Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 28, 2011; 17(44): 4858-4866
Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4858
Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4858
Figure 1 Experimental design for development of colitis-associated dysplasia in a rat model and timeline for administration of erlotinib (10 mg/kg, ip).
TNBS: Trinitrobenzene sulfonic acid.
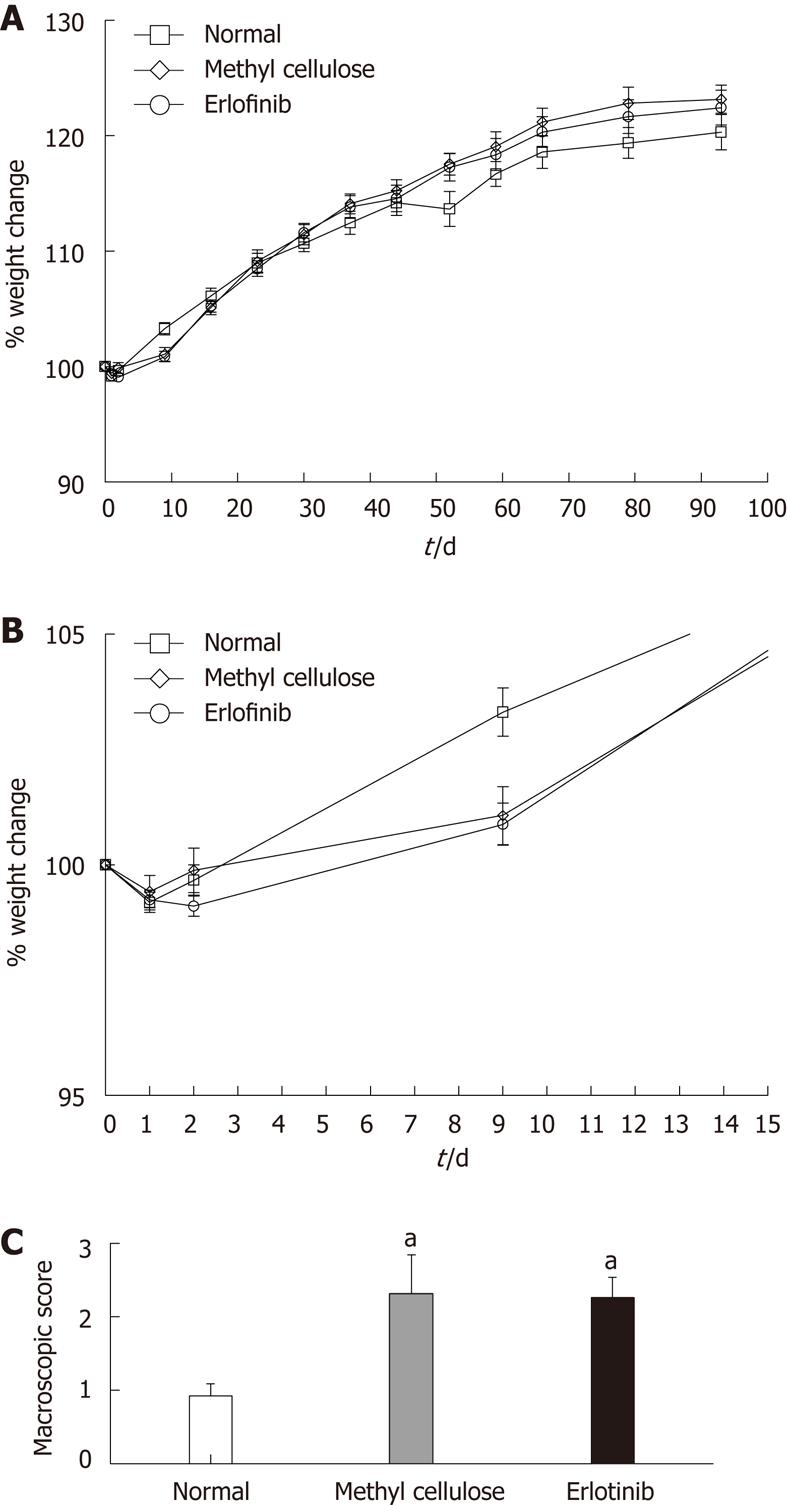
Figure 2 Erlotinib treatment did not worsen inflammation.
A: Effect of erlotinib treatment on weight change in an animal model of colitis-associated dysplasia (n = 10-20 per group ± SE); B: Weight change following initial reactivation of colitis; C: Effect of erlotinib treatment on macroscopic damage score in an animal model of colitis-associated dysplasia. The average macroscopic score (including the presence of adhesions, thickness of the tissue, presence or absence of diarrhea, and grade of ulceration) was significantly higher in all trinitrobenzene sulfonic acid-treated animals whether treated with vehicle or drug (aP < 0.05 vs normal animals, n = 12-20 ± SE).
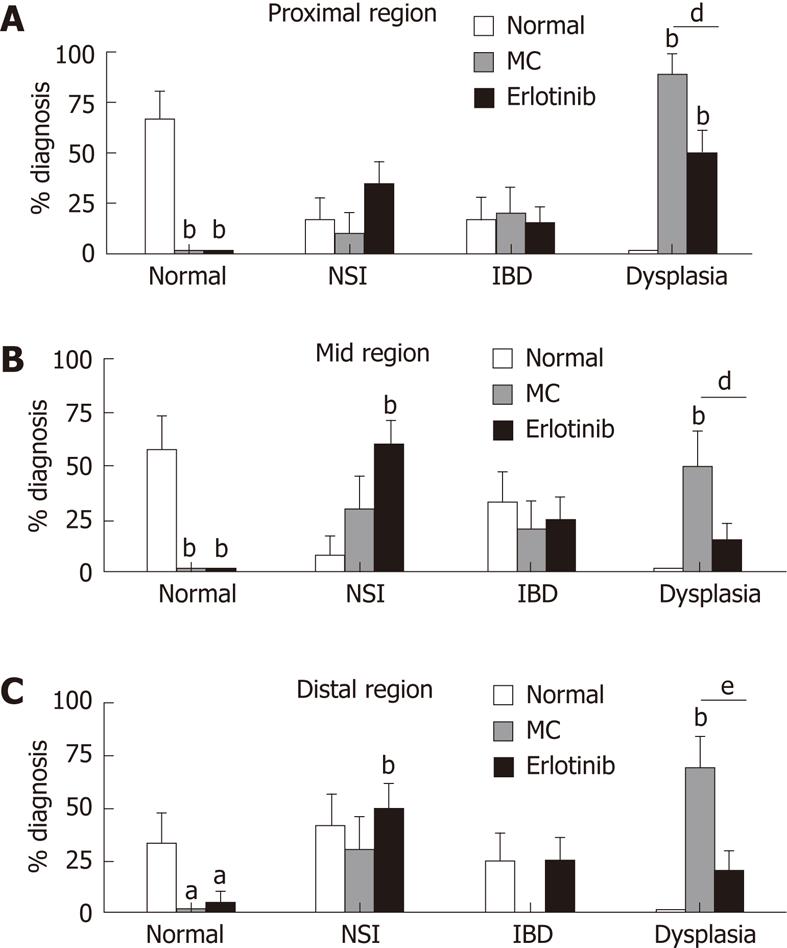
Figure 3 Effect of erlotinib treatment on pathological analysis in an animal model of colitis-associated dysplasia.
The percentage of animals with the most severe diagnosis found in each region of the colon is shown (aP < 0.05, bP < 0.01 vs normal animals; dP < 0.05, eP < 0.01 vs vehicle-treated animals; n = 10-20 ± SE). MC: Methyl cellulose; NSI: Non-specific inflammation; IBD: Inflammatory bowel disease.
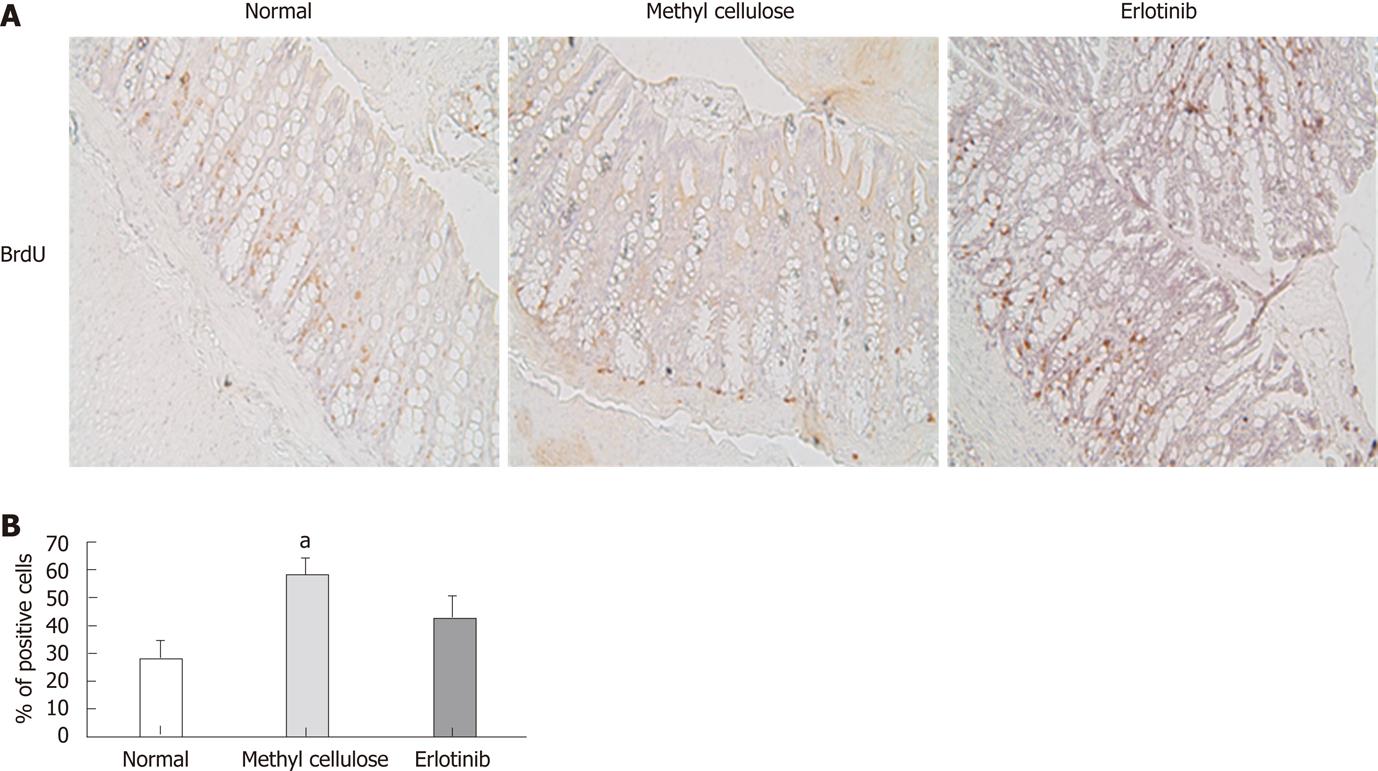
Figure 4 Erlotinib treatment inhibited cell proliferation.
A: Representative immunohistochemistry for BrdU incorporation in normal, vehicle-treated and erlotinib-treated animals (200 x). B: Cell proliferation in erlotinib-treated animals was less than that shown in vehicle-treated animals and not significantly different from normal animals (aP < 0.05; n = 10 ± SE).
Figure 5 Effect of erlotinib in an animal model of colitis-associated dysplasia on mitogen-activated protein kinase components.
Samples from (A) proximal, (B) mid, and (C) distal regions of normal rats and trinitrobenzene sulfonic acid (TNBS) + vehicle and TNBS + erlotinib treated animals were ground, and equal amounts of protein (30 μg) were separated by sodium dodecyl sulfate 12%-polyacrylamide gel electrophoresis before analysis by Western blotting. Antibodies against pErk1/2 and Erk1/2 were used. Lines are representative samples of 3 independent rats per group. Densitometry was performed by ImageQuant 5.2 Software (Typhoon 9410) (n = 10-20 ± SEM).
Figure 6 Immunohistochemistry analysis of c-Myc in rat colon tissue samples.
A: Slides of formalin-fixed, paraffin-embedded tissues were stained with an anti-c-Myc antibody, and the percentage of strong nuclear c-Myc stain was enumerated. Samples (10, 8 and 11 samples, respectively) from normal, methyl cellulose-treated, and erlotinib-treated rat colon were analyzed; B: Representative immunohistochemistry staining of samples.
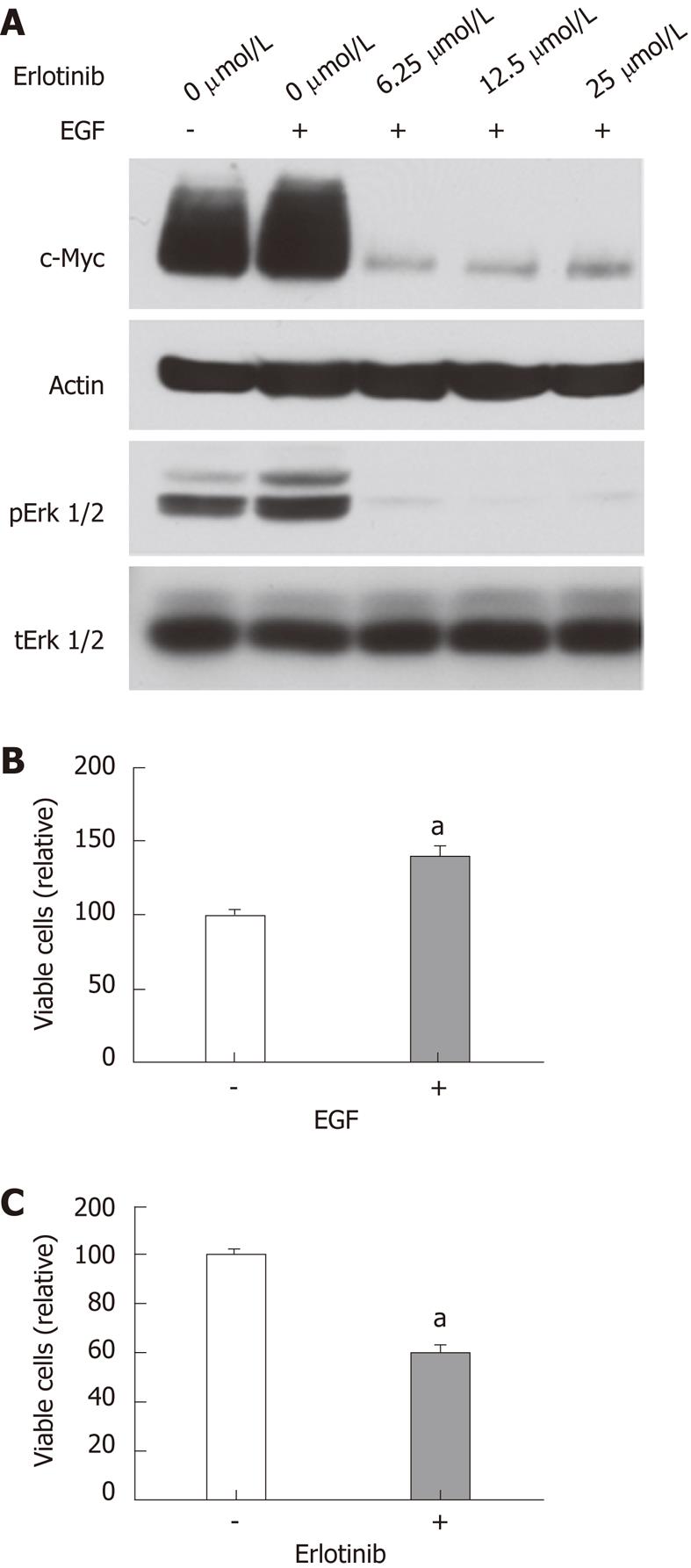
Figure 7 Effects of erlotinib on RIE-1 cells.
A: Cells were incubated in RPMI 1640-5% FBS with or without EGF (10 ng/mL) and the indicated concentrations of erlotinib for 24 h. Cells were also pretreated with indicated concentration of erlotinib for 24 h and then stimulated with EGF (10 ng/mL) for 5 min. Cell lysates were analyzed by immunoblotting with indicated antibodies; B and C: Cell proliferation was assayed in the presence or absence of EGF (10 ng/mL) or erlotinib (10 μmol/L) as described in the Materials and Methods. EGF: Epidermal growth factor.
- Citation: Pagán B, Isidro AA, Cruz ML, Ren Y, Coppola D, Wu J, Appleyard CB. Erlotinib inhibits progression to dysplasia in a colitis-associated colon cancer model. World J Gastroenterol 2011; 17(44): 4858-4866
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4858