Published online Dec 26, 2016. doi: 10.4330/wjc.v8.i12.735
Peer-review started: July 26, 2016
First decision: September 6, 2016
Revised: September 26, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 26, 2016
To investigate the clinical outcomes of transcatheter aortic valve implantation (TAVI) with the SAPIEN 3 transcatheter heart valve (S3-THV) vs the SAPIEN XT valve (XT-THV).
We retrospectively analyzed 507 patients that underwent TAVI with the XT-THV and 283 patients that received the S3-THV at our institution between March 2010 and December 2015.
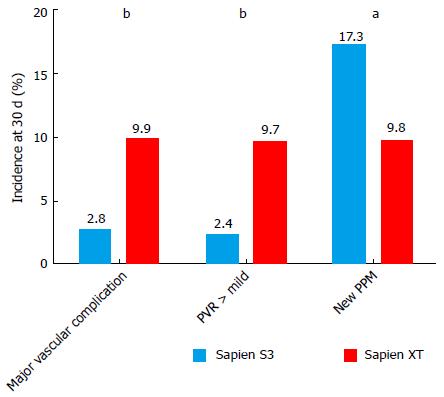
Thirty-day mortality (3.5% vs 8.7%; OR = 0.44, P = 0.21) and 1-year mortality (25.7% vs 20.1%, P = 0.55) were similar in the S3-THV and the XT-THV groups. The rates of both major vascular complication and paravalvular regurgitation (PVR) > 1 were almost 4 times lower in the S3-THV group than the XT-THV group (major vascular complication: 2.8% vs 9.9%, P < 0.0001; PVR > 1: 2.4% vs 9.7%, P < 0.0001). However, the rate of new pacemaker implantation was almost twice as high in the S3-THV group (17.3% vs 9.8%, P = 0.03). In the S3 group, independent predictors of new permanent pacemaker were pre-procedural RBBB (OR = 4.9; P = 0.001), pre-procedural PR duration (OR = 1.14, P = 0.05) and device lack of coaxiality (OR = 1.13; P = 0.05) during deployment.
The S3-THV is associated to lower rates of major vascular complications and PVR but higher rates of new pacemaker compared to the XT-THV. Sub-optimal visualization of the S3-THV in relation to the aortic valvular complex during deployment is a predictor of new permanent pacemaker.
Core tip: The SAPIEN 3 transcatheter heart valve (S3-THV) is associated to lower rates of major vascular complications and PVR but higher rates of new pacemaker compared to the SAPIEN XT valve (XT-THV). Sub-optimal visualization of the S3-THV in relation to the aortic valvular complex during deployment is a predictor of new permanent pacemaker (PPM). Our findings highlight the increased importance to adequately visualize the S3-THV in relation to the aortic valvular complex during deployment, in order to improve device positioning and potentially mitigate new PPM requirements.
- Citation: Sawaya FJ, Spaziano M, Lefèvre T, Roy A, Garot P, Hovasse T, Neylon A, Benamer H, Romano M, Unterseeh T, Morice MC, Chevalier B. Comparison between the SAPIEN S3 and the SAPIEN XT transcatheter heart valves: A single-center experience. World J Cardiol 2016; 8(12): 735-745
- URL: https://www.wjgnet.com/1949-8462/full/v8/i12/735.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i12.735
Transcatheter aortic valve implantation (TAVI) has gained rapid acceptance for patients with severe aortic stenosis[1-4] and has recently been associated with excellent short-, mid- and long-term outcomes in patients at intermediate risk[5-7]. However, TAVI is still associated with a higher incidence of paravalvular regurgitation (PVR), permanent pacemaker implantation (PPM) and vascular complications[8-12] when compared to surgical aortic valve replacement. In order to justify the extension of the procedure to lower risk patients, these adverse outcomes have to be mitigated. The development of novel transcatheter heart valves (THVs) and further iterations of delivery systems and prostheses have contributed to the decrease in complications rates in TAVI[13]. One of the recent developments is the balloon-expandable Sapien 3 transcatheter heart valve (S3-THV; Edwards Lifesciences, Irvine, CA). It has been designed with a lower profile to be delivered in a 14 French sheath (for sizes 23 and 26 mm), and with an external sealing cuff. The lower profile should diminish vascular complications while the sealing cuff should diminish PVL[14,15].
Despite positive procedural and short-term outcomes in small single center series and registries, large reports comparing the S3-THV to its predecessor, the Sapien XT (XT-THV), are lacking[16,17]. Recent reports suggest an increased rate of new PPM implantation following TAVI with the S3-THV, compared to the XT-THV[16,17]. Whether procedural characteristics such as depth of implant are related to PPM implantation with this new device remains unclear[18].
The objective of this analysis was to retrospectively compares the procedural outcomes, 30-d clinical outcomes and one-year mortality of TAVI with the S3-THV vs the XT-THV in patients with symptomatic severe aortic stenosis in a single high-volume center. We also explored clinical and procedural predictors of new PPM in the S3-THV group.
To compare clinical outcomes of patients undergoing TAVI with the S3-THV to those undergoing TAVI with the XT-THV, we retrospectively identified all patients treated with TAVI at our institution with either device. Patients underwent TAVI by the transfemoral, transaortic or transapical approach according to previously described techniques[17].
A multidisciplinary heart team involving at least one interventional cardiologist and one cardiac surgeon discussed all cases and consensus was achieved regarding therapeutic strategy. All patients provided informed written consent for the procedure and data collection, and the local ethics committee approved the study.
All patients underwent TTE examination and native valve function was assessed according to the recommended guidelines[19]. In addition, pre-procedural MSCT evaluation including measurements of the aortic annulus and aortic root was systematically performed. Aortic annulus dimensions were measured according to standard procedures using dedicated software (Philips Brilliance 64-slice multidetector computed tomography scanner, Philips Healthcare, Best, the Netherlands). Valve prosthesis size was selected in accordance with the manufacturer’s recommendations after taking into account other anatomic features such as the presence and location of calcification, eccentricity of the aortic annulus and dimensions of the sinuses of Valsalva and sino-tubular junction in case of borderline sizing ranges. In addition to dimensions, annulus orientation was assessed with MSCT. Implantation projection was selected so that the aortic valve would be seen coaxially, with the three cusps aligned. Cardiac catheterization and femoral angiography were performed prior to the procedure to assess for concomitant coronary artery disease and vessel narrowing or tortuosity.
The SXT-THV and the S3-THV designs have been described in detail previously[15,20]. Both consist of bovine pericardium sewn to a balloon-expandable cobalt-chromium tubular frame. The XT-THV was available in the 23, 26, and 29 mm sizes and was implanted with the use of the NovaFlex catheter, which employed an 18- or 19-F introducer sheaths. The S3-THV is available in the 23, 26, and 29 mm sizes. The device’s height is about 15% greater than that of the XT-THV. It was implanted with the use of the lower-profile Commander delivery catheter, which employed 14- (sizes 23 and 26 mm) or 16-F (size 29 mm) expandable sheaths (eSheath, Edwards Lifesciences, Inc.). The S3-THV stent was designed with a frame geometry that provides greater radial force. The difference in cell geometry between the inflow and the outflow causes the valve frame to foreshorten more from the ventricular side. The device also includes an outer polyethylene terephthalate fabric seal designed to minimize PVR.
The techniques of SAPIEN XT and SAPIEN S3 valve implantation have been described in detail elsewhere[15,20]. In our center, all trans-femoral cases were performed under local anesthesia and conscious sedation in the catheterization laboratory. The selected femoral artery was “pre-closed” with two 6-Fr suture-mediated closure devices Perclose ProGlide(Abbott Laboratories, Abbot Park, Illinois). With a pigtail in the right coronary cusp, aortography was performed to correct, if necessary, the implantation projection provided by MSCT. Pre-dilatation was performed routinely in the XT-THV group, but only in cases of severe calcification in the S3-THV group. Device positioning was based on fluoroscopy using annular calcification as a landmark along with serial 12 to 15 mL supra-annular aortography to validate its position. The XT-THV was implanted by means of a 2-step inflation technique[21]. The S3-THV was deployed during one-slow inflation (5-10 s). Prosthesis position and function, and patency of the coronary ostia were evaluated by angiography and transthoracic echocardiography. Significant aortic regurgitation was treated by post-dilatation adding 1 to 3 cc of contrast in the balloon delivery system or second valve implantation if the valve was positioned too high or too low. Removal of the sheath was cautiously achieved with serial contralateral angiograms to detect ilio-femoral complications. In the absence of any conduction abnormality, the pacing lead was removed at the end of the procedure. Patients were monitored in the intensive care unit for at least 24 h after valve implantation. For the transapical and transaortic cases, the SXT-THV and S3-THV were deployed with the Ascendra and Certitude delivery systems, respectively. These cases were performed in a hybrid room.
Clinical and echocardiographic data at baseline and follow-up were collected by dedicated personnel and entered in a local database and a national registry (FRANCE-TAVI)[22]. Data from the ECG and MSCT prior to the intervention were retrospectively collected by the co-authors and entered into the local database. The co-authors also retrospectively collected implant depth and device coaxiality from procedure fluoroscopy.
The primary endpoint was 30-d mortality. Secondary endpoints consisted of 1-year mortality, stroke, myocardial infarction, annulus rupture, new PPM implantation, major vascular complication, PVR greater than mild, annulus rupture, acute kidney injury and post-procedural mean gradient. Endpoints were defined according to the VARC-2 criteria[23].
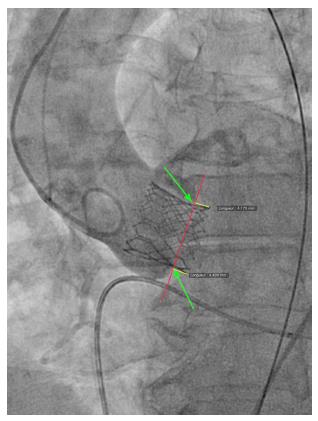
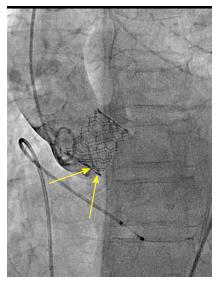
We reviewed procedural fluoroscopy of all patients in the S3-THV group to measure valve implant depth. A post-implant aortic angiogram with the device coaxial was required for implant depth measurement. First, on a single still frame, the hinge points between the device and the sinus of Valsalva on the septal and non-septal side were identified (Figure 1). Next, a line was drawn between both hinge points. The distances between this line and the bottom of the valve frame on both the septal and non-septal sides were then recorded as implant depth. Measurements were performed using the OsiriX sorftware, version 5.9.
In addition to depth, we also measured device lack of coaxiality during deployment. This was done on a single still frame at the end of valve deployment, while still under rapid pacing. The maximal perpendicular distance between the “front” and the “back” struts of the device was measured and recorded as device lack of coaxiality during deployment (Figure 2).
Continuous data are reported as mean ± SD, and categorical variables are reported as number of patients and percentages. Categorical data were compared using Fisher’s exact test, and continuous data using Student’s t test or Mann-Whitney’s U test, as appropriate. Events are reported as counts of first occurrence per type of event. Event probabilities at 30 d were compared for patients treated with the XT-THV vs the S3-THV using logistic regression. Crude and adjusted odds ratios (with 95%CI) are reported. Odds ratios are adjusted for procedure date (to account for a potential learning effect of time) and for baseline characteristics with a univariate P value < 0.10 for each individual outcome. One-year survival data was fitted in a Cox proportional hazards model and the XT-THV and S3-THV groups were compared using an adjusted hazard ratio. No adjusted analyses were performed for outcomes with less than 15 events overall. Patients with previous pacemaker implantation were excluded from analyses pertaining to the outcome of new pacemaker requirement. A P value < 0.05 was considered significant for adjusted models. Statistical analyses were performed with SPSS version 23 (IBM Corp, Armonk, NY).
Between March 2010 and December 2015, 790 patients underwent TAVI with the XT-THV (n = 507) or the S3-THV (n = 283) in our center. The XT-THV was used from March 2010 to September 2014, after which the S3-THV was used routinely. Patients in the S3-THV group had lower STS scores than those in the XT-THV group (STS score: 5.3% ± 3.5% vs 6.4% ± 4.0% respectively, P < 0.0001) (Table 1). Patients in the S3-THV group were also less likely to be in NYHA functional class 3 or 4 (59.1% vs 75.8%, P < 0.0001), and less likely to have peripheral vascular disease (19.8% vs 28.4%, P = 0.01) or chronic obstructive pulmonary disease (11.7% vs 21.9%, P < 0.0001). Baseline echocardiographic characteristics were similar between groups.
| Variable | S3-THV (n = 283) | XT-THV (n = 507) | P value |
| Age | 82.8 ± 7.1 | 83.5 ± 7.0 | 0.14 |
| Female sex | 137 (48.4) | 275 (54.3) | 0.12 |
| STS-PROM, % | 5.3 ± 3.5 | 6.4 ± 4.0 | < 0.0001 |
| Logistic EuroSCORE, % | 15.7 ± 10.8 | 18.8 ± 11.5 | < 0.0001 |
| NYHA class 3 or 4 | 162 (59.1) | 383 (75.8) | < 0.0001 |
| History of syncope | 1 (0.5) | 10 (2.1) | 0.19 |
| Atrial arrhythmia (flutter or fibrillation) | 80 (29.5) | 135 (27.8) | 0.67 |
| Diabetes | 71 (25.1) | 124 (24.5) | 0.86 |
| Hypertension | 161 (71.6) | 344 (68.8) | 0.49 |
| Dyslipidemia | 99 (44.0) | 263 (52.6) | 0.04 |
| Active smoker | 4 (1.4) | 18 (3.6) | 0.11 |
| Previous PPM | 35 (12.4) | 60 (11.8) | 0.91 |
| Previous PCI | 81 (29.3) | 114 (22.9) | 0.06 |
| Previous CABG | 25 (9.0) | 51 (10.3) | 0.62 |
| Previous SAVR | 2 (0.7) | 7 (1.4) | 0.5 |
| Previous stroke | 25 (8.8) | 39 (7.7) | 0.59 |
| Peripheral vascular disease | 56 (19.8) | 143 (28.4) | 0.01 |
| eGFR, mL/min per 1.73 m2 | 62.8 ± 24.6 | 61.4 ± 22.6 | 0.42 |
| eGFR < 40 mL/min per 1.73 m2 | 82 (16.2) | 41 (14.5) | 0.61 |
| Dialysis | 4 (1.5) | 13 (2.6) | 0.44 |
| Chronic obstructive pulmonary disease | 33 (11.7) | 110 (21.9) | < 0.0001 |
| Body mass index, kg/m2 | 26.5 ± 5.1 | 26.3 ± 4.9 | 0.61 |
| LVEF, % | 54.9 ± 14.8 | 53.6 ± 14.2 | 0.24 |
| LVEF < 30% | 55 (11.1) | 31 (11.4) | 0.91 |
| Mean aortic gradient, mmHg | 46.7 ± 15.3 | 46.9 ± 15.3 | 0.92 |
| AVA, cm2 | 0.67 ± 0.17 | 0.65 ± 0.14 | 0.31 |
| Pulmonary artery systolic pressure, mmHg | 44.5 ± 13.0 | 46.5 ± 12.9 | 0.06 |
| Pulmonary artery systolic pressure > 50 mmHg | 64 (28.3) | 123 (28.5) | 1 |
The use of the transfemoral approach increased from 54% in XT-THV group to more than 80% in the S3-THV group (P < 0.0001) (Table 2).
| Procedural characteristic | S3-THV (n = 283) | XT-THV (n = 507) | P value |
| Transfemoral approach | 232 (82.6) | 273 (53.8) | < 0.0001 |
| Local anesthesia | 232 (82.6) | 271 (54.2) | < 0.0001 |
| Predilatation | 50 (17.7) | 440 (86.8) | < 0.0001 |
| Postdilatation | 45 (15.9) | 61 (12.0) | 0.13 |
| Implanted device size | < 0.0001 | ||
| 23 mm | 111 (39.8) | 127 (25.1) | |
| 26 mm | 101 (36.2) | 270 (53.4) | |
| 29 mm | 67 (24.0) | 109 (21.5) | |
| Valve area oversizing, % | 11.5 ± 9.8 | 22.9 ± 11.2 | < 0.0001 |
| Device diameter/annulus diameter (area-derived) | 1.05 ± 0.05 | 1.11 ± 0.05 | < 0.0001 |
| Need for seconde valve implantation | 7 (2.5) | 8 (1.6) | 0.42 |
| Annulus rupture | 0 (0) | 13 (2.6) | 0.01 |
| Conversion to SAVR | 2 (0.7) | 14 (2.8) | 0.06 |
| Contrast use (mL) | 108.2 ± 42.7 | 131.6 ± 60.9 | < 0.0001 |
| Fluoroscopy time (min) | 17.4 ± 9.9 | 16.5 ± 9.8 | 0.28 |
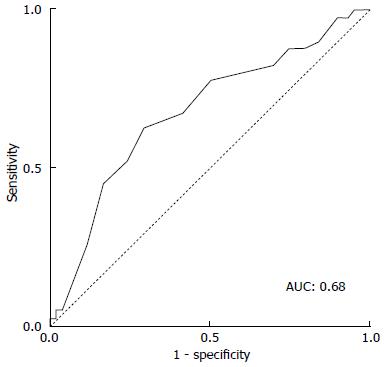
Predilatation was performed routinely in the XT-THV group (86.8%), which was not the case in the S3-THV group (17.7%, P < 0001) (Table 2). In the S3-THV group, predilatation was reserved for patients with an extensively calcified aortic valve. The lower use of predilatation in the S3-THV group did not translate into significantly more post-dilatation (S3-THV: 15.9% vs XT-THV: 12.0%; P = 0.13). As per manufacturer recommendations, device diameter to annulus diameter (area-derived) ratio was reduced from 1.11 ± 0.05 (XT-THV) to 1.05 ± 0.05 (S3-THV; P < 0.0001). As a result of this reduced oversizing, smaller device sizes were used in the S3-THV group (P < 0.0001). However, according to ROC curve analysis, a device diameter to annulus diameter ratio below the threshold of 1.03 increased the risk of post-dilatation or PVR > mild (area under the curve: 0.68; Figure 3).
While fluoroscopy time was similar between groups, contrast use decreased by more than 15% in the S3-THV group compared to the XT-THV group (131.6 ± 60.9 mL vs 108.2 ± 42.7 mL; P < 0.0001).
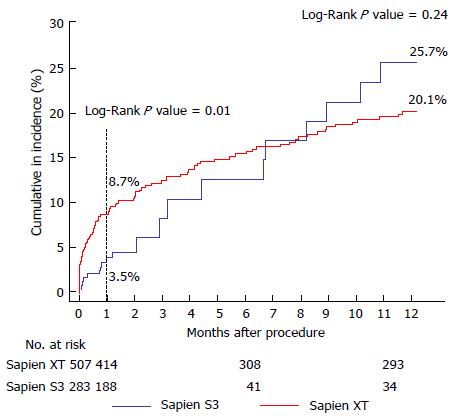
Thirty-day mortality was lower in the S3-THV group than the XT-THV group (3.5% vs 8.7%; univariate OR = 0.36; P = 0.01) (Figure 4 and Table 3). After adjustment for baseline characteristics, this difference was no longer statistically significant (adjusted OR = 0.44, P = 0.21). One-year mortality was also similar between groups (25.7% vs 20.1%, adjusted P =0.55) (Figure 4). In total, 20 deaths had occurred at 1 year in the S3-THV group. These are listed in Table 4 along with cause of death.
| 30-d outcomes | S3-THV (n =283) | XT-THV (n = 507) | Odds ratio (95%CI) | P value | Adjusted odds ratio (95%CI) | Adjusted P value |
| Death | 8 (3.5) | 42 (8.7) | 0.36 (0.16-0.81) | 0.01 | 0.44 (0.12-1.56) | 0.21 |
| Stroke | 4 (1.4) | 13 (2.8) | 0.51 (0.16-1.58) | 0.24 | 0.59 (0.08-4.33) | 0.6 |
| Myocardial infarction | 0 (0) | 2 (0.4) | 0 (0-∞) | 1 | ||
| New pacemaker implantation1 | 43 (17.3) | 44 (9.8) | 1.88 (1.19-2.97) | 0.007 | 1.68 (1.05-2.69) | 0.03 |
| Major vascular complication | 8 (2.8) | 50 (9.9) | 0.27 (0.13-0.57) | 0.001 | 0.20 (0.09-0.44) | < 0.0001 |
| Paravalvular regurgitation > mild | 6 (2.4) | 47 (9.7) | 0.23 (0.10-0.55) | 0.001 | 0.20 (0.08-0.47) | < 0.0001 |
| Acute kidney injury | 3 (1.1) | 69 (13.6) | 0.07 (0.02-0.22) | < 0.0001 | 0.12 (0.04-0.39) | < 0.0001 |
| Mean gradient > 20 mmHg | 7 (2.8) | 6 (1.3) | 2.48 (0.78-7.89) | 0.13 | ||
| Mean gradient, mmHg | 11.8 ± 5.8 | 10.0 ± 5.0 | < 0.0001 | |||
| Total hospital length of stay, d [median (IQR)] | 8 [5-13] | 9 [7-14] | < 0.0001 | |||
| 1-yr outcomes | P value | Adjusted hazard ratio (95%CI) | Adjusted P value | |||
| Death | 20 (25.7) | 87 (20.1) | 0.24 | 0.86 (0.52-1.42) | 0.55 |
| Patient | Days to death | Cause of death |
| 1 | 0 | Dissection of ascending aorta |
| 2 | 2 | Left main compression/cardiogenic shock |
| 3 | 3 | Iliac rupture |
| 4 | 5 | Sudden cardiac death |
| 5 | 10 | Cardiogenic shock |
| 6 | 22 | Heart failure |
| 7 | 24 | Subdural hematoma |
| 8 | 25 | Unknown |
| 9 | 31 | Stroke |
| 10 | 36 | Acute renal failure |
| 11 | 62 | Unknown |
| 12 | 87 | Heart failure |
| 13 | 96 | Heart failure |
| 14 | 133 | Unknown |
| 15 | 200 | Sudden cardiac death |
| 16 | 202 | Cancer |
| 17 | 247 | Myocardial infarction |
| 18 | 268 | Septic shock |
| 19 | 305 | Chronic obstructive pulmonary disease acute exacerbation |
| 20 | 326 | Major stroke |
The rates of major vascular complication and PVR > 1 were both almost 4 times lower in the S3-THV group than the XT-THV group (major vascular complication: 2.8% vs 9.9%, adjusted P < 0.0001; PVL > 1: 2.4% vs 9.7%, adjusted P < 0.0001) (Figure 5). However, the rate of new pacemaker implantation was almost twice as high in the S3-THV group (17.3% vs 9.8%, adjusted P = 0.03) (Figure 5).
Acute kidney injury was 10 times lower in the S3-THV group than the XT-THV group (1.1% vs 13.6%, P < 0.0001). There were no statistically significant differences between groups with respect to stroke, myocardial infarction and post-procedural mean gradient > 20 mmHg.
Electrocardiographic and angiographic characteristics of patients in the S3-THV group that required a new PPM are displayed in Tables 5 and 6. Implantation depth in the S3-THV group was 5.1 ± 2.5 mm on the septal side (non-coronary cusp) and 5.2 ± 2.0 mm on the non-septal side (left coronary cusp). According to multivariate analysis, independent predictors of new permanent pacemaker implantation were pre-procedural complete right bundle branch block (RBBB) (OR = 4.9; 95%CI: 1.88-12.95; P = 0.001), PR duration (OR = 1.14 per 10 ms increment; 95%CI: 1.00-1.29; P = 0.05) and device lack of coaxiality during deployment (OR = 1.13 per 1 mm increment; 95%CI: 1.00-1.29; P = 0.05). Device implantation depth was not a predictor of new pacemaker implantation in our series.
| Variable | New PPM (n = 43) | No PPM (n = 201) | P value |
| Complete RBBB | 12 (32.4) | 17 (9.5) | 0.001 |
| Complete LBBB | 0 (0) | 14 (7.8) | 0.14 |
| Fascicular block | 12 (32.4) | 33 (18.4) | 0.07 |
| QRS duration, ms | 108 ± 26 | 101 ± 23 | 0.1 |
| PR duration, ms | 196 ± 37 | 183 ± 30 | 0.04 |
| Implant depth (septal), mm | 5.3 ± 2.4 | 5.0 ± 2.6 | 0.67 |
| Implant depth (non-septal), mm | 4.9 ± 2.4 | 5.2 ± 1.9 | 0.64 |
| Device lack of coaxiality during deployment, mm | 4.0 ± 3.6 | 2.9 ± 2.5 | 0.06 |
| Parameter | Univariate analysis | Multivariate analysis | |||
| OR | P value | OR | 95%CI | P value | |
| Complete RBBB | 4.6 | < 0.001 | 4.9 | 1.88-12.95 | 0.001 |
| Complete LBBB | 1 | 1 | - | - | - |
| Fascicular block | 2.12 | 0.06 | 1.88 | 0.71-5.00 | 0.2 |
| QRS duration (per 10 ms increment) | 1.12 | 0.1 | 0.87 | 0.65-2.72 | 0.345 |
| PR duration (per 10 ms increment) | 1.14 | 0.05 | 1.14 | 1.00-1.29 | 0.05 |
| Implant depth (septal, per 1 mm increment) | 1.05 | 0.66 | - | - | - |
| Implant depth (non-septal, per 1 mm increment) | 0.94 | 0.63 | - | - | - |
| Device lack of coaxiality during implant (per 1 mm increment) | 1.13 | 0.07 | 1.13 | 1.00-1.29 | 0.049 |
To our knowledge, this is one of the largest observational studies to date comparing the newer balloon-expandable S3-THV to the XT-THV in an all-comer population. The major findings are as follows: (1) the S3-THV is associated with similar adjusted 30-d and one-year mortality rates compared to the XT-THV; (2) the S3-THV is associated with 4-fold lower rates of both major vascular complications and PVR compared to the XT-THV; (3) the S3-THV is associated with twice the rate of new PPM implantation compared to the XT-THV; and (4) independent predictors of new pacemaker included pre-procedural complete RBBB and PR duration, and lack of device coaxiality during implant.
In a recent study, all-cause 30-d mortality rates were reported between 0% and 17.5%, with a pooled estimate rate of 5.7% for all second-generation THVs[24]. Reported 30-d mortality rates with the S3-THV ranges from 0.5% to 4.5%[16,17,25]. We report also a low 30-d mortality of 3.5% in the S3-THV cohort that was not statistically lower than the 8.7% rate of the XT-THV group after covariates adjustment. The low 30-d mortality speaks to the advancement of TAVI in regard to valve design improvement, increased operator experience, improved patient selection and procedural pre-planning, but also the lower baseline risk profile of TAVI patients.
One of the shortcomings of TAVI is the association of major vascular complications with mortality[10]. Sheath size, severe ilio-femoral artery calcification, sheath external diameter to minimal femoral diameter artery ratio (≥ 1.05), early site experience and early operator experience, have all been previously associated with major vascular complications[13,26,27]. The S3-THV, with the lower profiles of its 14 and 16-F sheaths and the expanding properties of its E sheath, allows TAVI to be performed in patients with smaller arteries and for it to be safer in patients with larger arteries[28]. This is reflected in our series by the significant increase in proportion of transfemoral procedures. Three studies reported rates of major vascular complications of 4.5%, 5.2% and 3.6%, reflecting increased safety compared to the XT-THV[16,17,25]. We observed a similar rate of 2.9% in our S3-THV cohort, despite seeing the number operators performing TAVI increase from 4 to 9 between 2013 and 2015.
Patients with more than mild PVR have lower short- and long-term survival than those with trivial or mild PVR, making this an important echocardiographic outcome[29,30]. In the PARTNER trial, moderate or severe PVR was seen in 11.8% of patients implanted with the Edwards SAPIEN valve[31]. In the France 2 Registry, it was reported in 12.2%[32]. We found similar rates of PVR in the XT-THV group. In contrast, the S3-THV group had four times less PVR. Our 2.4% > mild PVR rate in the S3-THV group is comparable to other reports that showed a PVR range between 0% and 3.8%[25,33]. The reduced rate of PVR can be explained by improved annular sealing by the external cuff. Whether the decreased PVR rate with the S3 device could translate into improved long-term outcomes should be evaluated in long-term registries.
The need for new PPM implantation following TAVI may be correlated to prognosis[34-36]. As the S3-THV valve frame has greater height than the XT-THV, it may extend deeper into the LVOT after deployment[15,16]. Stent frame extension in the LVOT, i.e., depth of implant, has been shown to be a predictor of PPM implantation[37].
Preliminary data on the S3-THV device from the pivotal SAPIEN 3 trial have shown an increased 30-d PPM implantation rate (13.3%), despite excluding patients with LBBB, RBBB and PR > 200 ms[38]. A study by Tarantini et al[16] also showed an increased rate of PPM (20.7%) with the S3-THV. This increased risk for PPM was driven by deep implantation of the S3-THV (valve implantation depth ≥ 8 mm). Similarly, the Swiss registry showed an increased rate of PPM with the S3-THV of 17% compared to 11% with the XT-THV valve[16]. Our study showed similar results with a rate of 17.3% in S3-THV vs 9.8% in XT-THV (Table 7). As reported by others, independent predictors of new permanent pacemaker implantation in the S3-THV group included complete right bundle branch block and PR duration[25].
| PPM | S3 | XT | P value | Predictor/comments |
| Binder et al[40] 2015 Circulation interventions | 17% | 13% | 0.01 | Predictors: Depth, RBBB |
| Binder et al[14] 2013 JACC interventions | 13.30% | Excluded patient with LBBB, PR > 200 ms No predictors studied | ||
| Husser et al[25] 2015 JACC interventions | 15.20% | Predictors not studied | ||
| Binder et al[40] 2015 EuroIntervention | 20.70% | Predictor > 8 mm depth of implants | ||
| Nijhoff et al[17] 2015 Circulation interventions | 9.80% | 8.80% | 0.94 | High implants: 80/20 in aorta as mentioned by authors |
However, implant depth was not a predictor of new PPM in our study. Rather, lack of coaxiality of the device during its deployment was independently associated to new PPM. These findings may be explained by flaws in the way depth is estimated before the prosthesis is deployed, and by flaws in the way depth is measured after it is deployed.
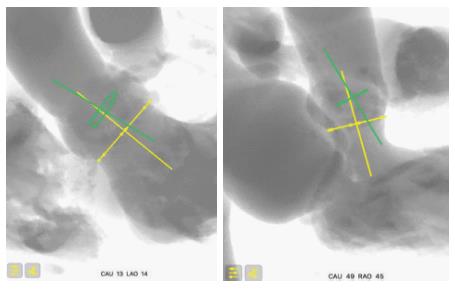
Before the prosthesis is deployed, the aortic annulus is seen in a coaxial projection, with the three cusps aligned. This projection is determined from the MSCT and confirmed during the procedure by aortography. However, the device positioned in the annulus, before deployment, is not necessarily coaxial. This may be difficult to appreciate because, unlike the Corevalve, the XT-THV and the S3-THV do not have a ring at their extremity. This lack of device coaxiality before deployment can induce flaws in the estimation of depth due to parallax error[18,39]. In our experience, lack of device coaxiality induces underestimation of implant depth. In other words, the less coaxial the device, the higher it will look, and the more the operator will want to push it deeper. This increases the true depth of implant and therefore risk of conduction disturbance and new PPM.
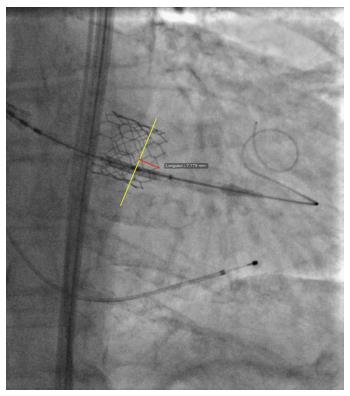
After the prosthesis is deployed, measurement of depth of implant can also be flawed by parallax error. As previously described, the projection in which depth is measured is not the one in which the device was deployed. Indeed, after deployment, the device is not necessarily coaxial. The projection is therefore modified to obtain device coaxiality and this is when final aortography is performed and depth is measured. In this new projection, however, the aortic annulus is no longer coaxial[18,39]. An example of this is provided in Figure 6, where two cusps are seen at different levels on the septal side. Proper localization of the hinge point between the device and sinus of Valsalva, and therefore proper implant depth measurement, can be difficult in such circumstances and prone to parallax error. To adequately measure device implantation depth, future studies should rely on post-procedural MSCT. This would allow measurement of depth all around the annulus, and not only on the septal and non-septal sides. Alternatively, computer programs that allow the operator to find the unique projection where both the device and the annulus are coaxial could be used. This would be the optimal projection to deploy the device, do the final aortography and measure depth.
The premise of this concept is that there is a slight angle between the un-deployed device and the aortic annulus. This is caused by patient anatomy and delivery catheter properties. As a result of this angle, even if the C-arm is perpendicular to the aortic annulus, it may not be perpendicular to the device. Figure 7 illustrates the coaxiality concept.
This retrospective study reflects a single-center experience. Groups had significant baseline characteristics differences and adjustment for these may be incomplete or flawed by residual confounding. Although PVR was assessed by experienced echocardiographers and reported according to VARC-2 criteria, the absence of a central core lab may lead to some heterogeneity in assessment of this outcome. In addition, we did not analyze the timing of conduction disturbances. Indeed, one of the possible reasons for higher PPM in the S3-THV group may be a delayed inflammatory process caused by the skirt polymer, in addition to its immediate mechanical effect on the conduction system. To reflect contemporary practice of TAVI, we collected ECG data, depth and device coaxiality only in the S3-THV group. As it is difficult to measure device coaxiality before implant on a crimped valve, we used the device coaxiality at the end of deployment. Measurements were taken as the balloon was deflated and the patient still under rapid pacing so that measurements reflected pre-deployment status. In addition, device coaxiality measurements were only available for procedures done in the catheterization laboratory, thereby excluding patients with non-transfemoral access.
The third generation Edwards S3-THV is associated to improved outcomes with lower rates of major vascular complications and PVR but higher rates of new PPM compared to its predecessor, the XT-THV.
These results are encouraging in the endeavor to take TAVI to lower risk populations. Our findings highlight the increased importance to adequately visualize the S3-THV in relation to the aortic valvular complex during deployment, in order to improve device positioning and potentially mitigate new PPM requirements.
Since its introduction in 2002, transcatheter aortic valve implantation (TAVI) has evolved tremendously and is now standard of care for high risk and inoperable aortic stenosis patients. However, TAVI is still associated with a higher incidence of paravalvular regurgitation (PVR), permanent pacemaker (PPM) and vascular complications when compared to surgical aortic valve replacement. In order to justify the extension of the procedure to lower risk patients, these adverse outcomes have to be mitigated. The development of novel transcatheter heart valves and refinement of technical skills have contributed to the decrease in complications rates associated with TAVI.
TAVI indication has now moved to intermediate and lower risk patients and it is crucial to continue careful evaluation of the newer generation devices aimed at improving patient outcomes. The study aimed to compare the different iterations between 2 valves on patient outcomes. New devices with lower profile and different designs have currently been introduced to further improve valve performance and efficacy.
TAVI is still associated with a higher incidence of PVR, PPM and vascular complications when compared to surgical aortic valve replacement. However, the third generation Edwards SAPIEN 3 transcatheter heart valve (S3-THV) the newest approved valve have improved TAVI outcomes by lowering complication rates and have recently been associated with improved outcomes compared to surgical aortic valve replacement in high risk patients. This breakthrough technology will without a doubt become the standard care of all patients in the near future with the continue improvement in device designs.
The third generation Edwards S3-THV is associated to improved outcomes with lower rates of major vascular complications and PVR but higher rates of new PPM compared to its predecessor, the SAPIEN XT transcatheter heart valve (XT-THV). These results are encouraging in the endeavor to take TAVI to lower risk populations. The authors’ findings highlight the increased importance to adequately visualize the S3-THV in relation to the aortic valvular complex during deployment, in order to improve device positioning and potentially mitigate new PPM requirements. Dedicated software devices that can align the annulus and the prosthesis during deployment could help in coaxial implantation of the valve.
TAVI: Transcatheter aortic valve implantation; PVR: Paravalvular regurgitation.
The paper is well written and offers a fairly large comparison of the performance of these 2 valves.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dizon JM, Sochman J, Said SAM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1000] [Cited by in F6Publishing: 970] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 2. | Lefèvre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 3. | Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Colombo A, Lange R. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 4. | Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1741] [Cited by in F6Publishing: 1709] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 5. | Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317-2326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 442] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 6. | Toggweiler S, Humphries KH, Lee M, Binder RK, Moss RR, Freeman M, Ye J, Cheung A, Wood DA, Webb JG. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:413-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1218] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 8. | Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4547] [Cited by in F6Publishing: 4639] [Article Influence: 356.8] [Reference Citation Analysis (0)] |
| 9. | Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;371:967-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol. 2012;60:1043-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 11. | Roten L, Wenaweser P, Delacrétaz E, Hellige G, Stortecky S, Tanner H, Pilgrim T, Kadner A, Eberle B, Zwahlen M. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V, Rihal CS, Zajarias A, Doshi D, Davidson M. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 13. | Barbanti M, Binder RK, Freeman M, Wood DA, Leipsic J, Cheung A, Ye J, Tan J, Toggweiler S, Yang TH. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention. 2013;9:929-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Binder RK, Schäfer U, Kuck KH, Wood DA, Moss R, Leipsic J, Toggweiler S, Freeman M, Ostry AJ, Frerker C. Transcatheter aortic valve replacement with a new self-expanding transcatheter heart valve and motorized delivery system. JACC Cardiovasc Interv. 2013;6:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Binder RK, Rodés-Cabau J, Wood DA, Webb JG. Edwards SAPIEN 3 valve. EuroIntervention. 2012;8 Suppl Q:Q83-Q87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Tarantini G, Mojoli M, Purita P, Napodano M, D’Onofrio A, Frigo A, Covolo E, Facchin M, Isabella G, Gerosa G. Unravelling the (arte)fact of increased pacemaker rate with the Edwards SAPIEN 3 valve. EuroIntervention. 2015;11:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Nijhoff F, Abawi M, Agostoni P, Ramjankhan FZ, Doevendans PA, Stella PR. Transcatheter aortic valve implantation with the new balloon-expandable Sapien 3 versus Sapien XT valve system: a propensity score-matched single-center comparison. Circ Cardiovasc Interv. 2015;8:e002408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Piazza N, Lauzier P, Mylotte D. Transcatheter Aortic Valve Replacement and New Conduction Abnormalities/Permanent Pacemaker: Can We Achieve the Intended Implant Depth? JACC Cardiovasc Interv. 2016;9:255-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 20. | Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol. 2009;53:1855-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Nijhoff F, Agostoni P, Samim M, Ramjankhan FZ, Kluin J, Doevendans PA, Stella PR. Optimisation of transcatheter aortic balloon-expandable valve deployment: the two-step inflation technique. EuroIntervention. 2013;9:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 967] [Cited by in F6Publishing: 938] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 23. | Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 704] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 24. | Athappan G, Gajulapalli RD, Tuzcu ME, Svensson LG, Kapadia SR. A systematic review on the safety of second-generation transcatheter aortic valves. EuroIntervention. 2016;11:1034-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Husser O, Pellegrini C, Kessler T, Burgdorf C, Thaller H, Mayr NP, Ott I, Kasel AM, Schunkert H, Kastrati A. Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Balloon-Expandable Transcatheter Heart Valve: A Single-Center Experience. JACC Cardiovasc Interv. 2015;8:1809-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 27. | Gurvitch R, Toggweiler S, Willson AB, Wijesinghe N, Cheung A, Wood DA, Ye J, Webb JG. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the Valve Academic Research Consortium (VARC) guidelines. EuroIntervention. 2011;7:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Hamm CW, Möllmann H, Holzhey D, Beckmann A, Veit C, Figulla HR, Cremer J, Kuck KH, Lange R, Zahn R. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2014;35:1588-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Généreux P, Kodali S, Hahn R, Nazif T, Williams M, Leon MB. Paravalvular leak after transcatheter aortic valve replacement. Minerva Cardioangiol. 2013;61:529-537. [PubMed] [Cited in This Article: ] |
| 30. | Jerez-Valero M, Urena M, Webb JG, Tamburino C, Munoz-Garcia AJ, Cheema A, Dager AE, Serra V, Amat-Santos IJ, Barbanti M. Clinical impact of aortic regurgitation after transcatheter aortic valve replacement: insights into the degree and acuteness of presentation. JACC Cardiovasc Interv. 2014;7:1022-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5086] [Cited by in F6Publishing: 5148] [Article Influence: 367.7] [Reference Citation Analysis (0)] |
| 32. | Auffret V, Bedossa M, Boulmier D, Verhoye JP, Ruggieri VG, Koning R, Laskar M, Van Belle É, Leprince P, Collet JP, Iung B, Lefèvre T, Eltchaninoff H, Gilard M, Le Breton H. From FRANCE 2 to FRANCE TAVI: are indications, technique and results of transcatheter aortic valve replacement the same? Presse Med. 1978;44:752-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Webb J, Gerosa G, Lefèvre T, Leipsic J, Spence M, Thomas M, Thielmann M, Treede H, Wendler O, Walther T. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol. 2014;64:2235-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 34. | Buellesfeld L, Stortecky S, Heg D, Hausen S, Mueller R, Wenaweser P, Pilgrim T, Gloekler S, Khattab AA, Huber C. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Urena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A, Dager AE, Serra V, Amat-Santos IJ, Barbanti M, Immè S. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 36. | Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 878] [Cited by in F6Publishing: 863] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 37. | Binder RK, Webb JG, Toggweiler S, Freeman M, Barbanti M, Willson AB, Alhassan D, Hague CJ, Wood DA, Leipsic J. Impact of post-implant SAPIEN XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv. 2013;6:462-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Binder RK, Rodés-Cabau J, Wood DA, Mok M, Leipsic J, De Larochellière R, Toggweiler S, Dumont E, Freeman M, Willson AB. Transcatheter aortic valve replacement with the SAPIEN 3: a new balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv. 2013;6:293-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Spaziano M, Thériault-Lauzier P, Meti N, Vaquerizo B, Blanke P, Deli-Hussein J, Chetrit M, Galatos C, Buithieu J, Lange R. Optimal fluoroscopic viewing angles of left-sided heart structures in patients with aortic stenosis and mitral regurgitation based on multislice computed tomography. J Cardiovasc Comput Tomogr. 2016;10:162-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Binder RK, Stortecky S, Heg D, Tueller D, Jeger R, Toggweiler S, Pedrazzini G, Amann FW, Ferrari E, Noble S. Procedural Results and Clinical Outcomes of Transcatheter Aortic Valve Implantation in Switzerland: An Observational Cohort Study of Sapien 3 Versus Sapien XT Transcatheter Heart Valves. Circ Cardiovasc Interv. 2015;8:pii: e002653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |