Published online Nov 15, 2017. doi: 10.4251/wjgo.v9.i11.444
Peer-review started: July 19, 2017
First decision: August 7, 2017
Revised: August 17, 2017
Accepted: September 4, 2017
Article in press: September 5, 2017
Published online: November 15, 2017
To test the validity of tumour thickness measurement in distinguishing between the different infiltration depths, especially when the duplication of muscularis mucosae cannot be demarcated clearly.
We re-evaluated 100 completely embedded Barrett’s adenocarcinomas regarding m-classification, maximum tumour thickness, and muscularis mucosae duplication. For validation, smoothelin staining was performed on a subset of cases.
The m1-, m2- and m3-classified adenocarcinomas showed a significant lower tumour thickness compared to the m4- and sm1-classified lesions (P < 0.001). Smoothelin staining determined a clear muscularis mucosae duplication in 64% of the tested samples and enabled the differentiation of the two layers in diffuse and merged splits.
Tumour thickness in early oesophageal adenocarcinoma significantly correlates with the depth of infiltration and demonstrates its worth as an accurate pT classification in non-polypoid lesions. We created a new algorithm, which combines histomorphology with morphometric analyses. It is noteworthy that it facilitates the assessment of mucosal vs submucosal infiltration depth. The smoothelin staining strengthened our results of the tumour thickness evaluation and can be used in cases of doubt.
Core tip: The aim of this study was to determine whether histomorphometric measurement of tumor thickness and immunohistochemical staining for smoothelin facilitate the exact pT substaging in early oesophageal adenocarcinoma. Our data showed that there is clear cut-off of 1000 μm to distinguish advanced early lesions (M4/sm1) from such lesions that do not reach the deep muscularis mucosae or the submucosa. Moreover, smoothelin staining is of help to distinguish the superficial from the deep muscularis mucosa by different staining intensities. Therfore, both methods could be shown to be of help for the often challenging task to T-classify early oesophageal adenocarcinomas.
- Citation: Endhardt K, Märkl B, Probst A, Schaller T, Aust D. Value of histomorphometric tumour thickness and smoothelin for conventional m-classification in early oesophageal adenocarcinoma. World J Gastrointest Oncol 2017; 9(11): 444-451
- URL: https://www.wjgnet.com/1948-5204/full/v9/i11/444.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i11.444
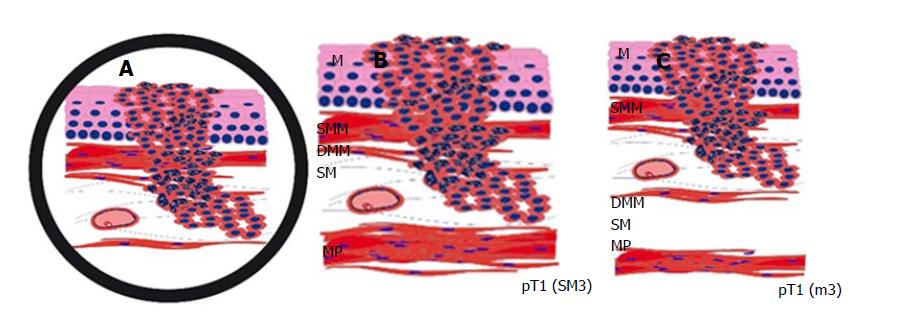
Early oesophageal adenocarcinoma, arising from Barrett Oesophagus, most frequently occurs in Caucasians and shows a rising incidence in recent decades[1-5]. The latest studies confirm that endoscopic submucosal dissection (ESD) is a safe and curative endoscopic method, yielding better R0 resection rates than endoscopic mucosal resections (EMR)[1,3,5,6]. Since the current endoscopic techniques allow for minimally invasive curative en-bloc resection of early carcinoma, it is essential to determine the accurate depth of infiltration. In particular, it is of crucial importance to distinguish between mucosal and submucosal invasion. While this is simple in most areas of gastrointestinal tumours, it can be a challenging problem in Barrett’s carcinoma. This is mainly caused by muscularis mucosae duplication, which was first described in 1990[6,7]. Based on Vieth and Stolte, the intramucosal carcinoma is divided into 4 subgroups, depending on the infiltration depth as follows: m1, limited to the Lamina propria mucosae; m2, the superficial muscularis mucosae (SMM); m3, the layer in between the superficial and deep muscularis mucosae (DMM) and m4, the DMM[7,8]. Submucosal invasion sm1 (< 500 μm), in association with poor differentiation (G3) or diameters > 20 mm, as well as sm2-invasion (> 500 μm) or deeper, are indications for a subsequent surgical resection[8,9]. Furthermore, significantly higher rates of lymphatic invasion and lymph node metastasis identify sm1-invasion and are important prognostic factors[9,10]. Due to the diffuse and confusing splits of the muscularis mucosae, poor material quality or piecemeal resections (EMR), the subclassification cannot be properly defined in each sample. When evaluating the slides, it is always important to keep in mind that the tumour beneath a layer of smooth muscle is not necessarily submucosal (sm) but could just as well be located within a splintered muscularis mucosae (m3) (Figure 1). Penetration of the SMM can be mistaken for an initial submucosal invasion and may have severe consequences to the further treatment plan, including mismanagement, such as oesophagectomy without adequate indication[10,11]. We, therefore, examined our ESD collection with early oesophageal adenocarcinoma and searched for a new parameter facilitating the exact subclassification as an adjunct to the conventional histomorphological method. With the help of a histomorphometric tumour thickness measurement, we intended to establish a cut-off value for distinguishing between the penetration of the SMM and the DMM, where the latter is equivalent to submucosal infiltration. The use of smoothelin immunohistochemistry for distinguishing between the muscularis mucosae and the muscularis propria is well established in bladder carcinoma[12]. In staging Barrett’s adenocarcinoma, it is not yet fully evaluated, but recent studies emphasized the different staining intensities in the superficial compared to DMM as an important discriminatory marker[13,14]. To confirm the reported findings and evaluate the additional discriminatory power, the smoothelin staining was used in a subset of cases in combination with the histomorphometric analysis.
The study was performed according to the national rules and was approved by the institutional ethical review board of the Klinikum Augsburg. All the ESDs of Barrett Oesophagus with adenocarcinoma between 2008 and the end of 2014 were retrieved from the archives of the Institute of Pathology, Klinikum Augsburg (Augsburg, Germany), and all the sections (the lesions were oriented and completely paraffin-embedded) were reviewed by at least two independent experienced pathologists without knowledge of the initial report (BM, DA, and HA). In discrepant cases, a consensus diagnosis was established after a re-evaluation on a multi-headed microscope. Haematoxylin and eosin-stained sections were reviewed regarding the pT (m/sm)-subclassification, grading, and resection margins. Neoplastic superficial lesions with a polypoid morphology (Paris endoscopic classification type I) were excluded from the study to avoid a bias of the measuring method. Flat, slightly elevated or slightly depressed tumours (Paris endoscopic classification type II) were included. A validation set of 25 cases (beginning of 2015 to mid-2015) was selected from our archives and was analysed as described.
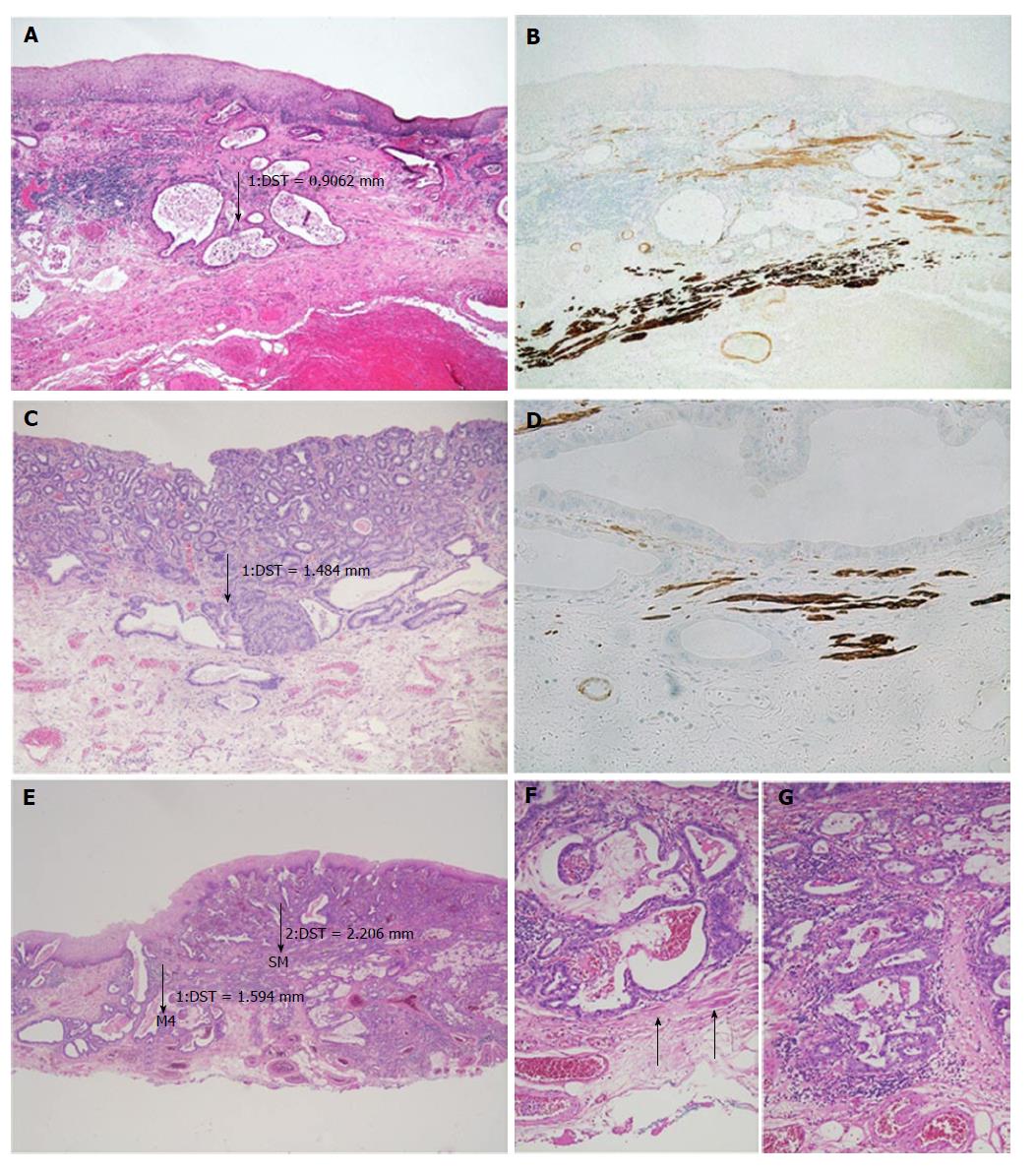
After detecting the area with the deepest infiltration, the tumour thickness was measured in millimetres using a digital camera with calibrated software (ProgRes C10, Jenoptik, Jena). It is important to mention that the invasion depth was not of interest, but we were interested in the complete thickness of the tumour throughout all the mucosal layers, from the surface to the invasion front. In case of undermining growth beneath the intact epithelium, tumour thickness was measured from the most superficial neoplastic cell layer to the point of deepest invasion (Figure 2). Size values were assigned to the subgroups depending on the infiltration level.
Slides showing the deepest infiltration level from 33 randomly selected cases were sectioned and stained for smoothelin (Cell Marque Clone R4 A, monoclonal mouse, dilution 1:100) and desmin (Dako, Clone D33, monoclonal mouse, dilution 1:200). The Ventana Ultraview detection system (Roche Diagnostics, Mannheim, Germany) was used for the development of the reactions. The slides were evaluated for the following parameters: Length of muscularis mucosae duplication within the Barrett’s lesion in percent (≤ 5%, > 5 and ≤ 50%, > 50 and ≤ 95%, > 95%); scattered split and notable difference in staining intensity of the SMM vs the DMM. Discrepant cases were reviewed together for a consensual diagnosis.
The Spearman Rank Order Correlation Test was used to calculate the correlation between tumour thickness and pT stage. A One-Way Repeated Measures Analysis of Variance was used to compare the numeric values of the pT subgroups. To isolate the groups that differed from the others, the Holm-Sidak method was applied. The mean values are given with ± 1 SD. A P-value of < 0.05 was considered significant. The sensitivity, specificity, and the positive and negative predictive values were calculated to measure the diagnostic accuracy of the morphometric evaluation. All the calculations were performed using the Sigma Plot 13.0 software package (Systat, Richmond, VA, United States).
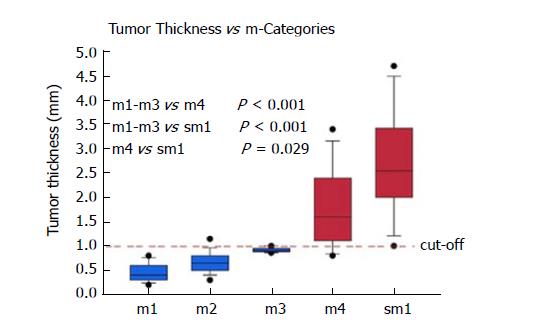
Cases were primarily included for analysis based on the diagnosis in the original pathology report. A total of 100/100 cases (100%) were confirmed as harbouring an early adenocarcinoma arising in Barrett’s mucosa. However, 12/100 cases (12%) had to be excluded due to a polypoid growth pattern. Thanks to the good quality of the ESD specimens, the overall orientation was excellent, and representative cross sections were available throughout most specimens. Only 5/100 cases (5%) had to be excluded due to technical issues, where rotated and tilted positions caused imprecise measurements and subclassifications. In 83/100 eligible cases (83%) with proper evaluation, the infiltration depth was divided as follows: m1: 13/83 (16%); m2: 36/83 (43%); m3: 4/83 (5%); m4: 14/83 (7%) and sm: 16/83 (19%). Table 1 shows the tumour thickness in the different pT subgroups. It ranged from 0.2 to 4.7 mm.
| pT1 subgroup | Total | m1 | m2 | m3 | m4 | sm |
| No. of cases (n) | 83 | 13 | 36 | 5 | 13 | 16 |
| Tumour thickness (mm) | 0.2-4.7 | 0.2-0.8 | 0.3-1.4 | 0.9-1.0 | 0.8-3.4 | 1.0-4.7 |
| Mean ± SD (mm) | - | 0.46 ± 0.19 | 0.64 ± 0.24 | 0.91 ± 0.05 | 1.78 ± 0.79 | 2.69 ± 1.03 |
pT1a Barrett’s carcinomas that were restricted to the mucosa and did not show any infiltration of the DMM (m1-m3), showed significantly (P < 0.001) lower levels of tumour thickness when compared to the pT1a (m4) and pT1b (sm1 or deeper) tumours (Figure 3). With increasing infiltration depth, the mean values of the tumour thickness steadily rose. Overall, there was a very strong correlation between the pT (m/sm) substages and tumour thickness (P < 0.0001). The values were summarized by two groups, either m1/m2/m3 or m4/sm. After separation, we detected six cases (7%) with a slightly overlapping result, where the tumour thickness did not fit the diagnosed pT stage. The mean tumour thickness of the subgroup m1-m3 was significantly different compared to group m4 (P < 0.001) and group sm1 and deeper (P < 0.001). The overlaps were observed at approximately 1000 μm. The statistical analysis, with a cut-off value of 1000 μm, showed high sensitivity (94%) and specificity (90%) for the distinction between m1/m2/m3 vs m4/sm. The negative predictive value was 94%, and the positive predictive value 91%, showing a strong correlation between the tumour thickness and infiltration depth. Only one case of this collection with a clear submucosal infiltration and tumour thickness of 3600 μm showed lymphatic invasion. Venous invasions were not identified. Therefore, correlations between tumour thickness and vascular invasion could not be calculated.
To confirm our findings, a validation set of 25 cases from the beginning of 2015 to mid-2015 was reviewed and analysed as described. In this smaller data set, we confirmed the findings of the evaluation set with a specificity of 100%, whereas the sensitivity was 83.3%.
Immunohistochemistry for desmin showed a similar staining intensity in the superficial and DMM, even though the SMM usually appeared finer and more delicate. In 21/33 cases (64%), desmin and smoothelin showed a clearly identifiable duplication, with at least a focal dissociation of the muscularis mucosae. Table 2 shows the details of the percentages of the detected duplication within these 21 specimens.
| Total cases | 33 (100) |
| Duplication | 21 (64) |
| Percent of duplication in the lesion | |
| ≤ 5% | 3 (9) |
| > 5% and ≤ 50% | 5 (15) |
| > 50% and ≤ 95% | 3 (9) |
| > 95% | 10 (30) |
A total of 9/33 cases (27%) did show a broadened muscular layer, where the superficial and DMM could not be demarcated. In 3/33 cases (9%), a single thin muscular layer appeared not to show any split at all.
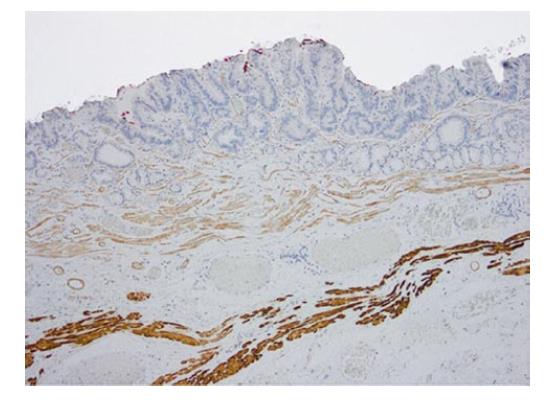
The staining pattern with smoothelin varies con-siderably from desmin, with a weak-to-moderate staining of the SMM, while the DMM resembles the strong staining with desmin (Figure 4). In the 21 cases described with duplication, this remarkable difference in staining intensity between the two muscular layers was determined. Cases without any definable muscularis mucosae duplication showed a uniform smoothelin staining intensity. It is noteworthy, that all the duplicated specimens, even if duplication was only detected in a minor fraction of the lesion, showed the different intensity with the smoothelin staining.
A significant observer variability is described for early oesophageal adenocarcinoma, leading to overdiagnosis followed by overtreatment[6,11,15]. In our experience, it is often challenging to determine the exact pT classification in early Barrett’s adenocarcinoma. Most difficulties are caused by duplication or diffuse branchlike splintering of the muscularis mucosae (Figure 1). The appearance of duplication was first described in the early 1990s[6,10,13-15], and since then, it has been analysed in several studies, showing a strict limitation to Barrett’s adenocarcinoma. It is reported in up to approximately 90% of Barrett-related neoplasia[10,13,14]. The origin and significance of the duplication still needs to be defined. Diffuse, irregular, and merged splits remain problematic in the histological assessment, leading to confusion and misinterpretation. In cases of inadequate orientation, an invasion of the space between the duplicated muscular layers (m3) could easily be mistaken as a submucosal invasion (sm). Since muscularis mucosae duplication is an inconsistent event and is usually not captured in all slides, the establishment of further standard evaluation criteria would be of great help.
This study aimed, therefore, to find a complementary tool to support the histomorphologic assessment. The superficial and DMM are the histological hallmark structures to determine pT subclassification. However, this is complicated by the morphological variability of these structures. The ESD is almost exclusively performed in specialized gastroenterological centres and therefore, most studies, to date, are limited to the more common piecemeal specimens gained from EMR. The basis for this study was a unique dataset of 100 ESD specimens of early oesophageal adenocarcinoma. Due to en-bloc resection, an outstanding benefit in the histological examination is given compared to the EMR specimens. The lesions can be oriented accurately regarding the horizontal diameter, invasion depth and basal as well as circumferential resection margins. Moreover, orthogonal cutting provides an excellent overview through the different tissue layers. The majority of slides in this study depicted a complete cross section through the mucosa and in parts the submucosa, whereas the muscularis propria was demonstrably not captured in any of the samples.
Our hypothesis was that tumour thickness, which is easy to measure, might facilitate the pT assessment. Exact pT staging is of crucial importance in early adenocarcinoma of the oesophagus as the risk of lymph node metastasis increases with infiltration depth[16,17]. In gastric and colorectal cancers, a morphometric measurement is already accepted in a different context for the evaluation of submucosal infiltration. An infiltration beyond the cut-off value indicates a higher risk for lymph node metastases and is defined as a limit for endoscopic treatment. Endoscopic mucosectomy is justified for a submucosal invasion of 500 μm or less (stomach) or 1000 μm or less (large bowel)[18-20]. In view of the morphometric measurement, it is important to mention that excessive stretching of the resected unfixed tissue can thin out the mucosal and submucosal layer and, therefore, lead to biased values[21]. To prevent this bias, all the specimens are pinned on a cork immediately after ablation, and no further tractions or tensions during the processing can influence the tumour thickness. For establishing our morphometric method, we investigated the tumour thickness from the surface to the invasion front by measuring the maximum infiltration depth. Since the initial point of invasion cannot always be defined and might lead to inter-observer variability, it appeared reasonable to not restrict our measurement to the invasive part of the tumour.
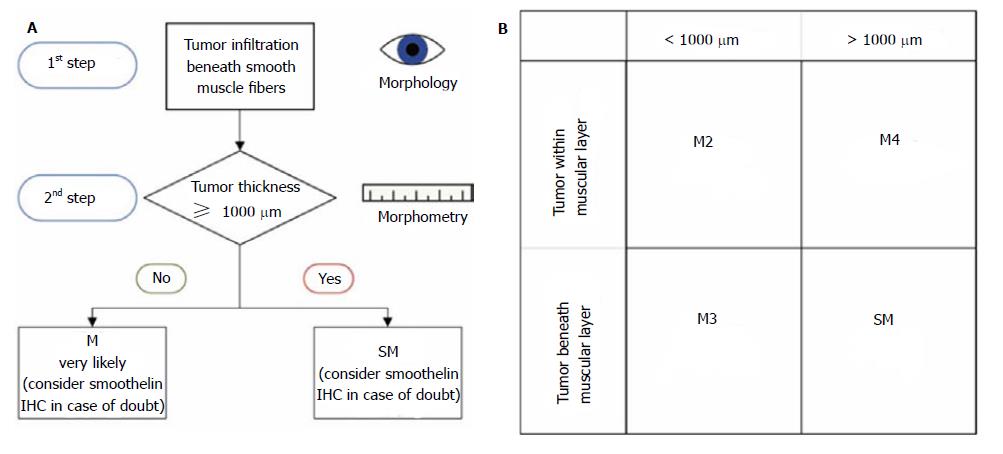
From a therapeutic point of view, the distinction between the mucosal and submucosal involvement is essential and usually the main issue at stake. In this study, we showed that the tumour thickness measurement, in combination with the histomorphology, is a robust method for the distinction of the infiltration depth. A Barrett adenocarcinoma with a muscular penetration and a tumour thickness < 1000 μm is most likely a mucosal lesion pT1a (m3), whereas a muscular penetration with a tumour thickness ≥ 1000 μm is most likely a submucosal lesion pT1b (sm). In certain cases, the immunohistochemical smoothelin staining is additionally helpful because it reveals the different muscle layers in the duplicated but irregularly merged or branchlike arranged muscle fibres. The detected cut-off value of 1000 μm will help, especially in small and fragmented specimens, such as EMR, or specimens where the duplication cannot be properly classified. It is noteworthy that our method is restricted to non-polypoid lesions, which account for more than 85% of the cases.
Approximately 60%-70% of the analysed Barrett’s adenocarcinomas harbour a distinctive muscularis mucosae split, which is close to the reported ranges of 92% (n = 50) and 66% (n = 110)[13,14]. Faragalla et al[10] did not report the frequency of duplication since their study is based merely on the cases showing duplication. Many cases in our study showed a clear identification of the two muscularis mucosae layers by smoothelin immunohistochemistry. The staining was, first of all, useful for determining the borders of the individual layer itself and was even more helpful in distinguishing the superficial from DMM, especially in the described critical cases with irregular splits. In those cases, the differentiation often seemed virtually impossible to assess in the haematoxylin and eosin-stained sections and with desmin staining but was quite obvious with the smoothelin staining. Some diffuse splits can only be demarcated with the help of immunohistochemical analyses. Faragalla et al[10] suggested the development of a modified smoothelin antibody that only stains the DMM, since, in his opinion, staining of the SMM could cause confusion. In our eyes, it was helpful to identify both layers with different staining intensities[10]. Solely staining with the smoothelin antibody can be insufficient for precise staging, but it offers an important additional benefit in complex and confusing areas of duplicated lesions. All three observers independently confirmed the diagnostic advantage of the smoothelin staining, in comparison with haematoxylin and eosin, as well as desmin.
In conclusion, our results show a very strong correlation between pT stage and tumour thickness. However, a clear cut-off was identified only for the discrimination of m1/m2/m3 vs m4/sm stages. At first glance, this seems of minor value because the clinically most relevant discrimination is the separation between the m- and sm-cases. Nevertheless, we developed an algorithm to overcome this problem. The histomorphological evaluation of the tumour invasion, in relation to the smooth muscle fibres in combination with the tumour thickness measurement, allows us to translate our results into a mucosal vs submucosal discrimination (Figure 5). This, therefore, considerably facilitates accurate pT staging. In cases of doubt, further immunohistochemical staining with smoothelin is advocated. Our data consequently indicate that a morphometric measurement of tumour thickness and smoothelin staining supplement the histomorphological discrimination of mucosal vs submucosal invasion in non-polypoid oesophageal adenocarcinoma. It has to be emphasized that the morphometric analysis is an adjunct to the conventional histomorphological evaluation and not its replacement.
The main problem of determine the infiltration depth in early oesophageal adenocarcinoma is caused by the duplication of the muscularis mucosae in early oesophageal adenocarcinoma. Based on Vieth and Stolte, the intramucosal carcinoma is divided into 4 subgroups, depending on the infiltration depth as follows: m1, limited to the Lamina propria mucosae; m2, the superficial muscularis mucosae (SMM); m3, the layer in between the superficial and deep muscularis mucosae (DMM) and m4, the DMM.
There are still controversies concerning the criteria for the histological diagnosis of barrett mucosa. Further research topics deal with the techniques to treat early oesophageal lesions.
This work provides helpful adjuncts to evaluate the pT stage in early oesophageal adenocarcinoma. Because of its high clinical relevance this can be seen as a major advance.
Histomorphometric measurement of the tumor thickness and smoothelin staining can be used as an adjunct to the conventional pT evaluation which is solely based on morphology.
EMR: Endoscopic mucosa resection; ESD: Endoscopic submucosa dissection; M: Mucosa; SMM: Superficial muscularis mucosae; DMM: Deep muscularis mucosae.
This is a well-conducted and well-written study.
The authors thank Kathrin Ferstl-Blahetek and her team for excellent technical assistance.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guner A, Kataoka K, Ladic A, Nishida T S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
| 1. | Probst A, Messmann H. Endoskopische Submukosadissektion: Aktuelle Indikationen und Grenzen. Viszeralmedizin. 2012;28:395-401. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7953] [Cited by in F6Publishing: 8022] [Article Influence: 534.8] [Reference Citation Analysis (2)] |
| 3. | Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;47:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 944] [Cited by in F6Publishing: 938] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 5. | Chevaux JB, Piessevaux H, Jouret-Mourin A, Yeung R, Danse E, Deprez PH. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett’s neoplasia. Endoscopy. 2015;47:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Tada T, Suzuki T, Iwafuchi M, Watanabe H, Katayanagi N, Aizawa K, Nishimaki T, Tanaka O, Muto T. Adenocarcinoma arising in Barrett’s esophagus after total gastrectomy. Am J Gastroenterol. 1990;85:1503-1506. [PubMed] [Cited in This Article: ] |
| 7. | Vieth M, Stolte M. Pathology of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:857-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Koop H, Fuchs KH, Labenz J, Lynen Jansen P, Messmann H, Miehlke S, Schepp W, Wenzl TG; Mitarbeiter der Leitliniengruppe. [S2k guideline: gastroesophageal reflux disease guided by the German Society of Gastroenterology: AWMF register no. 021-013]. Z Gastroenterol. 2014;52:1299-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Liu L, Hofstetter WL, Rashid A, Swisher SG, Correa AM, Ajani JA, Hamilton SR, Wu TT. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005;29:1079-1085. [PubMed] [Cited in This Article: ] |
| 10. | Faragalla HF, Marcon NE, Yousef GM, Streutker CJ. Im-munohistochemical staining for smoothelin in the duplicated versus the true muscularis mucosae of Barrett esophagus. Am J Surg Pathol. 2011;35:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sangle NA, Taylor SL, Emond MJ, Depot M, Overholt BF, Bronner MP. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: a multicenter, international study. Mod Pathol. 2015;28:758-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Paner GP, Shen SS, Lapetino S, Venkataraman G, Barkan GA, Quek ML, Ro JY, Amin MB. Diagnostic utility of antibody to smoothelin in the distinction of muscularis propria from muscularis mucosae of the urinary bladder: a potential ancillary tool in the pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol. 2009;33:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Abraham SC, Krasinskas AM, Correa AM, Hofstetter WL, Ajani JA, Swisher SG, Wu TT. Duplication of the muscularis mucosae in Barrett esophagus: an underrecognized feature and its implication for staging of adenocarcinoma. Am J Surg Pathol. 2007;31:1719-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Lewis JT, Wang KK, Abraham SC. Muscularis mucosae duplication and the musculo-fibrous anomaly in endoscopic mucosal resections for barrett esophagus: implications for staging of adenocarcinoma. Am J Surg Pathol. 2008;32:566-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Takubo K, Sasajima K, Yamashita K, Tanaka Y, Fujita K. Double muscularis mucosae in Barrett’s esophagus. Hum Pathol. 1991;22:1158-1161. [PubMed] [Cited in This Article: ] |
| 16. | Zemler B, May A, Ell C, Stolte M. Early Barrett’s carcinoma: the depth of infiltration of the tumour correlates with the degree of differentiation, the incidence of lymphatic vessel and venous invasion. Virchows Arch. 2010;456:609-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Estrella JS, Hofstetter WL, Correa AM, Swisher SG, Ajani JA, Lee JH, Bhutani MS, Abraham SC, Rashid A, Maru DM. Duplicated muscularis mucosae invasion has similar risk of lymph node metastasis and recurrence-free survival as intramucosal esophageal adenocarcinoma. Am J Surg Pathol. 2011;35:1045-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 19. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 567] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 20. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1197] [Article Influence: 57.0] [Reference Citation Analysis (3)] |
| 21. | Park S, Chun HJ, Kwon YD, Keum B, Seo YS, Kim YS, Jeen YT, Um SH, Kim CD, Ryu HS. Stretching Causes Extensive Changes of Gastric Submucosa: Is It Acceptable to Define 500 microm as the Safe Margin? Gut Liver. 2008;2:199-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |