Published online Dec 28, 2011. doi: 10.4329/wjr.v3.i12.289
Revised: September 7, 2011
Accepted: October 11, 2011
Published online: December 28, 2011
Congenital airway anomalies can be asymptomatic or may cause severe respiratory distress requiring immediate treatment. These anomalies can present early in life, or may be just incidental findings. It is important to recognize these entities to realize their clinical significance and to avoid false diagnosis. In this article, the various congenital airway anomalies and their imaging features by multidetector computed tomography (MDCT) are reviewed in order of occurrence during the embryological timeline. This pictorial essay reviews the various distinct congenital airway lesions and their MDCT manifestations. It also provides insight into the embryological basis of the congenital airway lesions encountered.
- Citation: Sundarakumar DK, Bhalla AS, Sharma R, Gupta AK, Kabra SK, Jagia P. Multidetector computed tomography imaging of congenital anomalies of major airways: A pictorial essay. World J Radiol 2011; 3(12): 289-297
- URL: https://www.wjgnet.com/1949-8470/full/v3/i12/289.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i12.289
Imaging modalities for pediatric tracheo-bronchial lesions have vastly improved over time. Frontal and lateral neck and chest X-rays were the radiological investigations used in the past which provided limited diagnostic yield[1]. With the advent of multidetector computed tomography (MDCT) scanners and continued refinement in the 3-D reconstruction software algorithms, newer options for non-invasive imaging of these lesions have become available. These high resolution images demonstrate exquisite details of the airways down to the segmental bronchi, can depict the adjacent mediastinal structures, and result in an improvement in diagnostic confidence. In addition, decreased scan time, and therefore decreased need for prolonged sedation in the pediatric population, are advantageous in scanning children, where motion artifact is an issue.
In this pictorial essay, congenital airway lesions are depicted using axial MDCT images and reconstructed imaging techniques such as multiplanar reformatted images, minimal intensity projection images, and virtual bronchoscopy images.
The lower respiratory system originates as a diverticulum from the ventral wall of the foregut during the 4th week of life and continues to develop until 2 years of life. The respiratory epithelium originates from the endodermal lining of the respiratory diverticulum. The cartilaginous and muscular components of the trachea and lungs are derived from the surrounding splanchnic mesoderm. As the diverticulum elongates in the caudal direction, it divides into the trachea and esophagus by a trachea-esophageal septum. The ventral portion forms the trachea and lung buds, while the dorsal portion forms the esophagus. At 28-30 d, the lung buds form the primary bronchi, from which develop the segmental bronchi.
Tracheal agenesis and atresia are rare congenital anomalies which cause respiratory distress in the newborn immediately after delivery. In the most common variety (type II), there is complete tracheal atresia with normal bronchi and carina. Rarely, there is normal distal trachea and bronchi with a tracheo-esophageal fistula and atretic proximal trachea (type I) or no trachea at all, with the bronchi arising directly from the esophagus (type III)[2]. Tracheal agenesis is sometimes associated with syndromic conditions, such as VATER (vertebrae, anus, trachea, esophagus, and renal) (Figure 1 and Table 1).
| Tracheal agenesis type | Features |
| Type I | Agenesis of proximal trachea with short segment normal distal trachea, carina and bronchi. Fistula is present between the distal trachea and esophagus |
| Type II | Agenesis of entire trachea. There may be a communication between esophagus and carina, from which the bronchi originate |
| Type III | Atresia of entire trachea and carina, the bronchi originate individually from the esophagus |
Foregut duplication cysts are not often classifiable as either esophageal or bronchogenic. Bronchogenic cysts are by far the most common mediastinal cysts. Approximately 80% of cysts are located in the paratracheal or subcarinal location. These foregut duplication cysts close to the airway can cause compression and narrowing of the lumen, thereby causing hyperinflation of the lung[3]. On CT, these cysts appear as thin walled unilocular fluid attenuation masses situated close to the airway (Figure 2).
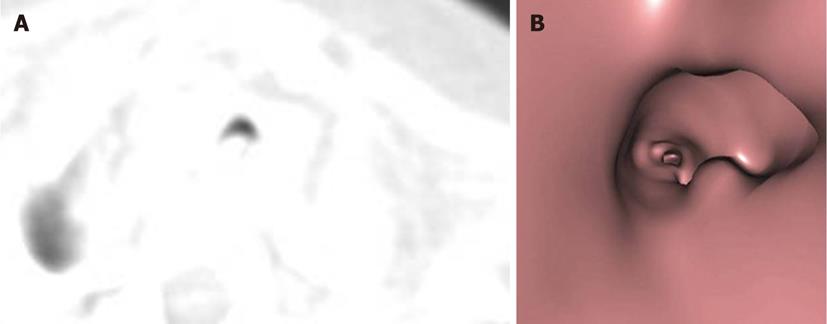
A tracheal web consists of a thin layer of membrane encircling the tracheal lumen. The narrowing of the lumen caused by the membrane is variable. Webs and stenosis result from a failure of complete resorption of the epithelium during the seventh and eighth weeks of intrauterine development. Congenital webs occur in the larynx, usually at the glottis level, and affect the vocal cords[4] (Figure 3).
This condition accounts for 15% of all laryngeal anomalies and is the most common laryngeal anomaly requiring tracheotomy in infants. Incomplete recanalization of the laryngotracheal tube during the third month of gestation leads to different degrees of congenital subglottic or tracheal stenosis. Congenital subglottic stenosis can be membranous or rarely cartilaginous, and results from an abnormal shape of the cricoid cartilage (Figure 4). Congenital tracheal stenosis can be generalized as follows: hypoplasia, funnel-shaped stenosis or segmental stenosis[5].
Tracheomalacia is a common cause of stridor and respiratory distress in neonates and infants, second only to laryngomalacia. Tracheomalacia is caused by abnormal collapsibility of the C-cartilages of the trachea. CT imaging features include opposition of the tracheal wall and widening of the C-cartilage with buckling of the posterior wall during the expiratory scan, i.e. the “expiratory frown sign”. Often, imaging may not reveal the narrowing due to the dynamic nature of the narrowing[6] (Figure 5).
Tracheoesophageal fistula (TEF) is due to an incomplete separation of pulmonary and esophageal anlage during early embryogenesis. There are five types of esophageal atresia (EA) and TEF, the most common abnormality being EA with a distal TEF (84%). Isolated atresia without a fistula is the next most common finding (8%), followed by H-type TEF without atresia (4%). EA with proximal and distal fistulas (3%) and EA with a proximal fistula (1%) are less common[7] (Figure 6).
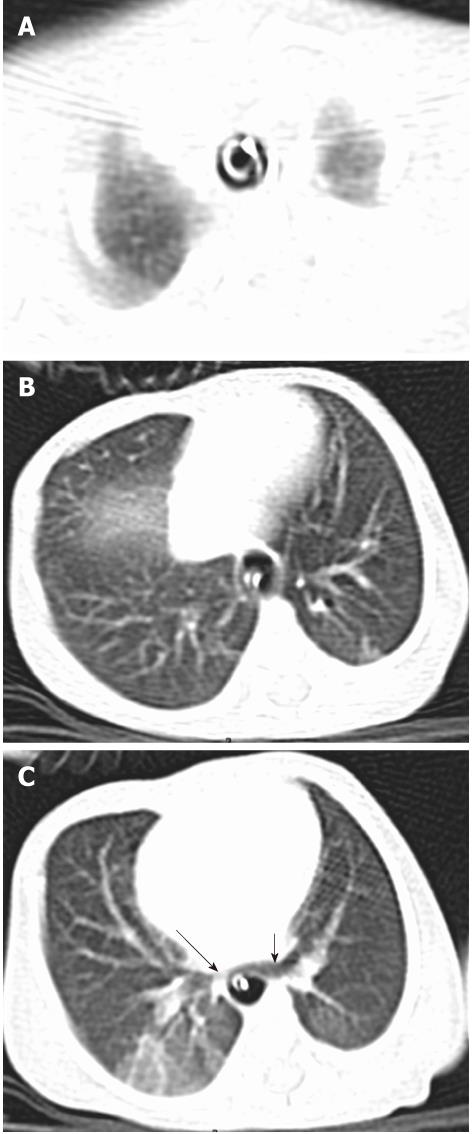
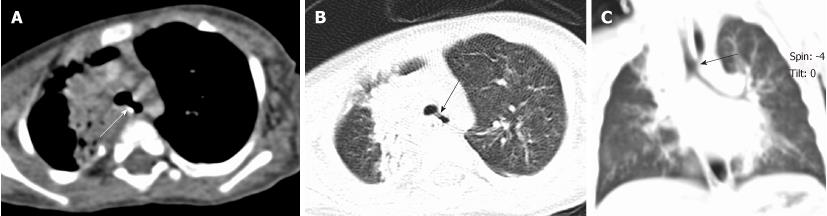
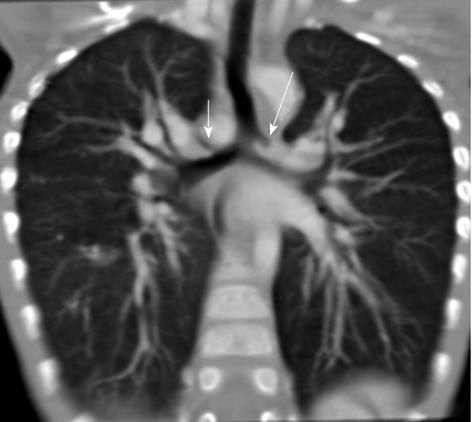
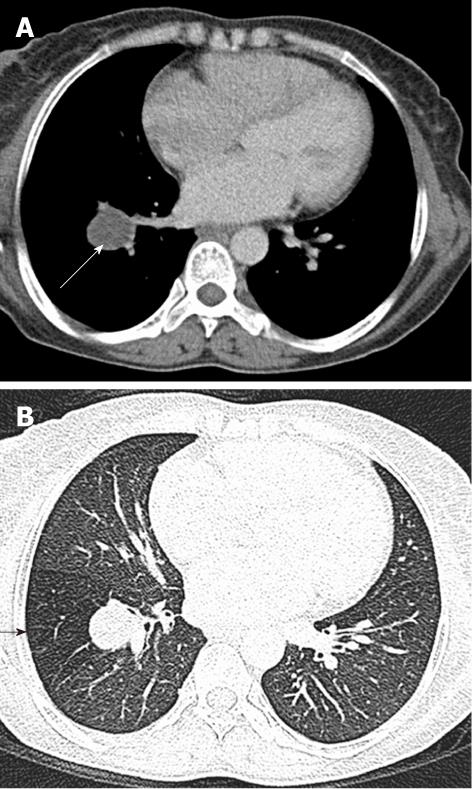
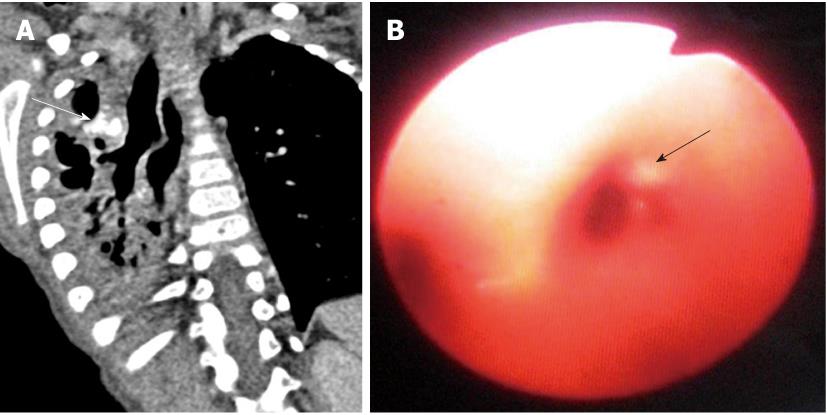
Tracheal bronchus refers to an aberrant bronchus arising from the tracheal wall above the carina, usually on the right side, caused by abnormal additional branching in early embryonic life. The incidence of tracheal bronchus is reported to be between 0.1% and 5%. Rarely, it might cause recurrent infection of the involved upper lobe[8] (Figure 7).
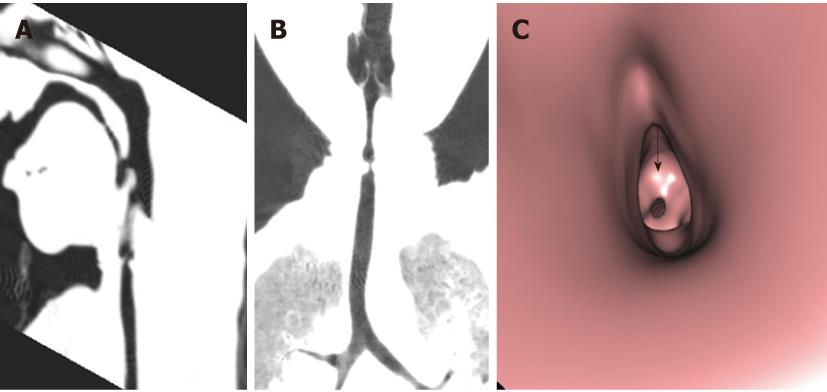
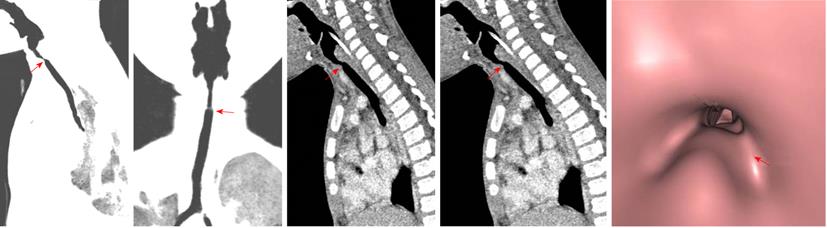
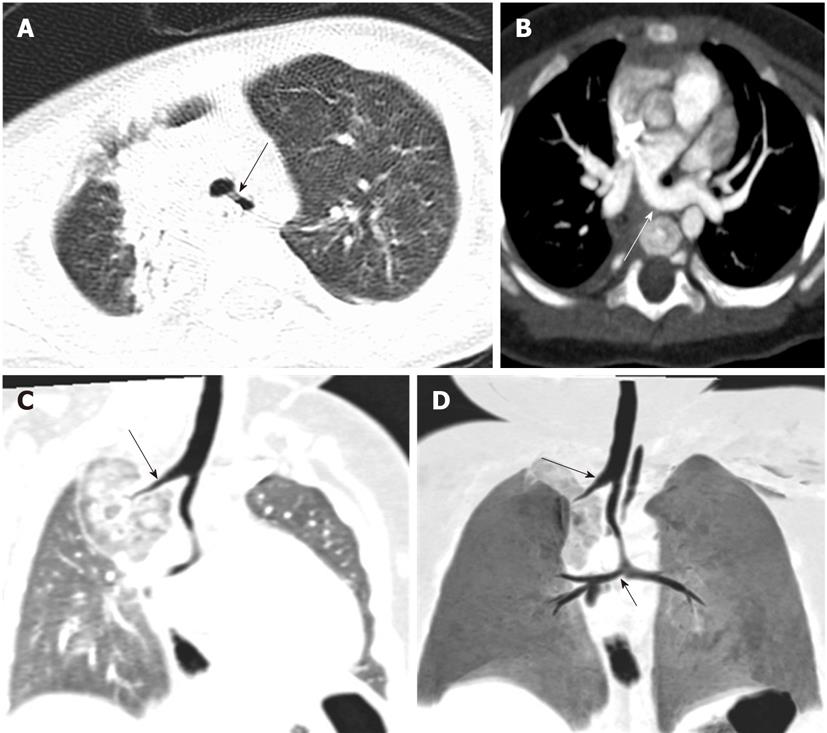
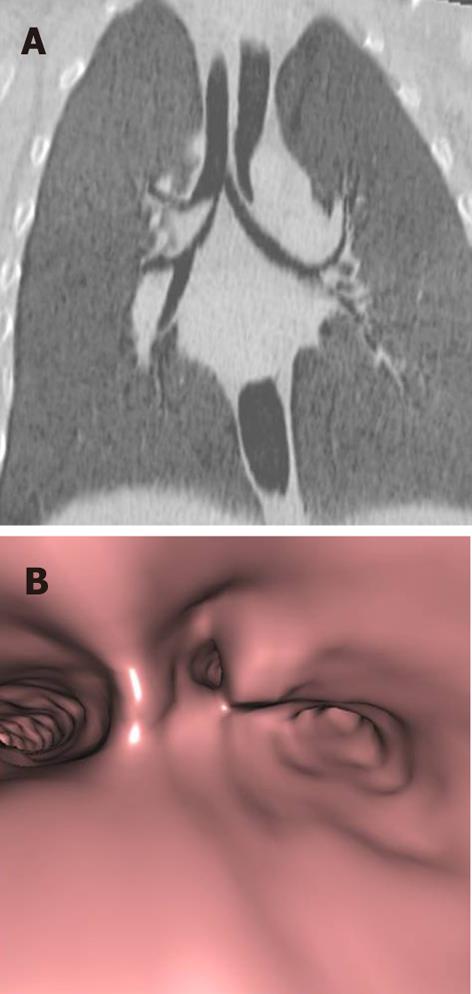
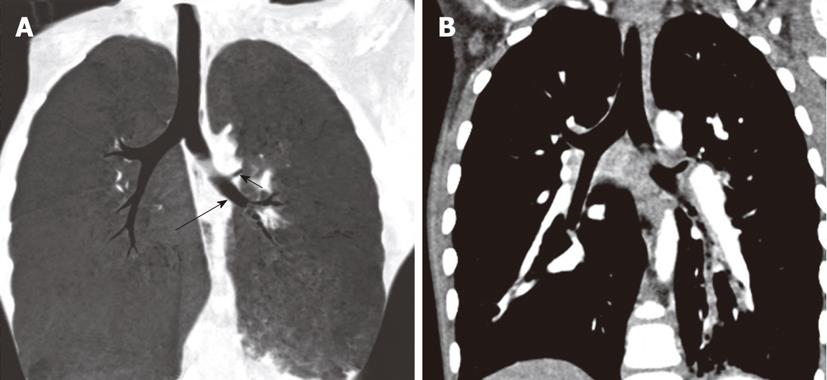
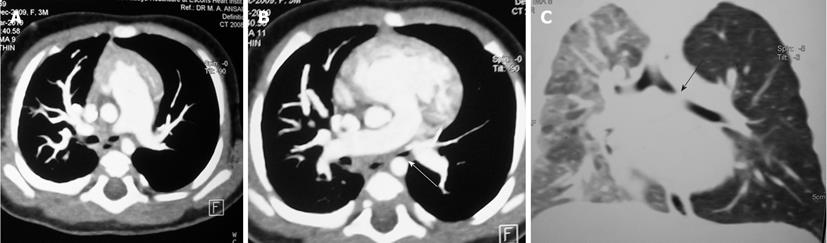
Tracheal trifurcation develops when there is an abnormal division of tracheal segments into three segments instead of the normal two divisions[9] (Figure 8).
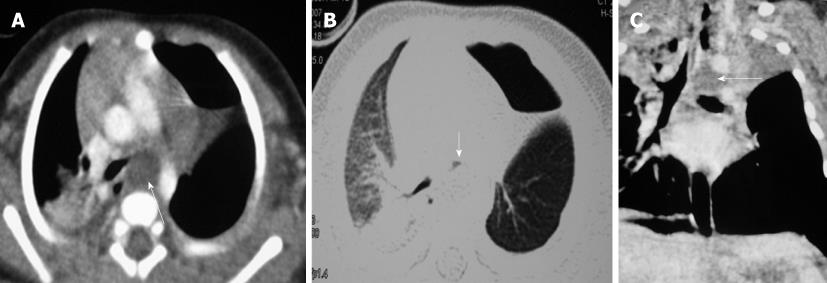
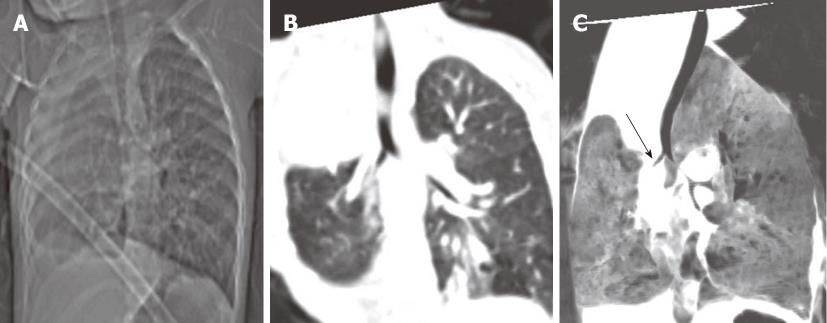
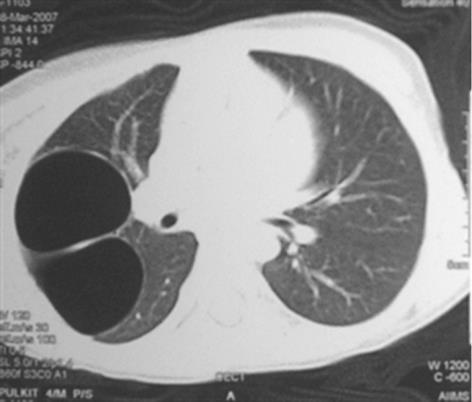
Congenital tracheal diverticula are rare developmental lesions which are due to abnormal supernumerary branches arising from the trachea during development. The diverticulum is lined by respiratory mucosa and usually communicates with the tracheal lumen. The most common location of the lesion is the right postero-lateral wall of the trachea at the cervicodorsal junction[10] (Figure 9).
Pulmonary isomerism is an anomaly of the number of lung lobes. In this anomaly, the right lung has 2 lobes, whereas the left has three. This condition may be associated with situs inversus, asplenia, polysplenia, and/or anomalous pulmonary venous drainage (Figure 10).
The absence of development of bronchial buds leads to pulmonary agenesis. The presence of a blind-ending bronchial bud with absence of lung parenchyma is called aplasia. Pulmonary arteries may be absent in both of the above-mentioned conditions. In very rare instances, there may be absence of the right upper lobe bronchus, as well as the upper branch of the pulmonary artery and vein[11] (Figure 11).
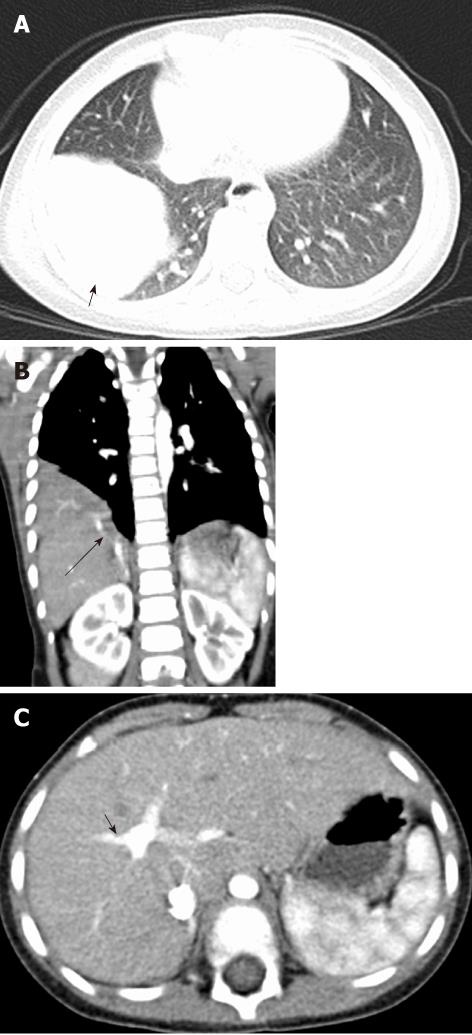
There are four types of congenital broncho-esophageal communication. Type I is a large congenital diverticulum with an inflammatory fistula with the bronchus. Type II is a fistula between the esophagus and the lobar or segmental bronchus. Type III is an esophageal communication with a pulmonary cyst which then communicates with the bronchus. In type IV, the fistula enters a sequestered segment and divides into smaller tracks[12] (Figure 12).
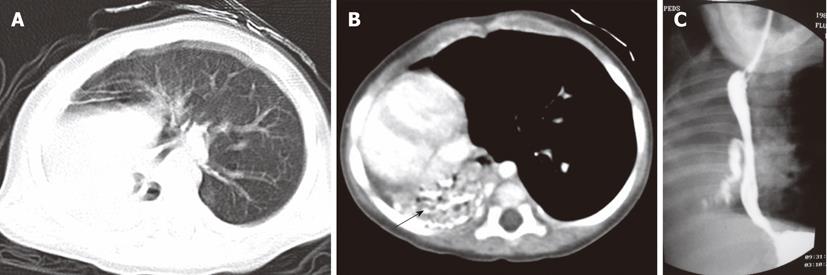
Bronchial atresia is caused by the obliteration of a segment of bronchus and mucus impaction of the proximal lumen of the bronchus with preservation of distal branches[13]. It is usually an asymptomatic lesion, detected incidentally during imaging for other purposes. The distal lung is aerated from collateral bronchial airways and appears hyperlucent on CT[14]. CT shows a tubular mass-like fluid-filled dilated bronchus, usually an apico-posterior segment of the left upper lobe with peripheral segmental hyperinflation. A dilated tubular-shaped opacity associated with segmental hypoattenuation and decreased vascularity is the characteristic CT finding of bronchial atresia (Figure 13).
CCAM is a developmental malformation caused by adenomatous proliferation of the bronchiole-like epithelium which is usually not sequestered by the airways. In type 1 CCAM, there are multiple cysts with mass effect on the adjacent lung. Type II and III have increasingly solid components in addition to the cystic component[15] (Figure 14).
A pulmonary sequestration is a bronchopulmonary segment without a normal bronchial communication and with normal or anomalous vascular supply. The sequestered segment may be intralobar if it is not surrounded by pleura, or extralobar if it is covered by a layer of pleura. Intralobar sequestration usually presents late in childhood due to repeated infections and is generally situated in the lower lobes. Extralobar sequestration usually presents in infancy, is less common than the intralobar variety, and has an upper lobe predilection (Figure 15).
These conditions are not part of the congenital airway lesions and are beyond the scope of this article. However, these conditions may give rise to airway symptoms due to extrinsic compression.
The left main bronchus can be compressed by an anteriorly placed descending aorta or enlarged pulmonary artery[16]. Tracheal compression can be due to a pulmonary arterial sling (Figure 7) or aortic ring[17] (Figure 16).
Pulmonary hamartomas are generally seen sub-pleurally in the peripheral lung parenchyma. Occasionally, they may arise from the mesenchyme of the bronchial wall causing bronchial narrowing or intraluminal growth and can lead to hyperinflation, collapse, pneumonia and hemoptysis[18] ( Figure 17).
Congenital major airway anomalies differ in their stage of development in the embryological sequence, severity of symptoms, time of presentation, and prognosis. MDCT is a valuable adjunct to bronchoscopy, especially in patients with suboptimal bronchoscopy examination. In this review, the utility of MDCT in the diagnosis of congenital airway anomalies is highlighted.
Peer reviewer: Patrick K Ha, MD, Assistant Professor, Johns Hopkins Department of Otolaryngology, Johns Hopkins Head and Neck Surgery at GBMC, 1550 Orleans Street, David H Koch Cancer Research Building, Room 5M06, Baltimore, MD 21231, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Berrocal T, Madrid C, Novo S, Gutiérrez J, Arjonilla A, Gómez-León N. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics. 2004;24:e17. [PubMed] |
| 2. | Effmann EL, Spackman TJ, Berdon WE, Kuhn JP, Leonidas JC. Tracheal agenesis. Am J Roentgenol Radium Ther Nucl Med. 1975;125:767-781. [PubMed] |
| 3. | Madhusudhan KS, Seith A, Srinivas M, Gupta AK. Esophageal duplication cyst causing unilateral hyperinflation of the lung in a neonate. Acta Radiol. 2007;48:588-590. [PubMed] |
| 4. | Cohen SR. Congenital glottic webs in children. A retrospective review of 51 patients. Ann Otol Rhinol Laryngol Suppl. 1985;121:2-16. [PubMed] |
| 6. | Boiselle PM, Ernst A. Tracheal morphology in patients with tracheomalacia: prevalence of inspiratory lunate and expiratory "frown" shapes. J Thorac Imaging. 2006;21:190-196. [PubMed] |
| 7. | Holder TM, Ashcraft KW, Sharp RJ, Amoury RA. Care of infants with esophageal atresia, tracheoesophageal fistula, and associated anomalies. J Thorac Cardiovasc Surg. 1987;94:828-835. [PubMed] |
| 8. | Barat M, Konrad HR. Tracheal bronchus. Am J Otolaryngol. 1987;8:118-122. [PubMed] |
| 9. | Beigelman C, Howarth NR, Chartrand-Lefebvre C, Grenier P. Congenital anomalies of tracheobronchial branching patterns: spiral CT aspects in adults. Eur Radiol. 1998;8:79-85. [PubMed] |
| 10. | Goo JM, Im JG, Ahn JM, Moon WK, Chung JW, Park JH, Seo JB, Han MC. Right paratracheal air cysts in the thoracic inlet: clinical and radiologic significance. AJR Am J Roentgenol. 1999;173:65-70. [PubMed] |
| 11. | Tsunezuka Y, Oda M, Ohta Y, Watanabe G. Congenital absence of the right upper lobe of the lung. Ann Thorac Surg. 2002;74:571-573. [PubMed] |
| 12. | BRAIMBRIDGE MV, KEITH HI. OESOPHAGO-BRONCHIAL FISTULA IN THE ADULT. Thorax. 1965;20:226-233. [PubMed] |
| 13. | Lucaya J, Strife J. Pediatric chest imaging: chest imaging in infants and children. Berlin: Springer-Verlag 2002; 93-112. |
| 14. | Zylak CJ, Eyler WR, Spizarny DL, Stone CH. Developmental lung anomalies in the adult: radiologic-pathologic correlation. Radiographics. 2002;22 Spec No:S25-S43. [PubMed] |
| 15. | CH'IN KY, TANG MY. Congenital adenomatoid malformation of one lobe of a lung with general anasarca. Arch Pathol (Chic). 1949;48:221-229. [PubMed] |
| 16. | Hungate RG, Newman B, Meza MP. Left mainstem bronchial narrowing: a vascular compression syndrome? Evaluation by magnetic resonance imaging. Pediatr Radiol. 1998;28:527-532. [PubMed] |
| 17. | Park CD, Waldhausen JA, Friedman S, Aberdeen E, Johnson J. Tracheal compression by the great arteries in the mediastinum. Report of 39 cases. Arch Surg. 1971;103:626-632. [PubMed] |
| 18. | Jain V, Goel P, Kumar D, Seith A, Sarkar C, Kabra S, Agarwala S. Endobronchial chondroid hamartoma in an infant. J Pediatr Surg. 2009;44:e21-23. |