Published online Oct 28, 2014. doi: 10.4329/wjr.v6.i10.756
Revised: July 4, 2014
Accepted: August 27, 2014
Published online: October 28, 2014
Deep brain stimulation (DBS) is emerging as a powerful tool for the alleviation of targeted symptoms in treatment-resistant neuropsychiatric disorders. Despite the expanding use of neuropsychiatric DBS, the mechanisms responsible for its effects are only starting to be elucidated. Several modalities such as quantitative electroencephalography as well a intraoperative recordings have been utilized to attempt to understand the underpinnings of this new treatment modality, but functional imaging appears to offer several unique advantages. Functional imaging techniques like positron emission tomography, single photon emission computed tomography and functional magnetic resonance imaging have been used to examine the effects of focal DBS on activity in a distributed neural network. These investigations are critical for advancing the field of invasive neuromodulation in a safe and effective manner, particularly in terms of defining the neuroanatomical targets and refining the stimulation protocols. The purpose of this review is to summarize the current functional neuroimaging findings from neuropsychiatric DBS implantation for three disorders: treatment-resistant depression, obsessive-compulsive disorder, and Tourette syndrome. All of the major targets will be discussed (Nucleus accumbens, anterior limb of internal capsule, subcallosal cingulate, Subthalamic nucleus, Centromedial nucleus of the thalamus-Parafasicular complex, frontal pole, and dorsolateral prefrontal cortex). We will also address some apparent inconsistencies within this literature, and suggest potential future directions for this promising area.
Core tip: Deep brain stimulation (DBS) is emerging as a powerful tool for the alleviation of targeted symptoms in treatment-resistant neuropsychiatric disorders. Most recently, functional magnetic resonance imaging has been used to examine the effects of focal DBS on activity in a distributed neural network. The purpose of this review is to summarize the current functional neuroimaging findings from neuropsychiatric DBS implantation and to discuss some apparent inconsistencies within this literature, and to suggest potential future directions for this promising area.
- Citation: Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol 2014; 6(10): 756-778
- URL: https://www.wjgnet.com/1949-8470/full/v6/i10/756.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i10.756
Deep brain stimulation (DBS) is a form of invasive neuromodulation in which electrodes are implanted into a neuroanatomical target (nucleus or fiber tract) in an effort to modulate activity throughout a neural network[1]. Neuropsychiatric disorders can also be modulated through stimulation of cortical nodes in a neural network with similar efficacy and reduced risk[2,3]. The cortex can be stimulated either non-invasively from outside the skull with techniques like transcranial magnetic stimulation (TMS), or by placing stimulation paddles close to the surface of the brain under the skull but outside of the dura (epidural). For the purpose of this review, these cortical stimulation studies with invasive stimulation paddles and external generators are also labeled DBS, even though the electrodes are not deep. The imaging issues with these invasive cortical stimulation methods are the same as for traditionally defined DBS. These network modulations are driven by stimulation parameters that can be optimized for maximal therapeutic benefits. DBS was first developed for movement disorders wherein high frequency electrical stimulation of the thalamus has the capacity to reduce tremors[4]. Since that time, DBS has been investigated for treatment-resistant neuropsychiatric disorders[5,6]. In this context, high frequency electrical stimulation of limbic system targets has the capacity to alter mood and alleviate affective symptoms. Similar to DBS for movement disorders[7], invasive neuromodulation for neuropsychiatric disorders has similar reported open-label efficacy rates as lesion surgeries (although never directly compared) but unlike lesion surgery offers reversibility and fewer side-effects[8,9].
Neuropsychiatric disorders are beginning to be classified based on limbic, cognitive, and motoric[10,11] circuit dysfunction[12]. DBS likely exerts its effects by directly modulating the target node(s) and indirectly modulating the regions connected to that node[1,13]. Functional neuroimaging studies have attempted to capture these downstream network effects induced by DBS[13]. While traditional nuclear medicine techniques such as positron emission tomography (PET) imaging have successfully captured gross DBS-induced changes in neural networks, functional magnetic resonance imaging (fMRI) is poised to offer more refined insights[14]. Additionally, basic science techniques like optogenetics are now being used to explore the precise neural changes associated with DBS in animal models of disease[15].
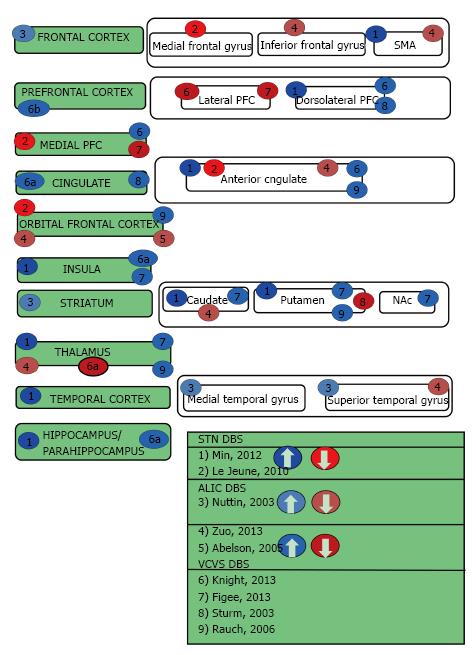
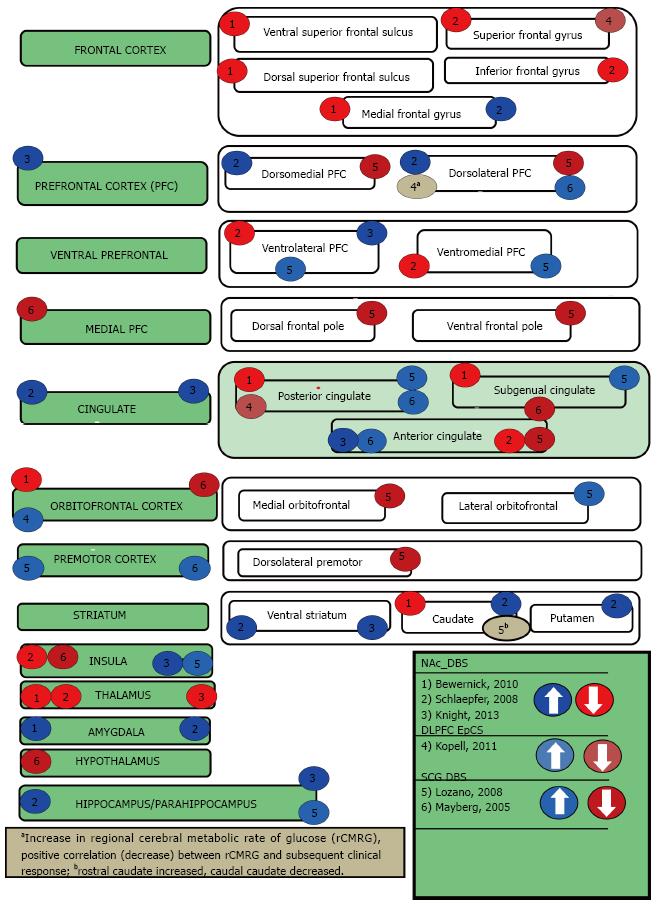
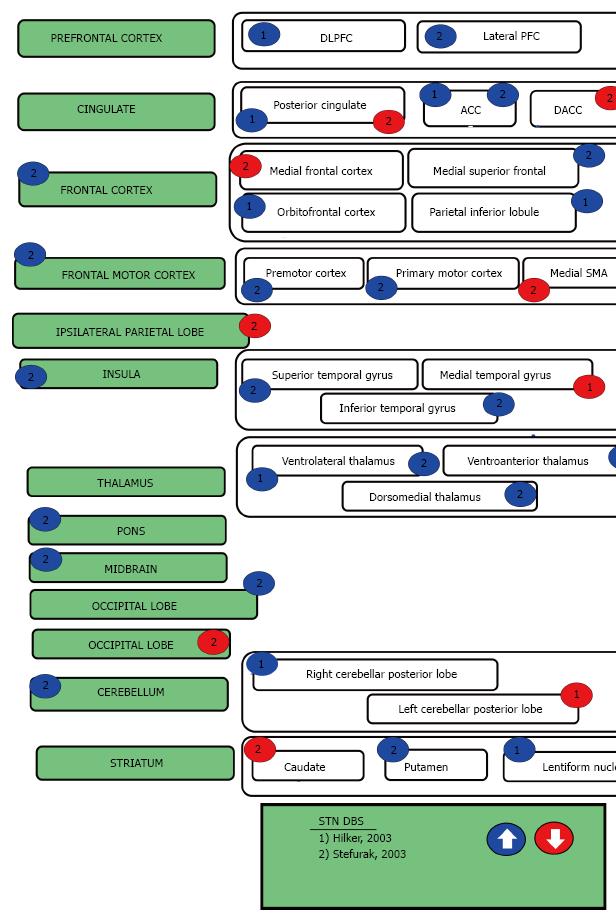
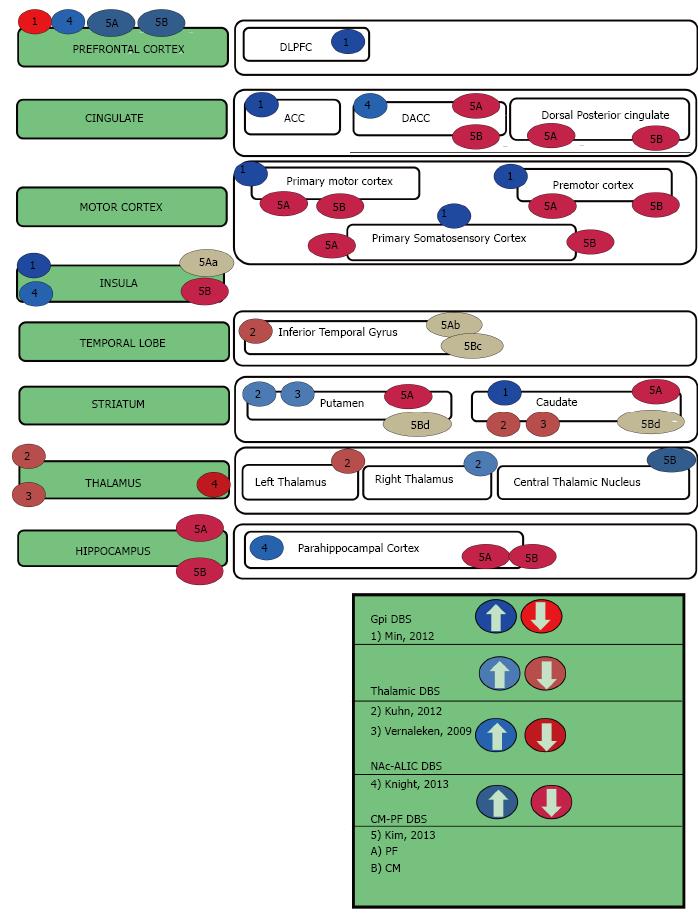
This review begins by describing the functional imaging techniques that have been used to examine the effects of focal DBS on activity in a distributed neural network. Thereafter the three neuropsychiatric syndromes of interest will be introduced and their DBS targets briefly identified. The subsequent sections describe the cortical and subcortical targets for each disorder in further detail, as well as discuss their imaging results and relevance to neuropsychiatric symptoms. Table 1 summarizes advantages, disadvantages and cautions for each imaging method. Tables 2, 3, 4, 5 and Figures 1-4 are categorized by neuropsychiatric disease and illustrate the effect each DBS target has on downstream nodes.
| Modality | Advantages | Disadvantages |
| PET | Unlikely to disrupt implant placement or function Widely available Technically simple to combine with DBS (relative to fMRI) Customized radiotracers to measure binding capacity | Cost, availability and health risks of radiotracers Static data (averaged over set time interval) Poor temporal and spatial resolution (relative to fMRI) Difficult to interpret data (receptor binding vs density) |
| MRI | Time-locked data High temporal and spatial resolution (relative to PET) Flexible data acquisition, processing and analysis Possible analysis of ON and OFF settings | BOLD signal interpretation (temporal lag, anatomical imprecision) DBS safety considerations (lead migration, secondary lesioning) Possible deactivation of implanted impulse generators Potential DBS-related artifacts on images |
| STN_DBS | ALIC_DBS | VCVS_DBS | |
| Frontal cortex | ↑Nuttin1a | ||
| Medial frontal gyrus | ↓Le Jeune1f | ||
| Inferior frontal gyrus | ↓Zuo1b | ||
| Supplementary motor area | ↑Min1b,5 | ↓Zuo2 | |
| Prefrontal cortex | ↑ Knight1d,7 | ||
| Lateral PFC | ↓Figee1b,2,↓ Knight | ||
| Dorsolateral PFC | ↑Min1b,5 | ↑Sturm, ↑ Knight | |
| Medial PFC | ↓Le Jeune2 | ↓Figee1a,2 , ↑ Knight | |
| Cingulate | ↑Sturm; ↑ Knight1d,7 | ||
| Anterior cingulate cortex | ↑Min1b; ↓Le Jeune1f | ↓Zuo1b,2 | ↑ Knight1d; ↑Rauch1f |
| Orbitofrontal cortex | ↓Le Jeune2 | ↓Zuo1b; ↓Abelson3 | ↑Rauch1f |
| Insula | ↑Min1b | ↑ Figee; ↑Knight1d,6 | |
| Striatum | ↑ Nuttin1a | ||
| Caudate | ↑Min1b | ↓Zuo1b | ↑Figee |
| Putamen | ↑Min1b | ↑Figee; ↓Sturm;↑Rauch1f | |
| Nucleus accumbens | ↑Figee1c | ||
| Temporal cortex | ↑ Min1b,4 | ||
| Superior temporal gyrus | ↓Zuo1b ;↑Nuttin1a | ||
| Medial temporal gyrus | ↑Nuttin1a | ||
| Thalamus | ↑ Min1b,4 | ↓Zuo1b | ↑Figee; ↓Knight1d,6; ↑Rauch1f |
| Pons | ↑Nuttin1a | ||
| Hippocampus/parahippocampus | ↑ Min1b,4 | ↑ Knight1d,6 | |
| Globus pallidus | ↑ Rauch1f |
| NAc_DBS | SCG_DBS | DLFPC_ EpCS | |
| Frontal cortex | |||
| Ventral superior frontal sulcus | ↓Bewernick1c | ||
| Dorsal superior frontal sulcus | ↓Bewernick1c | ||
| Medial frontal gyrus | ↑Schlaepfer; ↓Bewernick1c | ||
| Superior frontal gyrus | ↑Schlaepfer | ↓Kopell2 | |
| Inferior frontal gyrus | ↓Schlaepfer1a | ||
| Prefrontal cortex (PFC) | ↑Knight1d,11 | ||
| Dorsomedial PFC | ↑Schlaepfer1a | ↓Lozano4 | |
| Dorsolateral PFC | ↑Schlaepfer1a | ↑Mayberg8; ↓ Lozano4,6f | ↑Kopell1f;↓Kopell2 |
| Ventral prefrontal | |||
| Ventrolateral prefrontal | ↓Schlaepfer1a; ↑Knight | ↑Lozano6f | |
| Ventromedial prefrontal | ↓Schlaepfer1a | ↑Lozano4 | |
| Medial prefrontal | ↓Mayberg8 | ||
| Dorsal frontal pole | ↓Lozano4 | ||
| Ventral frontal pole | ↓Lozano4 | ||
| Cingulate | ↑Schlaepfer1a; ↑Knight1d,11 | ||
| Posterior cingulate | ↓Bewernick1c | ↑Mayberg7b; ↑Lozano5 | ↓Kopell2 |
| Subgenual cingulate | ↓Bewernick1c,2 | ↓Mayberg7b; ↑Lozano4 | |
| Anterior cingulate | ↓Schlaepfer1a; ↑Knight1d | ↑Mayberg8; ↓Lozano6f | |
| Orbitofrontal cortex | ↓Bewernick1e | ↓Mayberg9b | ↑Kopell1f |
| Lateral orbitofrontal | ↑Lozano4 | ||
| Medial orbitofrontal | ↓Lozano6f | ||
| Premotor cortex | ↑Mayberg7b; ↑Lozano3 | ||
| Dorsolateral premotor | ↓Lozano3 | ||
| Striatum | |||
| Ventral striatum | ↑Schlaepfer1a; ↑Knight | ||
| Caudate | ↓Bewernick1c; ↑Schlaepfer1c | ↑(rostral) ↓(caudal)Lozano4 | |
| Putamen | ↑Schlaepfer | ||
| Insula | ↓Schlaepfer1a; ↑Knight1d | ↓Mayberg7b; ↑Lozano4 | |
| Amygdala | ↑Schlaepfer1a; ↑Bewernick1c,2 | ||
| Pons | ↓Lozano5 | ||
| Thalamus | ↓Bewernick1c; ↓Schlaepfer1a↓Knight1d,10 | ||
| Hypothalamus | ↓Mayberg7b | ||
| Hippocampus/parahippocampus | ↑Schlaepfer; ↑Knight1d | ↑Lozano45 |
| Gpi_DBS | Thalamic_DBS | Nac-ALIC_DBS | Pf_DBS | CM_DBS | |
| Prefrontal cortex | ↓Min6 | ↑Knight1d,5 | ↑Kim7,8b,9,10b | ↑Kim7,8a,9,10a | |
| Dorsolateral prefrontal cortex | ↑Min1b | ||||
| Cingulate | |||||
| Anterior cingulate cortex | ↑Min1b | ||||
| Dorsalanterior cingulate cortex | ↑Knight4 | ↓Kim7810 | ↓Kim7a,8,9,10 | ||
| Dorsal Posterior cingulate cortex | ↓Kim7,8a,10 | ↓Kim7d,8,9,10 | |||
| Motor cortex | |||||
| Primary motor cortex | ↑Min1b | ↓Kim7,8a ,9,10a | ↓Kim7,8b,9,10a | ||
| Premotor cortex | ↑Min1b | ↓Kim7,8a,9,10a | ↓Kim7a,e,f, 8ba,9a,10a | ||
| Primary somatosensory cortex | ↑Min1b | ↓Kim7,8a,9,10a | ↓Kim7,8,9,10a | ||
| Insula | ↑Min1b | ↑Knight1d,4 | ↓Kim79↑ 8a,10a | ↓Kim78910 | |
| Temporal lobe | |||||
| Inferior temporal gyrus | ↓Kuhn | ↓Kim7↑8a,10 | ↓Kim79↑810 | ||
| Striatum | |||||
| Caudate | ↑Min1b | ↓Kuhn ↓Vernaleken | ↓Kim810 | ↑Kim7↓810 | |
| Putamen | ↑Kuhn ↑Vernaleken | ↓Kim810 | ↑Kim7↓810 | ||
| Thalamus | ↓Kuhn ↓Vernaleken | ↓Knight1d,4 | |||
| Right thalamus | ↑ Kuhn2 | ||||
| Left thalamus | ↓Kuhn3 | ||||
| Central thalamic nucleus | ↑Kim8 | ||||
| Hippocampus | ↓Kim7810 | ↓Kim8 | |||
| Parahippocampal cortex | ↑Knight1d,4 | ↓Kim7810 | ↓Kim7810 |
| STN_DBS | |
| Prefrontal | |
| Dorsolateral prefrontal cortex | ↑Hilker1b |
| Lateral prefrontal cortex | ↑Stefurak1b |
| Cingulate | |
| Posterior cingulate | ↓Stefurak1b; ↑Hilker1b |
| Anterior cingulate cortex | ↑Stefurak1b; ↑Hilker1b |
| Dorsal anterior cingulate cortex | ↓Stefurak1b |
| Frontal cortex | ↑Stefurak |
| Medial frontal cortex | ↓Stefurak1b |
| Medial superior frontal | ↑Stefurak1b |
| Orbitofrontal cortex | ↑Hilker1b |
| Parietal Inferior lobule | ↑Hilker1b |
| Frontal motor cortex | ↑Stefurak1b |
| Premotor cortex | ↑Stefurak1b |
| Primary motor cortex | ↑Stefurak1b |
| Medial supplementary motor area | ↓Stefurak1b |
| Ipsilateral parietal lobe | ↓Stefurak1b |
| Insula | ↑Stefurak1b |
| Superior temporal gyrus | ↑Stefurak1b |
| Middle temporal gyrus | ↓Hilker1b |
| Inferior temporal gyrus | ↑Stefurak1b |
| Striatum | |
| Caudate | ↓Stefurak1b |
| Putamen | ↑Stefurak1b |
| Lentiform nucleus | ↑Hilker1b |
| Thalamus | |
| Ventrolateral thalamus | ↑Stefurak; ↑Hilker1a |
| Ventroanterior thalamus | ↑Stefurak1b |
| Dorsomedial nuclei of Thalamus | ↑Stefurak1b |
| Pons | ↑Stefurak1b |
| Midbrain | ↑Stefurak1b |
| Cerebellum | ↑Stefurak1b |
| Right Cerebellar posterior lobe | ↑Hilker1a |
| Left Cerebellar posterior lobe | ↓Hilker1b |
| Parahippocampal cortex | ↑Stefurak1b |
| Occipital lobe | ↓Stefurak1b |
PET can safely and effectively probe the effects of DBS without disrupting the function of the device or its electrodes. PET uses short-lived radioisotopes to create a static image of averaged neural activity over time based on changes in oxygen metabolism, glucose, blood flow or neurotransmitter receptor binding[16,17]. PET data are static in that they are averaged over time. Thus, PET cannot yield information about the immediate (e.g., second to second) time course of change induced by an experimental manipulation, although oxygen PET can image activity for one minute and can thus show changes over a few minutes.
PET is the predominant functional imaging technique in the psychiatric DBS literature, in part because it was available much earlier than modalities like fMRI, and because it is technically less difficult to combine DBS with PET[14]. Although PET continues to make significant contributions to clinical Neuroscience, the technique has a number of limitations that are relevant to DBS research. First, PET has poor temporal and spatial resolution relative to fMRI. These resolution limitations might make it difficult to accurately assess DBS-induced changes in small subcortical nodes in limbic neural networks. Second, PET requires injections of radioactive tracers into patients who have no medical indication for such exposure. Furthermore, the cost and availability of these radiotracers can be prohibitive. Third, PET studies typically have methodological variations that make it difficult to compare data between studies. For example, a study that uses repeated injections of 15O-H2O PET to measure cerebral blood perfusion over 60-120 s intervals[18] cannot be contextualized with a study that uses a single injection of 18F-fluorodeoxyglucose (18F-FDG) PET to measure glucose metabolism over a 60 min interval[19]. Fourth, PET measures of binding capacity are not specific for receptor occupancy and could reflect altered receptor density[20,21]. This lack of clarity might make it difficult to assess the pharmacological effects of DBS. These are just a few of the considerations that should be kept in mind when considering PET DBS research.
On the other hand, there are few if any problems with performing PET studies in patients with DBS electrodes implanted, unlike with fMRI. Also, single photon emission computed tomography imaging, with perfusion tracers that capture activity summed over a minute or two, offers a less expensive and readily available option[18,22,23].
One of the most popular ways to assess brain function is to employ fMRI. Most fMRI studies use blood oxygen level dependent (BOLD) signals that are based on magnetization differences between oxygenated and deoxygenated hemoglobin[24]. Thus, fMRI can be used to make inferences about neural activity based on time-locked alterations in neurovascular coupling. Although BOLD fMRI is widely used, it does have limitations. The BOLD hemodynamic response takes about 1-3 s to peak, which is a lifetime in terms of neuronal firing. Also, the BOLD signal reflects general vascular changes arising from new metabolic demands, and is not precise to the actual neurons driving the signal. That is, the BOLD response is anatomically imprecise or “smudged” with respect to the actual activated neurons, like “the garden being flooded for want of one thirsty flower”[25]. Finally, BOLD changes occur both when a brain region is acting to excite activity, and when it is attempted to regulate of inhibit brain activity. Thus the precise interpretation of the BOLD signal direction is problematic.
In the DBS literature, fMRI is often used to examine the function of neural network(s) before and after targeted stimulation[26,27]. Safety considerations such as MRI-induced lead migration and secondary heat lesioning have greatly slowed the utilization of this technology in patients with DBS implanted electrodes[28]. There is also some concern that fMRI may deactivate implanted impulse generators, although this phenomenon has been linked to low battery[29] and more recent studies have not observed it[30]. To address some of these concerns, DBS-fMRI studies have typically been conducted utilizing externalized leads connected to pulse generators that are housed in the fMRI control room. This setup may reduce the risk of hardware malfunction[31]. This also means that most DBS fMRI studies are performed in humans immediately after surgical implantation and before connecting the DBS electrodes to the generator implanted in the chest wall. This makes longitudinal studies problematic.
Many of the original DBS-fMRI studies were performed during the roughly 2-3-wk interval between lead implantation and IPG placement. During this time, stimulator extension wires can be accessed for externalized operation and stimulation[32]. While this approach is useful for examining the short-term effects of DBS on the limbic/cognitive networks, it cannot be used to assess long-term effects that may evolve or change over time[33]. Furthermore, in the weeks after surgery, a “microlesion effect” may result from mechanical manipulation at the site of lead placement. Such a lesion could itself have a unique functional imaging signature[34] and therefore complicate predictions about the long-term effects of limbic DBS. These microlesion effects have also been shown to have unanticipated effects on adjacent neural circuits[35,36]. IPG replacement surgery has been proposed as an optimal window of time during which the chronic effects of DBS may be explored[37].
There are several sources of potential MRI artifacts in patients with DBS implants: 1-the skull-cap, 2- the metallic portion of the electrode contact, 3- the electrical stimulation itself, and 4-movement[38,39]. Some of these artifacts can be reduced or eliminated. Some of these artifacts are disease specific, such as the participant having tics in the scanner[40]. The artifact generated by the skull-cap, for example, can be addressed by replacing ferromagnetic screws with non-ferromagnetic options[41] or by post-processing techniques normally used for sinus artifacts[30]. Other artifacts, like those generated by the metallic portion of the lead during echoplanar acquisition[29,42], are more difficult to address. The deep brain stimulation itself can also create MR artifact in the area around the tissue-lead interface. This has been addressed by turning off the device minutes before the scan[13]. This artifact, if present, prevents analysis of DBS-induced activity in the area surrounding the electrode. Some authors have chosen to address these issues with strict statistical thresholding in offline analysis[43,44].
Despite its limitations, DBS-fMRI can provide unique and valuable information regarding the neurobiological effects of DBS[45,46]. Most psychiatric DBS-fMRI studies have only utilized the device in the OFF condition[13]. Newer protocols, however, have begun to explore the possibility that DBS-fMRI could be performed in the ON condition in patients with fully-implanted DBS hardware[30,43]. One recent study successfully imaged Parkinson’s disease (PD) patients with subthalamic nucleus (STN) DBS in the ON and OFF setting with no reported adverse effects[43]. This protocol utilized a 1.5 Tesla MRI scanner as well as a special transmit-receive (T/R) head coil. The specific absorption ratio in the head was limited to under 0.1 W/kg for this experiment. This protocol was first performed in a phantom, demonstrating no significant heating with the sequences utilized in the human study[30]. We encourage anyone considering such a technique to consult with a magnetic resonance physicist and take great caution as this is a risky undertaking. While this protocol appears to be safe, it is yet to be utilized in patients with DBS for psychiatric conditions.
This portion of the review will focus on the imaging of DBS in three neuropsychiatric syndromes: 1-obsessive-compulsive disorder (OCD), 2-major depressive disorder (MDD), and 3-Tourette syndrome (TS). The following sections will identify common DBS targets for each disorder and review relevant functional imaging data.
OCD is a neuropsychiatric condition characterized by functionally impairing obsessions and compulsions. The obsessions occur as recurrent and ego-dystonic ideas, images, or impulses. By contrast, the compulsions are stereotyped, repetitive mental acts or behaviors that are performed with the intention of reducing anxiety generally related to the obsessions. OCD affects approximately 2%-3% of the population and 20%-40% of patients with OCD remain severely disabled despite conventional treatments like exposure/response prevention as well as medications such as selective serotonin reuptake inhibitors. DBS for severe, treatment-resistant OCD was recently approved through a humanitarian device exemption[47]. Several targets have been explored for this indication, including the nucleus accumbens (NAc)[48,49], the anterior limb of the internal capsule (ALIC)[50], the ventral capsule/ventral striatum (VCVS)[51-53] and the STN[54,55]. Four of the main OCD targets that have been imaged with PET include the ALIC[56], VCVS[57], STN[58], and NAc[50,59](Table 2, Figure 1). Functional MRI has been performed following NAc DBS in both animals[26] and more recently in humans with OCD[13].
Depression is a psychiatric disorder characterized by extreme sadness and/or melancholia that is of sufficient length and interferes with activities of daily living as well as socialization. One in five people will experience an episode of major depression at some point in their lifetime. The World Health Organization has indicated that major depressive disorder is one of the four most disabling illnesses worldwide[60]. Currently, antidepressants and psychotherapy are the primary modes of treatment, although that is evolving[61]. Minimally invasive brain stimulation methods like electroconvulsive therapy (ECT) and TMS are typically reserved for patients with treatment-resistant depression[62]. DBS is currently being utilized in a clinical research setting for patients who do not respond to the aforementioned therapies. Three of the main depression targets that have been imaged with PET include the dorsolateral prefrontal cortex[3], subcallosal cingulate[63,64], and the nucleus accumbens[65,66] (Table 3, Figure 2). Two of the depression targets are also OCD targets have been imaged for OCD, but not depression include the anterior limb of the internal capsule[56], ventral capsule/ventral striatum)[57]. Functional MRI has been performed in NAc in non-depressed animals[26].
TS is a neuropsychiatric movement disorder that affects approximately 1% of the world’s population, typically in childhood and/or adolescence. TS is characterized by at least two motor tics and one or more vocal tics that persist for more than a year. Approximately 90% of individuals with TS have at least one comorbid psychiatric symptom. These symptoms include attention-deficit/hyperactivity disorder, OCD, anxiety disorders, or impulse control disorders. DBS for TS has been shown to reduce the frequency and severity of motor tics as well as their associated psychiatric symptoms[47]. Although the precise mechanism of action remains unclear, DBS for TS appears to diminish dopamine signaling in the thalamus and the striatum[16]. Multiple targets have been introduced for TS DBS, including the thalamic centromedian nucleus and parafasicular complex (CM-pf)[67], the globus pallidus internus (GPi)[68], the ALIC, and NAc[69] (Table 4, Figure 4) While three of these targets (GPi[70], ALIC, and NAc) have had functional neuroimaging performed in other conditions (as described above), only the medial thalamus has been imaged in the TS population[16,17]. In a large animal model, both the CM-Pf and GPi have been imaged using fMRI[27,71].
Dorsolateral prefrontal cortex (BA 9 and BA 46): The dorsolateral prefrontal cortex (DLPFC) (BA 9 and 46)[72] is a critical node in the mesocortical system, a dopaminergic tract that modulates anticipation, goal selection, planning monitoring, and the use of feedback in task performance[73]. The DLPFC is critical for working memory of both spatial and non-spatial information[74]. There has been some suggestion that direct stimulation (activation) of the DLPFC may serve to modulate parietal attentional networks involved in the automatic processing of salient environmental stimuli. For example, a recent meta-analysis of 162 imaging studies revealed that co-activation of BA 9 (which spans the dorsolateral and medial prefrontal cortices) and midbrain regions like the periaqueductal gray (PAG) is essential for assigning emotional valence[75]. These results may partially explain how the DLPFC plays such a critical role in mood regulation[76]. In depression, the left DLPFC is hypoactive and thought to be associated with negative emotional judgment. The right DLPFC is hyperactive and this is linked to attentional modulation[77]. The DLPFC has been demonstrated to have various subcortical connections, including but not limited to the mediodorsal nucleus of the thalamus[78], the caudate[79], hippocampus[80], subcallosal cingulate[81], and amygdala[82] to name a few.
Unilateral stimulation of the DLPFC has bilateral effects[83] as long as the corpus callosum is intact. While there have been varied downstream neural network changes in response to non-invasive magnetic stimulation of the DLPFC, it is clear that the effects of DLPFC stimulation are not isolated to a single node in the network. There is some suggestion that the antidepressant efficacy of non-invasive, repetitive transcranial magnetic stimulation of the left DLPFC sites is related to the anticorrelation of the subgenual cingulate, further supporting that the effects of DLPFC stimulation modulated deep structures in the neural network[81]. It is also important to note that there are individual differences in DLPFC connectivity and these difference appear to affect efficacy of stimulation at this node[84].
Left as well as bilateral epidural DLPFC stimulation has been shown to have a positive effect on depression[2,3], and when combined with bilateral epidural frontopolar epidural cortical stimulation (EpCS) appears to be a durable therapy for treatment-resistant depression[85]. Left DLPFC EpCS causes contralateral activation of the right DLPFC as well as superior frontal gyrus, cuneus, and posterior cingulate. In the Van Laere study, chronic, bilateral ALIC stimulation reduced right DLPFC activity on PET[56]. Conversely, in the Sturm study, DBS of the right NAc was shown to increase the activity of the right DLPFC in one patient on PET[59]. Subcallosal cingulate gyrus (SCG) DBS has been demonstrated to increase metabolism in bilateral DLPFC[63] in an earlier study, then decrease DLPFC bilaterally in a later study[64] at essentially the same time points of 3 and 6 mo[63,64]. An increase in activity of the DLPFC was also seen with fMRI of NAc DBS in large animals[26]. In a functional connectivity analysis of NAc DBS, the efficacy of the stimulation was related to reduced hyperconnectivity between the lateral (and medial) prefrontal cortex and the NAc[13].
Frontopolar cortex (BA 10): Frontopolar cortex (FPC) is the most uniquely human area in the hominid brain and is the only part of the prefrontal cortex (PFC) that has no direct inputs from the sensory cortex[86,87]. Several studies have demonstrated the frontal pole has a significant role in self-reflection, long-term goals, past or future events, or hypothetical scenarios[87-89]. Damage to this area impairs an individual’s ability to multitask[90]. The FPC functions to consider events beyond the present moment[87]. Pathological patterns of rumination and self-reflection are core features of depression. The FPC is being more and more implicated in the pathogenesis of depression as well as in its recovery[91,92]. A recent neuroimaging meta-analysis demonstrated a consistent finding of increased resting-state activity in the frontal pole (BA10) in patients with depression[93].
Invasive neuromodulation over the FPC (along with DLPFC) bilaterally has been implanted in 5 individuals with treatment-resistant depression, termed epidural cortical stimulation[2]. Furthermore, subgenual cingulate DBS appears to only be effective when it contacts the white matter tracts that cause downstream changes in the frontal polar cortex[47,94,95]. The tractographic connection to the frontal pole was the invasive neuromodulation target common between the ALIC target and the SCG target[2,96]. Chronic deep brain stimulation of NAc reduces activity in the gyri that terminate in the area of the frontal polar cortex[65]. The therapeutic effects of NAc DBS for OCD are related to reducing functional connectivity between the NAc and the FPC[13].
The frontal pole can be divided into a medial (ventromedial prefrontal cortex (vmPFC) part that is seen in all primates and a lateral part (the lateral frontopolar cortex (lFPC)) that is seen only in humans[86]. Activity of the vmPFC has been shown to be increased with NAc deep brain stimulation in an animal model, but these animals were not noted to be depressed[26]. Furthermore, it is unclear the function of the vmPFC in non-primate mammals, although it has been consistently demonstrated to have connections with the NAc[97]. vmPFC has been implicated in the pathogenesis of obsessive-compulsive disorder through its neural network connections to nodes involved in affective and reward processing[13,98]. A PET study investigating the effects of STN DBS for OCD demonstrated that a decrease in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score, a 10-item scale designed to both determine severity of OCD and to monitor improvement during treatment, was correlated with a decrease of metabolic activity in the vmPFC[58].
Orbitofrontal cortex (BA 11 and BA 12/47): The orbitofrontal prefrontal cortex (OFC) includes BA 12 caudally (or more recently 47[99] and BA 11 anteriorly[100]. Orbitofrontal hyperactivity/hyperconnectivity has been demonstrated on numerous functional imaging studies of obsessive-compulsive disorder[101] and Tourette[102]. Numerous neuroimaging studies in idiopathic depression and Parkinson’s-related depression have also implicated this area[93,103]. Reduction of OFC activity is correlated with symptom improvement in medication and psychotherapy trials[104,105]. With DBS to the inferior thalamic peduncle (connection from OFC to thalamus), it has been demonstrated to have significant reduction in OCD and depression symptoms[106]. OFC activity has been demonstrated to be reduced in chronic NAc stimulation[65]. In ALIC DBS for OCD, reduction in OFC activity was associated with clinical response in one study[50] and regardless of clinical response in another[107], as well as in larger studies of VCVS DBS[57], ALIC DBS[56], and capsulotomy[108]. It is also reduced in SCG stimulation[63,64], potentially explaining its anti-obsessional effects[109]. A PET study investigating the effects of STN DBS for OCD revealed that the decrease in Y-BOCS score was correlated with a decrease of metabolic activity in the orbitofrontal cortex[58]. Given that decreased orbitofrontal metabolism is associated with Parkinson’s related depression, this may explain why STN is associated with increased depressive symptoms[47], although that is likely not the whole story[110]. Reduction in the activity of the OFC appears to be clearly linked to improvements from OCD surgery regardless of the target or methodology chosen[57,58,108].
Cingulate cortex (BA 23, BA 24, BA 25, BA 31, BA 32): The SCG is the portion of the cingulum that lies ventral to the corpus callosum[111,112]. The SCG has been recognized for more than two decades to be an important node in the neural networks that contribute to mood regulation. This network includes cortical structures such as the DLPFC and the FPC as well as the subcortical structures such as the limbic system, thalamus, hypothalamus, and brainstem nuclei[111]. It has been demonstrated that activity in the SCG can reflect antidepressant efficacy, regardless of the nature of the intervention[81,113-115]. The SCG has reciprocal connections to the orbitofrontal, FPC, anterior cingulate cortex (ACC), intralaminar thalamus, hypothalamus and amygdala. The SCG also has afferents from BA 9/46 and efferents to numerous brainstem nuclei[111,116,117]. The SCG was the first target chosen because of functional neuroimaging data and not as the result of lesioning studies[63]. SCG was first targeted for depression DBS (unipolar and bipolar)[63,64,118], but has also been targeted for anorexia[109]. The gray matter of the subgenual cingulate has reduced activity with chronic NAc DBS[65], ALIC DBS[56] and SCG (white matter) DBS[63]. The white matter of the subgenual cingulate has increased activity with SCG DBS[63] and VCVS DBS[57]. It appears that in order to achieve an antidepressant response, the active contact has to interface with the white matter tracts to the ventral pallidum, the mediodorsal (MD) thalamus, and to the FPC[47,94].
The ACC has been heavily implicated in the neural circuitry of mood and anxiety regulation as well as the pathogenesis of TS[119]. In depression, the ACC is underactive while the SCG is overactive[120]. Conversely, the ACC is overactive in OCD during error processing[121,122]. The ACC is involved in self-referential processing. It is an important area for determining treatment response[120]. The higher the activity in the ACC, the higher likelihood of depression remission[123]. Anterior cingulotomy has been demonstrated to be quite effective in the treatment of OCD and to a lesser degree depression, but with significant side effects[9,124]. Success with anterior capsulotomy has been associated with significant reductions in dorsal anterior cingulate[108]. DBS of the right NAc has been shown to increase the activity of the right ACC[59] and animal studies have confirmed the laterality of these findings in a porcine model[26]. VCVS DBS increases activity of ACC acutely[57]. Conversely, DBS of the SCG demonstrated decreases in ACC metabolism[63,64]. Interestingly, limbic STN DBS in appears to reduce activity in the ACC[71]. The ACC has significant connections to the posterior cingulate cortex (PCC) and the medial PFC, another target for invasive neuromodulation for depression[2].
The PCC is a key node in the default mode network. It has been hypothesized that the posterior cingulate cortex has a role in supporting internally-directed cognition and increases in activity when individuals retrieve autobiographical memories or plan for the future[125]. The PCC has been shown to be decreased with depression[126] and obsessive-compulsive disorder[127] and is associated with treatment response to multiple modalities[128,129]. PCC activity is increased in the neural activity of the PCC is correlated with good response in DBS, but additionally in medication trials[125]. Cingulate activity was increased with NAc DBS in a large animal model[26] and increased in another case with OCD[59], but appears to decrease after chronic NAc DBS[65]. YBOCS scores for ALIC DBS were negatively correlated with PCC activity on PET[56]. Activity of the PCC increases with SCG stimulation[63,64]. The activity of SCG was reduced with limbic STN DBS[58]. The PCC has been demonstrated to have reduced activity with chronic NAc stimulation for depression[65], but this may reflect homeostatic mechanisms during chronic stimulation.
Insula: The anterior insula appears to be an important node in mood and anxiety regulation networks. A recent study demonstrated that relative hypo- or hyperactivity of the anterior insula is correlated with response to depression treatment whether cognitive behavioral therapy or medication[130]. Disgust associated with OCD is thought to be mediated by the insula[131]. The insula has also been implicated in tic generation in TS[132]. The metabolism of the insula has been demonstrated to have a significant correlation preoperative Y-BOCS score in patients undergoing capsulotomy[108]. Anterior insular activity is reduced with ALIC DBS stimulation [56] and NAc DBS stimulation[66]. It is similarly reduced in SCG stimulation[63,64] as well as in STN DBS (limbic)[58]. Conversely, the insular signal has been demonstrated to also be decreased with a model of porcine CM-Pf DBS[27], but increased in porcine NAc DBS[26]. It should be noted that both porcine DBS studies utilized non-depressed animal subjects[26,27].
Pre-supplementary motor area and supplementary motor area (BA 6): While the supplementary motor area (SMA) is an area of motor planning, the pre-SMA is an area related to response inhibition[133]. Lesions to the SMA cause reduction of spontaneous movements and difficulty in performing voluntary motor acts[134]. Inhibitory transcranial magnetic stimulation over the SMA and pre-SMA can reduce motor tics and OCD symptoms, respectively[10,11]. The effects of ALIC DBS and anterior capsulotomy have been demonstrated to spread to the pre-SMA/SMA[56,108]. SCG DBS has been shown to have effects on BA 6[63,64].
The NAc is an important node in the human reward system. It can be divided into two principal parts; the core and the shell[135]. The NAc core projects to the pallidal and nigral complexes and the NAc shell projects to the lateral hypothalamic areas, dopaminergic cell groups, and caudal mesencephallic areas[136]. Hyperactivity of the NAc has been shown to have a negative correlation with recovery from depression[123]. DBS has been implanted in the NAc not only for depression and OCD[59], but also TS[137], addiction[138-142], eating disorders[143], and even suggested for pain[144].
DBS of the ALIC normalized pre-op hyperactivity of the NAc[56]. The caudate has been shown to be involved in reward processing and hypoactive caudate responses to reward may be the functional explanation for anhedonia. The caudate has been shown to have reduced activity with chronic NAc stimulation[65]. Furthermore, it has been implicated in the pathophysiology of OCD where specific symptom profiles can be mapped to subregions within the caudate nucleus[145]. The caudate has been targeted for OCD DBS with apparent efficacy[145]. VCVS has been shown to activate striatum[57]. Right NAc inhibited the activity of the right dorsolateral rostral putamen[59]. Recently, the white matter tract afferent from the ventral tegmental area, medial forebrain bundle, to the NAc was targeted for treatment-resistant depression with marked success[146].
The amygdala is a brain nucleus that responds to novelty, salience and a variety of emotional stimuli. The amygdala has been implicated as an important component of the neural network that underlies social behavior[147]. The most commonly referenced role of the amygdala is in mediating fear and anxiety[148]. The amygdala has been shown to be overactive in patients with both MDD and Bipolar Disorder[149] and its activity is elevated in anticipatory anxiety in patients with generalized anxiety disorder[150] and in contamination fear with OCD[151]. Patients with Tourette appear to have altered functional connectivity of the amygdala[152] as well as amygdala response to negative face pictures[153,154]. Furthermore, the mean amygdala metabolism decreases in antidepressant treatment responders for depression, but persistence of elevated amygdala metabolism following remission is associated with a high risk for depressive relapse[155].
DBS stimulation of the ALIC was shown to normalize baseline hyperactivity of the amygdala and trended towards significant correlation with reductions in YBOCS[56], while this is not seen after anterior capsulotomy[108]. Acutely (1 wk), there is an increase in amygdalar activity with NAc DBS[66], which may explain the acute panic that has been observed[156]. Reduction in amygdalar hyperactivity was correlated with response to chronic NAs DBS for depression and anxiety[65]. In a functional connectivity analysis of NAc DBS, positive coupling was found with the amygdala[13]. CM-Pf DBS in a large animal model demonstrated reductions in amydalar signal which may explain some of the positive psychiatric improvements from CM-Pf DBS for TS[27].
The bed nucleus of the stria terminalis (BNST) is part of the extended amygdaloid complex and a relay/integral regulator of the hypothalamic-pituitary-adrenal stress axis[157,158]. The BNST is the major output of the basolateral (BL) amygdala and has been associated with sustained fear[159]. Some have suggested that the bed nucleus of the stria terminalis has been responsible for the positive effects of DBS that was targeted at the VCVS[160]. In a recent world-wide analysis, it was noted that when the “VCVS target” was shifted posterior (i.e., closer to the BNST), lower voltages were required to achieve efficacy[51].
The amygdala has been implicated in the pathogenesis of autism[161] and its hyperarousal has been seen on fMRI[162]. There is one case of BL amygdalar DBS, which was performed for self-injurious behavior in autism. In addition to improving self injurious behavior, DBS of the BL amygdala was found to improve the core symptoms of the autism spectrum in the emotional, social, and cognitive domains[163].
The sensorimotor STN has been demonstrated to be an efficacious target for the treatment of PD, although stimulation in this node can cause a range of neuropsychiatric symptoms[164]. In addition to the sensorimotor region, the STN also has limbic and executive regions with segregated circuitry involved in affective and cognitive processes[164]. Several PET studies have identified activity changes in limbic and executive regions during STN-DBS in Parkinson’s patients, including the dorsolateral prefrontal cortex and cingulate gyrus[70,165,166]. The ventromedial or limbic STN has been demonstrated to have profound anti-OCD effects through two serendipitous findings where STN DBS treated both PD and OCD[47].
Limbic STN DBS has been utilized successfully utilized for OCD[54,55] and TS[167]. Neuroimaging studies of the limbic and cognitive STN circuits have proven valuable, as these help with the STN DBS target refinement. Several of the STN DBS OCD patients underwent PET imaging[58]. When compared to OFF stimulation, DBS ON resulted in reduction in activity of the cingulate, orbitofrontal cortex, and supplementary motor areas (BA 6). The cingulate and orbitofrontal cortex are major nodes in the emotional and executive networks and the SMA is intimately involved in OCD/TS pathophysiology as was described above. The cingulate, SMA, and orbitofrontal cortex are hyperfunctional in untreated OCD, suggesting that a possible mechanism of DBS action may be the normalization of the activity of the SMA, OFC and cingulate[98,133,168,169].
The pallidal areas have been demonstrated to be immensely important in the treatment of movement disorders, such as Parkinson’s disease. This target has been associated with much better neuropsychiatric side effect profiles for PD DBS than STN[164]. The GPi has been implicated in the pathophysiology of TS[170] and OCD[171]. While the GPi has been implicated as a motor target, it is clear that the anterio-medial portions of the GPi are limbic. In fact the ventral pallidum has been implicated to be the limbic pleasure generator[172]. Both TS and OCD are successfully treated with GPi DBS[68,173] and there has even been some report of mood improvement with GPi DBS[174]. The globus pallidus externa has also been demonstrated to treat TS[175]. Surprisingly, many of the functional imaging studies do not demonstrate changes in the pallidum with DBS[26,50,63]. The exception is PET of patients with VCVS DBS[57]. The efficacy of SCG DBS is optimized when the active contact interfaces with the white matter tract to the ventral pallidum[47,94].
Centromedian nucleus of the thalamus and parafascicular complex: The CM-Pf nuclei complex is located in the posterior part of the intralaminar thalamus and serves as the main basal ganglia input station. Additionally, the CM-Pf controls striatal dopamine function and provides a majority of the thalamic input to the striatum[176-178]. CM-Pf lesions have been shown to cause complex attention deficits. This has been hypothesized to be due to the role of the CM-Pf in directing attention to motivationally relevant stimuli[179]. The CM-Pf DBS target has been utilized in TS, but has also been shown to have positive effects on mood, anxiety, and OCD[180].
In addition to the striatum, the CM-Pf has been demonstrated to have connections with the amygdala[181], hippocampus[182], globus pallidus[183], nucleus accumbens[184], insular cortex, and anterior cingulate cortex[176]. A recent large animal study looking at DBS-fMRI in the CM-Pf study was recently completed[27]. In this study, DBS at the Pf was shown to have negative BOLD downstream effects on the hippocampus as well as the dorsal anterior cingulate cortex and posterior cingulate cortex. DBS at the CM was shown to have negative BOLD downstream effects on the sensorimotor areas and the associative area. The observed differences in structural connections of CM versus Pf in non-human primate topographical analyses were verified by this large animal CM-Pf DBS-fMRI study[27]. The Pf has been demonstrated to innervate associative and limbic striatal areas[185-187]. This large animal study also demonstrated a primarily limbic downstream effect with high frequency stimulation[27]. Similarly, the CM innervates the sensorimotor striatal area[185,188,189]. The large animal study also confirmed that the CM is has primarily sensorimotor downstream effects with high frequency stimulation[27].
The limbic nucleus of the thalamus, the medial dorsal nucleus of the thalamus, is an important node in the limbic circuitry. Using high-resolution 7T fMRI, Metzger, et.al. demonstrated general emotional arousal is localized to the mediodorsal nucleus while preceding attention and expectancy were localized to the intralaminar centromedian/parafascicular complex[190]. A component of several of the frontal-striatal circuitry, the MD thalamus has been shown to be hyperactive in depression. In the depression associated with Parkinson’s, volumetric studies have shown that the MD nucleus volume is larger than non-depressed controls, but hypoactive on the left in this population suggesting that the increase in volume is compensatory[191]. There has been four cases where the MD was targeted for TS[16,17]. In addition to improvement in TS symptoms, there was a hint of improvement in mood and obsessive thinking for at least one of these individuals[16]. During chronic NAc stimulation, MD nucleus activity is reduced[65]. This reduction in MD activity is also observed with ALIC stimulation[56]. In large animal studies looking at porcine NAc DBS stim, similar reductions were seen[26]. In a recent analysis of SCG DBS, tractography interfacing with the active contact was compared to presence or absence of remission and only individuals with white matter projections extending to the MD thalamus were able to achieve remission[47]. It appears that the MD thalamus is a critical node in mood/affect regulation as well as mediating anxiety disorders.
It is possible to non-invasively determine the anatomical connection pathways using diffusion tractography imaging. These pathways are assumed to be consistent with known anatomy[192,193]. This MRI technology has been utilized in determining the elements that contact and are stimulated with DBS[194]. The benefits observed in patients with DBS appear to heavily depend on exact electrode position, which can only be truly verified with sophisticated tractography techniques[94,95,146,194-196]. In the case of medial forebrain bundle DBS, the target can only be identified with sophisticated tractography imaging as one cannot visualize this target on conventional imaging[146]. Slight alterations in the position of the electrode in the SCG appear to change whether this target is efficacious or not[95]. The white matter tracts have to be completely intact for DBS to be efficacious where any alteration in the macroanatomy of the limbic white matter tracts can cause the stimulation to be ineffective[197,198].
Conversely, verification of correct position with EpCS has been shown to only require a postoperative computed tomography (CT) scan coregistered with the presurgical MRI scan. This method will allow for all four contact electrodes on each paddle lead to be identified. In all 5 subjects of the Nahas study, contact electrodes were over the bilateral frontal pole and dorsolateral prefrontal cortex[2]. In the Kopell study, the responders were noted to have the contacts of the electrodes placed at or anterior to the target site (DLPFC). Non-responders or partial responders had their electrodes placed posterior to the DLPFC target site[3].
Recent developments in noninvasive functional connectivity analysis demonstrate that this allows for a powerful network-level view of the neuropsychiatric disease[199] and the effects DBS on that network[13]. In this technique, the spontaneous fluctuations in BOLD are identified as the intrinsic marker for functional connectivity. A seed region is then identified and correlated with nodes in the connected neural network in an effort to determine areas where there is functional connectivity[200]. The utility of this approach in DBS research comes from its ability to not only identify the aberrant functional connectivity of a particular neuropsychiatric disorder, but also normalize this aberrant functional connectivity through neuromodulation. This technique has been utilized to probe the resting state connectivity changes in patients receiving NAc DBS for OCD[13]. In this study, the NAc DBS was shown to normalize aberrant hyperconnectivity between the NAc and lateral/medial prefrontal cortices. This reduction in connectivity correlated with the reductions in the YBOCS. While NAc is also a target for treatment-resistant depression (TRD) and TS, there have been no functional connectivity analysis studies in TRD and TS to date.
Invasive neuromodulation holds promise as a tool for treatment-resistant neuropsychiatric conditions[47]. DBS resides at an interface between neurophysiology, Neurosurgery, movement disorders Neurology, and interventional psychiatry[201]. DBS has been demonstrated to be an effective therapy for essential tremor, dystonia and Parkinson’s disease, and is being investigated in many other neuropsychiatric diseases[47]. Advanced neuroimaging techniques aid in targeting, investigate the effects of, and further refine targeting of DBS all in a non-invasive manner. Although many of the functional imaging studies to date are limited to small case series and have had conflicting results, a growing trend is emerging. For most neuropsychiatric DBS imaging studies, the effects of DBS appears to have downstream effects on both cortical areas (DLPFC, FP, SCG, ACC, PCC, insula, SMA) as well as subcortical areas (thalamus, pallidum, STN, amygdala, striatum). These findings affirm that the therapeutic properties of DBS reside not only in local effects, but also downstream effects on the distributed neural network.
The effects and apparent efficacy of DBS for OCD appears to be related its downstream effects on the prefrontal cortex[13], more specifically orbitofrontal cortex[50,58]. This also appears to be true for invasive neuromodulation in treatment-resistant depression where the tractography and the functional imaging of depression DBS targets all converge on PFC[13], more specifically Brodmann 10[47,94-96]. Recent optogenetics findings have confirmed the critical role of the medial prefrontal cortex in mood regulation[92]. While there has only been one study investigating the effects of direct BA 10 stimulation [2], this technique appears remarkably durable[85]. The critical role of the frontopolar cortex in mood regulation was further demonstrated when its activation was demonstrated to be associated with acute, stimulation-induced depressive symptoms in a patient with Parkinson’s and a mispositioned lead[31]. Furthermore, this cortical stimulation approach seems to avoid many of the methodological challenges seen with other techniques such as the need for advanced tractography[95,146] and the risk of cerebral hemorrhage[198]. The therapeutic effects of TS DBS appears to be correlated with the therapy’s ability to reduce dopamine levels in various downstream targets[17].
While the apparent efficacy of DBS for neuropsychiatric disease is promising, there are several remaining questions. Are cortical or subcortical targets better for invasive neuromodulation? Will sophisticated tractography techniques be essential to the widespread implementation of this therapy for TRD[95,196,202]? How do symptom clusters of a given neuropsychiatric disease correlate with the pre-operative functional imaging and the DBS-induced functional activation? Will imaging eventually dictate the target with the best chance of efficacy? Will DBS move beyond its current role to affect plasticity[203]? Will DBS confirm findings that the basal ganglia may in fact be organized in a gradient of medial to lateral where emotional/reward (most medial)-cognitive-and motor subcomponents of the nuclei are in fact highly interconnected.
As this field develops both scientifically and technically, we anticipate that larger studies will be conducted and our collective knowledge will converge. In addition to extending ongoing functional imaging studies in this area, future investigations will likely temporally pair functional MRI with information on structural integrity (including diffusion tensor imaging and voxel based morphometry) which will enable us to have a more comprehensive understanding of the effects of DBS on neural network integrity among DBS patients. Some disease specific issues to consider in future studies include inherent microstructural pathology in a given disease may influence the effect of DBS outcomes[204,205], the role of acute vs chronic stimulation on neural network plasticity[13], and baseline firing rates in areas downstream from the DBS target[206]. DBS is an extremely nonselective method of stimulation and this non-specific nature may be harmful in one disorder[207], while that same neural element being efficacious in treating another[146]. Currently, imaging studies are unable to distinguishing between circuits of interest and those being activated unintentionally. Current steering technologies coupled with functional imaging will potentially allow for isolation of a single neural element and the ability to image that neural element[208,209]. Such an approach when coupled with the selective circuit manipulation of optogenetics[15] may allow for further verification of findings. It is truly an exciting time to be embarking on a journey into the interface between advanced imaging and invasive neuromodulation.
We would also like to thank the Drug Abuse Research Track (DART) program at the Medical University of South Carolina with special thanks to Drs. Back and Brady, along with the grant that supports it [NIDA R25 DA020537-06 (PI’s Back and Brady)].
P- Reviewer: Arsalidou M, Llamas EP, Sun Z, Tang GH S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Okun MS, Oyama G. Mechanism of action for deep brain stimulation and electrical neuro-network modulation (ENM). Rinsho Shinkeigaku. 2013;53:691-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Nahas Z, Anderson BS, Borckardt J, Arana AB, George MS, Reeves ST, Takacs I. Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biol Psychiatry. 2010;67:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Kopell BH, Halverson J, Butson CR, Dickinson M, Bobholz J, Harsch H, Rainey C, Kondziolka D, Howland R, Eskandar E. Epidural cortical stimulation of the left dorsolateral prefrontal cortex for refractory major depressive disorder. Neurosurgery. 2011;69:1015-1029; discussion 1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 278] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 491] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353:724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 334] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Hooper AK, Okun MS, Foote KD, Fernandez HH, Jacobson C, Zeilman P, Romrell J, Rodriguez RL. Clinical cases where lesion therapy was chosen over deep brain stimulation. Stereotact Funct Neurosurg. 2008;86:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Yang JC, Ginat DT, Dougherty DD, Makris N, Eskandar EN. Lesion analysis for cingulotomy and limbic leucotomy: comparison and correlation with clinical outcomes. J Neurosurg. 2014;120:152-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Jenike MA, Rauch SL. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Berlim MT, Neufeld NH, Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2013;47:999-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry. 2002;10:86-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1084] [Cited by in F6Publishing: 1053] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 16. | Vernaleken I, Kuhn J, Lenartz D, Raptis M, Huff W, Janouschek H, Neuner I, Schaefer WM, Gründer G, Sturm V. Bithalamical deep brain stimulation in tourette syndrome is associated with reduction in dopaminergic transmission. Biol Psychiatry. 2009;66:e15-e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kuhn J, Janouschek H, Raptis M, Rex S, Lenartz D, Neuner I, Mottaghy FM, Schneider F, Schaefer WM, Sturm V. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette’s syndrome. Biol Psychiatry. 2012;71:e11-e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | George MS, Ketter TA, Post RM. SPECT and PET imaging in mood disorders. J Clin Psychiatry. 1993;54 Suppl:6-13. [PubMed] [Cited in This Article: ] |
| 19. | Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K; European Association of Nuclear Medicine Neuroimaging Committee. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103-2110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000-10006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 340] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 658] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 22. | George MS, Ring HA, Costa DC, Ell PJ, Kouris K, Jarritt P. Neuroactivation and Neuroimaging with SPET. London: Springer-Verlag 1991; 1-100. [Cited in This Article: ] |
| 23. | Wyckhuys T, De Geeter N, Crevecoeur G, Stroobants S, Staelens S. Quantifying the effect of repetitive transcranial magnetic stimulation in the rat brain by μSPECT CBF scans. Brain Stimul. 2013;6:554-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868-9872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4233] [Cited by in F6Publishing: 3614] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 25. | Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 26. | Knight EJ, Min HK, Hwang SC, Marsh MP, Paek S, Kim I, Felmlee JP, Abulseoud OA, Bennet KE, Frye MA. Nucleus accumbens deep brain stimulation results in insula and prefrontal activation: a large animal FMRI study. PLoS One. 2013;8:e56640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kim JP, Min HK, Knight EJ, Duffy PS, Abulseoud OA, Marsh MP, Kelsey K, Blaha CD, Bennet KE, Frye MA. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry. 2013;74:917-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Gupte AA, Shrivastava D, Spaniol MA, Abosch A. MRI-related heating near deep brain stimulation electrodes: more data are needed. Stereotact Funct Neurosurg. 2011;89:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Georgi JC, Stippich C, Tronnier VM, Heiland S. Active deep brain stimulation during MRI: a feasibility study. Magn Reson Med. 2004;51:380-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Carmichael DW, Pinto S, Limousin-Dowsey P, Thobois S, Allen PJ, Lemieux L, Yousry T, Thornton JS. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. Neuroimage. 2007;37:508-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, Saint-Cyr J, Lozano A. Deep brain stimulation for Parkinson’s disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003;18:1508-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Arantes PR, Cardoso EF, Barreiros MA, Teixeira MJ, Gonçalves MR, Barbosa ER, Sukwinder SS, Leite CC, Amaro E. Performing functional magnetic resonance imaging in patients with Parkinson’s disease treated with deep brain stimulation. Mov Disord. 2006;21:1154-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005;46:1444-1454. [PubMed] [Cited in This Article: ] |
| 34. | Pourfar M, Tang C, Lin T, Dhawan V, Kaplitt MG, Eidelberg D. Assessing the microlesion effect of subthalamic deep brain stimulation surgery with FDG PET. J Neurosurg. 2009;110:1278-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Rozanski VE, Lieb M, Vollmar C, Mehrkens JH, Bötzel K. Evidence of a non-motor microlesion effect following deep brain surgery: a case report. Acta Neurochir (Wien). 2012;154:835-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Lefaucheur R, Derrey S, Martinaud O, Wallon D, Chastan N, Gérardin E, Hannequin D, Maltête D. Early verbal fluency decline after STN implantation: is it a cognitive microlesion effect? J Neurol Sci. 2012;321:96-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Swan BD, Grill WM, Turner DA. Investigation of deep brain stimulation mechanisms during implantable pulse generator replacement surgery. Neuromodulation. 2014;17:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Rezai AR, Baker KB, Tkach JA, Phillips M, Hrdlicka G, Sharan AD, Nyenhuis J, Ruggieri P, Shellock FG, Henderson J. Is magnetic resonance imaging safe for patients with neurostimulation systems used for deep brain stimulation? Neurosurgery. 2005;57:1056-1062; discussion 1056-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Rezai AR, Phillips M, Baker KB, Sharan AD, Nyenhuis J, Tkach J, Henderson J, Shellock FG. Neurostimulation system used for deep brain stimulation (DBS): MR safety issues and implications of failing to follow safety recommendations. Invest Radiol. 2004;39:300-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry. 2011;168:1326-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | Giller C, Mehta S, Yanasak N, Jenkins P. Avoidance of electrode related MRI artifact during staged deep brain stimulator implantation. J Neurol Surg A Cent Eur Neurosurg. 2012;73:320-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Rezai AR, Lozano AM, Crawley AP, Joy ML, Davis KD, Kwan CL, Dostrovsky JO, Tasker RR, Mikulis DJ. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Kahan J, Mancini L, Urner M, Friston K, Hariz M, Holl E, White M, Ruge D, Jahanshahi M, Boertien T. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson’s disease. PLoS One. 2012;7:e50270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Liu HL, Chen HM, Wu YC, Lim SN, Huang CM, Hsu YY, Wai YY, Wu T. False-positive analysis of functional MRI during simulated deep brain stimulation: a phantom study. J Magn Reson Imaging. 2008;27:1439-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Chhabra V, Sung E, Mewes K, Bakay RA, Abosch A, Gross RE. Safety of magnetic resonance imaging of deep brain stimulator systems: a serial imaging and clinical retrospective study. J Neurosurg. 2010;112:497-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Jech R, Urgosík D, Tintera J, Nebuzelský A, Krásenský J, Liscák R, Roth J, Růzicka E. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson’s disease. Mov Disord. 2001;16:1126-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123:4546-4556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Huff W, Lenartz D, Schormann M, Lee SH, Kuhn J, Koulousakis A, Mai J, Daumann J, Maarouf M, Klosterkötter J. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg. 2010;112:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 49. | Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, Bosch A, Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 50. | Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 51. | Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 52. | Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384-2393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 620] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 53. | Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, Shapira NA, Wu SS, Hill CL, Rasmussen SA. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Chabardès S, Polosan M, Krack P, Bastin J, Krainik A, David O, Bougerol T, Benabid AL. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg. 2013;80:S31.e1-S31.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 675] [Cited by in F6Publishing: 581] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 56. | Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, Cosyns P. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47:740-747. [PubMed] [Cited in This Article: ] |
| 57. | Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, Alpert NM, Haber SN, Stypulkowski PH, Rise MT. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Le Jeune F, Vérin M, N’Diaye K, Drapier D, Leray E, Du Montcel ST, Baup N, Pelissolo A, Polosan M, Mallet L. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68:1016-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Williams N, Simpson AN, Simpson K, Nahas Z. Relapse rates with long-term antidepressant drug therapy: a meta-analysis. Hum Psychopharmacol. 2009;24:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, Boggio PS, Lotufo PA, Benseñor IM, Fregni F. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383-391. [PubMed] [Cited in This Article: ] |
| 62. | Schlaepfer TE, George MS, Mayberg H. WFSBP Guidelines on Brain Stimulation Treatments in Psychiatry. World J Biol Psychiatry. 2010;11:2-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2616] [Cited by in F6Publishing: 2391] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 64. | Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 612] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 65. | Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 567] [Cited by in F6Publishing: 527] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 66. | Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 602] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 67. | Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, Robertson MM. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Cannon E, Silburn P, Coyne T, O’Maley K, Crawford JD, Sachdev PS. Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette’s syndrome. Am J Psychiatry. 2012;169:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Sachdev PS, Cannon E, Coyne TJ, Silburn P. Bilateral deep brain stimulation of the nucleus accumbens for comorbid obsessive compulsive disorder and Tourette’s syndrome. BMJ Case Rep. 2012;2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol. 1997;42:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 289] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 71. | Min HK, Hwang SC, Marsh MP, Kim I, Knight E, Striemer B, Felmlee JP, Welker KM, Blaha CD, Chang SY. Deep brain stimulation induces BOLD activation in motor and non-motor networks: an fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage. 2012;63:1408-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23:2677-2689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 73. | Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141-51. [PubMed] [Cited in This Article: ] |
| 74. | Fuster JM. The prefrontal cortex. 4th ed. Amsterdam; Boston: Academic Press/Elsevier 2008; 293. [Cited in This Article: ] |
| 75. | Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 737] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 76. | Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 77. | Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 78. | Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010;51:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 79. | Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 80. | Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 570] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 81. | Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 665] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 82. | Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049-4068. [PubMed] [Cited in This Article: ] |
| 83. | Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, Repella JD, Danielson AL, Willis MW, Benson BE. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 84. | Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2012;66C:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 85. | Williams NR, Short EB, Borckardt JJ, Schmidt M, Jeffery A, Korte JE, George MS, Takacs I, Nahas ZH. Five-Year Follow-up of Bilateral Epidural Prefrontal Cortical Stimulation for Treatment- Resistant Depression [Conference Poster]. Biol Psychiatry. 2014;. [Cited in This Article: ] |
| 86. | Koechlin E. Frontal pole function: what is specifically human? Trends Cogn Sci. 2011;15:241; author reply 3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 88. | Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 89. | Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1100] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 90. | Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3:e3227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010;44:683-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082-16090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 93. | Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 94. | Mayberg H, editor . Optimizing Subcallosal Cingulate DBS for Treatment Resistant Depression [Conference Poster]. Society of Biological Psychiatry;. Amsterdam; Boston: Academic Press/Elsevier 2013; Biol Psychiatry. [Cited in This Article: ] |
| 95. | Lujan JL, Chaturvedi A, Choi KS, Holtzheimer PE, Gross RE, Mayberg HS, McIntyre CC. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul. 2013;6:737-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 97. | Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 742] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 98. | Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 518] [Cited by in F6Publishing: 528] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 99. | Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, Giraud AL. How the brain repairs stuttering. Brain. 2009;132:2747-2760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 100. | Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 735] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 101. | Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, Kathmann N. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 102. | Weeks RA, Turjanski N, Brooks DJ. Tourette’s syndrome: a disorder of cingulate and orbitofrontal function? QJM. 1996;89:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, Wagner HN, Robinson RG. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol. 1990;28:57-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 237] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME, Baxter LR. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 105. | Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, Yoshizato C, Yoshioka K, Tomita M, Kanba S. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. 2008;163:236-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Jiménez F, Velasco F, Salin-Pascual R, Hernández JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585-593; discussion 585-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 107. | Nuttin BJ, Gabriëls LA, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels JM, Gielen F. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263-1272; discussion 1263-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 108. | Zuo C, Ma Y, Sun B, Peng S, Zhang H, Eidelberg D, Guan Y. Metabolic imaging of bilateral anterior capsulotomy in refractory obsessive compulsive disorder: an FDG PET study. J Cereb Blood Flow Metab. 2013;33:880-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, Sutandar K, Staab R, Elias G, Lyman CH. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381:1361-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 110. | Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci USA. 2007;104:17087-17092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 111. | Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 112. | Riva-Posse P, Holtzheimer PE, Garlow SJ, Mayberg HS. Practical considerations in the development and refinement of subcallosal cingulate white matter deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80:S27.e25-S27.e34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, Price BH, Mayberg HS, Fischman AJ, Rauch SL. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 600] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 115. | Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001;158:305-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 116. | Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 117. | Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1006] [Cited by in F6Publishing: 1019] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 118. | Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 119. | Müller-Vahl KR, Kaufmann J, Grosskreutz J, Dengler R, Emrich HM, Peschel T. Prefrontal and anterior cingulate cortex abnormalities in Tourette Syndrome: evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009;10:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 500] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 121. | Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 122. | Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 123. | Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 124. | Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR, Eskandar EN, Dougherty DD. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg. 2013;118:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 125. | Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1291] [Cited by in F6Publishing: 1423] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 126. | Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675-682. [PubMed] [Cited in This Article: ] |
| 127. | Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, Baker SK, Baxter LR. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 128. | Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, Nierenberg AA, Mayberg HS, Baer L, Jenike MA. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry. 2001;50:659-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SC, Speckens A, Phillips ML. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry. 2003;53:482-493. [PubMed] [Cited in This Article: ] |
| 130. | McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 131. | Stein DJ, Arya M, Pietrini P, Rapoport JL, Swedo SE. Neurocircuitry of disgust and anxiety in obsessive-compulsive disorder: a positron emission tomography study. Metab Brain Dis. 2006;21:267-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, Frith CD, Frackowiak RS, Dolan RJ. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741-748. [PubMed] [Cited in This Article: ] |
| 133. | de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, van Balkom AJ, Veltman DJ, van den Heuvel OA. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169:1100-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 134. | Bannur U, Rajshekhar V. Post operative supplementary motor area syndrome: clinical features and outcome. Br J Neurosurg. 2000;14:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 481] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 136. | Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 791] [Cited by in F6Publishing: 827] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 137. | Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007;254:963-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 138. | Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, Sturm V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 139. | Müller UJ, Voges J, Steiner J, Galazky I, Heinze HJ, Möller M, Pisapia J, Halpern C, Caplan A, Bogerts B. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann N Y Acad Sci. 2013;1282:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 140. | Kuhn J, Möller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TO, Maarouf M, Brosig A, Barnikol UB, Klosterkötter J. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry. 2014;19:145-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 141. | Heldmann M, Berding G, Voges J, Bogerts B, Galazky I, Müller U, Baillot G, Heinze HJ, Münte TF. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS One. 2012;7:e36572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Voges J, Müller U, Bogerts B, Münte T, Heinze HJ. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 2012;80:S28.e21-S28.e31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Wu H, Van Dyck-Lippens PJ, Santegoeds R, van Kuyck K, Gabriëls L, Lin G, Pan G, Li Y, Li D, Zhan S. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2013;80:S29.e1-S29.10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 144. | Plow EB, Malone DA, Machado A. Deep brain stimulation of the ventral striatum/anterior limb of the internal capsule in thalamic pain syndrome: study protocol for a pilot randomized controlled trial. Trials. 2013;14:241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 145. | Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 147. | Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 460] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 148. | Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2060] [Cited by in F6Publishing: 1981] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 149. | Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 150. | Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 151. | van den Heuvel OA, Veltman DJ, Groenewegen HJ, Dolan RJ, Cath DC, Boellaard R, Mesina CT, van Balkom AJ, van Oppen P, Witter MP. Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res. 2004;132:225-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 152. | Werner CJ, Stöcker T, Kellermann T, Wegener HP, Schneider F, Shah NJ, Neuner I. Altered amygdala functional connectivity in adult Tourette’s syndrome. Eur Arch Psychiatry Clin Neurosci. 2010;260 Suppl 2:S95-S99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Neuner I, Kellermann T, Stöcker T, Kircher T, Habel U, Shah JN, Schneider F. Amygdala hypersensitivity in response to emotional faces in Tourette’s patients. World J Biol Psychiatry. 2010;11:858-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 154. | Via E, Cardoner N, Pujol J, Alonso P, López-Solà M, Real E, Contreras-Rodríguez O, Deus J, Segalàs C, Menchón JM. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry. 2014;204:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 155. | Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 411] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 156. | Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, Springer US, Lang PJ, Greenberg BD, Haber SN, Goodman WK. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 157. | Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025-2034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 158. | Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 159. | Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1078] [Cited by in F6Publishing: 1004] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 160. | Nuttin B, Gielen F, van Kuyck K, Wu H, Luyten L, Welkenhuysen M, Brionne TC, Gabriëls L. Targeting bed nucleus of the stria terminalis for severe obsessive-compulsive disorder: more unexpected lead placement in obsessive-compulsive disorder than in surgery for movement disorders. World Neurosurg. 2013;80:S30.e11-S30.e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 162. | Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 163. | Sturm V, Fricke O, Bührle CP, Lenartz D, Maarouf M, Treuer H, Mai JK, Lehmkuhl G. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2012;6:341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 164. | Williams NR, Foote KD, Okun MS. STN vs. GPi Deep Brain Stimulation: Translating the Rematch into Clinical Practice. Mov Disord Clin Pract. 2014;1:24-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 165. | Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, Lehrke R, Koulousakis A, Herholz K, Sturm V. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab. 2004;24:7-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 166. | Geday J, Østergaard K, Johnsen E, Gjedde A. STN-stimulation in Parkinson’s disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp. 2009;30:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 167. | Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T, Limousin P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology. 2009;72:1787-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 168. | Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Front Integr Neurosci. 2012;6:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 169. | Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain Cogn. 2004;55:220-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 170. | Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 171. | Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 696] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 172. | Ho CY. The Ventral Pallidum As a Limbic Pleasure Generator (Doctoral dissertaion). Available from: http: // www.deepblue.lib.umich.edu/handle/2027.42/78836. [Cited in This Article: ] |
| 173. | Nair G, Evans A, Bear RE, Velakoulis D, Bittar RG. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J Clin Neurosci. 2014;21:815-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 174. | Kosel M, Sturm V, Frick C, Lenartz D, Zeidler G, Brodesser D, Schlaepfer TE. Mood improvement after deep brain stimulation of the internal globus pallidus for tardive dyskinesia in a patient suffering from major depression. J Psychiatr Res. 2007;41:801-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 175. | Piedimonte F, Andreani JC, Piedimonte L, Graff P, Bacaro V, Micheli F, Vilela Filho O. Behavioral and motor improvement after deep brain stimulation of the globus pallidus externus in a case of Tourette’s syndrome. Neuromodulation. 2013;16:55-58; discussion 58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 176. | Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 691] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 177. | Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 178. | Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 179. | Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol. 2001;85:960-976. [PubMed] [Cited in This Article: ] |
| 180. | Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 181. | Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 184] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 182. | Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gülçebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat. 2008;212:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 183. | Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 405] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 184. | Jayaraman A. Organization of thalamic projections in the nucleus accumbens and the caudate nucleus in cats and its relation with hippocampal and other subcortical afferents. J Comp Neurol. 1985;231:396-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 185. | Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 2009;78:122-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 186. | Sadikot AF, Parent A, François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 228] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 187. | Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 2005;481:127-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 188. | Sidibé M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol. 1997;382:323-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 189. | Sidibé M, Paré JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 190. | Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front Neuroanat. 2010;4:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 191. | Cardoso EF, Maia FM, Fregni F, Myczkowski ML, Melo LM, Sato JR, Marcolin MA, Rigonatti SP, Cruz AC, Barbosa ER. Depression in Parkinson’s disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage. 2009;47:467-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 192. | Parker GJ, Stephan KE, Barker GJ, Rowe JB, MacManus DG, Wheeler-Kingshott CA, Ciccarelli O, Passingham RE, Spinks RL, Lemon RN. Initial demonstration of in vivo tracing of axonal projections in the macaque brain and comparison with the human brain using diffusion tensor imaging and fast marching tractography. Neuroimage. 2002;15:797-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 193. | Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1807] [Cited by in F6Publishing: 1719] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 194. | Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 195. | Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392-10402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 196. | Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Mädler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 2012;24:223-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 197. | McNab JA, Voets NL, Jenkinson N, Squier W, Miller KL, Goodwin GM, Aziz TZ. Reduced limbic connections may contraindicate subgenual cingulate deep brain stimulation for intractable depression. J Neurosurg. 2009;111:780-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 198. | Coenen VA, Mädler B, Schlaepfer TE. Reply to: medial forebrain bundle stimulation-speed access to an old or entry into a new depression neurocircuit? Biol Psychiatry. 2013;74:e45-e46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 199. | Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, Huang X, Kemp GJ, Mechelli A, Gong Q. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168:642-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 200. | Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735-2747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 830] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 201. | Williams NR, Taylor JJ, Snipes JM, Short BE, Kantor EM, George MS. Interventional Psychiatry: How should we incorporate neuromodulation into psychiatric education? Acad Psychiatry. 2014;38:168-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 202. | Cho ZH, Law M, Chi JG, Choi SH, Park SY, Kammen A, Park CW, Oh SH, Kim YB. An anatomic review of thalamolimbic fiber tractography: ultra-high resolution direct visualization of thalamolimbic fibers anterior thalamic radiation, superolateral and inferomedial medial forebrain bundles, and newly identified septum pellucidum tract. World Neurosurg. 2013;Aug 22; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 203. | Schweder PM, Joint C, Hansen PC, Green AL, Quaghebeur G, Aziz TZ. Chronic pedunculopontine nucleus stimulation restores functional connectivity. Neuroreport. 2010;21:1065-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 204. | Liu Y, Miao W, Wang J, Gao P, Yin G, Zhang L, Lv C, Ji Z, Yu T, Sabel BA. Structural abnormalities in early Tourette syndrome children: a combined voxel-based morphometry and tract-based spatial statistics study. PLoS One. 2013;8:e76105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 205. | Neuner I, Kupriyanova Y, Stöcker T, Huang R, Posnansky O, Schneider F, Tittgemeyer M, Shah NJ. White-matter abnormalities in Tourette syndrome extend beyond motor pathways. Neuroimage. 2010;51:1184-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 206. | Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson’s disease. J Neurosci. 2013;33:7220-7233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 207. | Coenen VA, Honey CR, Hurwitz T, Rahman AA, McMaster J, Bürgel U, Mädler B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery. 2009;64:1106-1114; discussion 1106-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 208. | Chaturvedi A, Foutz TJ, McIntyre CC. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain Stimul. 2012;5:369-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 209. | Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34:3031-3054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |