matrix gel is polymerised within the insert of a standard migration assay plate. A: Cancer and pancreatic stellate cells are seeded on top of the gel and allowed to attach. The media is then removed and then replaced only in the bottom of the well to again create a chemotactic gradient cells are cultured for 7-10 d; B: HE image demonstrating a similar pattern of cell proliferation and invasion is seen, as in the raised model; C: Immunofluorscence in the same gel showing strong cytokeratin expression in the cancer cells and vimentin expression in the stellate cells which form a layer below the cancer cells and invade into the gel ahead of cancer cells.
- Citation: Coleman SJ, Watt J, Arumugam P, Solaini L, Carapuca E, Ghallab M, Grose RP, Kocher HM. Pancreatic cancer organotypics: High throughput, preclinical models for pharmacological agent evaluation. World J Gastroenterol 2014; 20(26): 8471-8481
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8471