Copyright
©The Author(s) 2004.
World J Gastroenterol. Sep 15, 2004; 10(18): 2756-2758
Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2756
Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2756
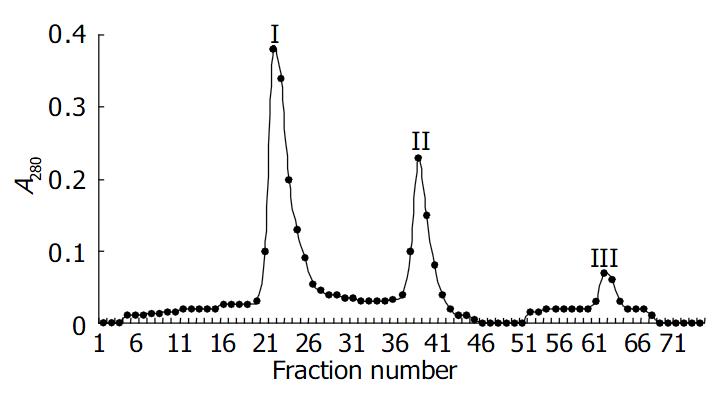
Figure 1 Purification of Clostridium difficile toxin A by DEAE-Toyopearl 650 mol/L chromatography.
Fractions 1-34 were eluted with 50 mmol/L Tris-HCl buffer (pH7.5) containing a linear NaCl gradient (50 mmol/L-250 mmol/L), then fractions 35-50 were eluted with 300 mmol/L NaCl and fractions 51-75 were eluted with linear gradient (300 mmol/L-600 mmol/L NaCl) in the same buffer.
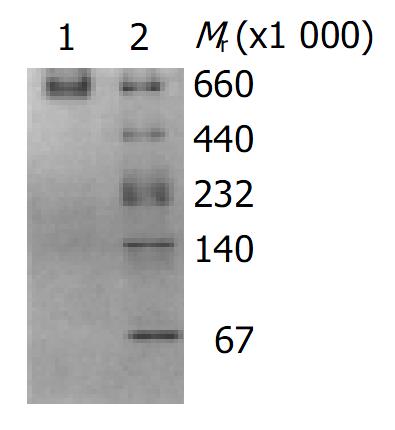
Figure 2 Analysis of toxin A preparations by native-PAGE (4%-30%).
Line 1: Purified C. difficile toxin A; Line 2: High molecular mass markers, thyroglobulin (Mr 669000), ferritin (Mr 440000), catalase (Mr 232000), lactate dehydrogenase (Mr 140000), bovine serum albumin (Mr 67000).
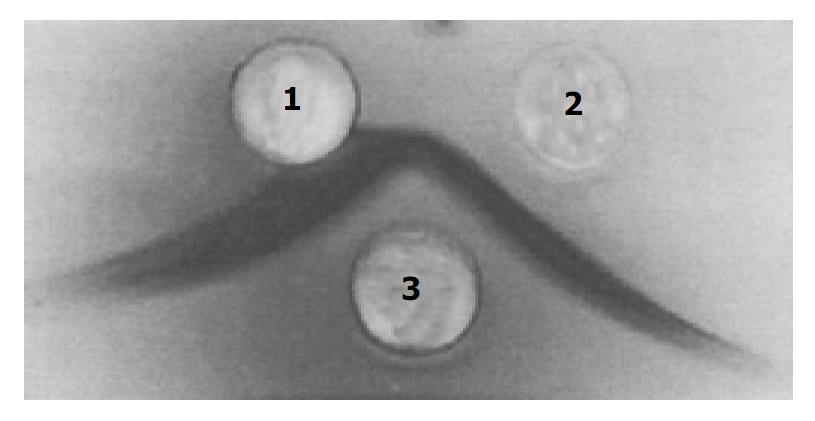
Figure 3 Analysis of toxin A by Ouchterlony double immunodiffusion.
Well 3 contained 20 μL of antiserum against strain 10463 culture filtrate, well 1 and well 2 contained 20 μL of purified toxin A by acid precipitation and DEAE-Toyopeal 650 mol/L chromatography respectively.
-
Citation: Fu SW, Xue J, Zhang YL, Zhou DY. Simplified purification method for
Clostridium difficile toxin A. World J Gastroenterol 2004; 10(18): 2756-2758 - URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2756