Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.327
Peer-review started: August 31, 2014
First decision: November 14, 2014
Revised: December 27, 2014
Accepted: January 9, 2015
Article in press: January 9, 2015
Published online: March 27, 2015
Processing time: 215 Days and 16.6 Hours
The clinical course of chronic hepatitis C virus (HCV) infection is characterized by possible development of both liver and extrahepatic disorders. The tropism of HCV for the lymphoid tissue is responsible for several immune-mediated disorders; a poly-oligoclonal B-lymphocyte expansion, commonly observed in a high proportion of patients with HCV infection, are responsible for the production of different autoantibodies and immune-complexes, such as mixed cryoglobulins. These serological alterations may characterize a variety of autoimmune or neoplastic diseases. Cryoglobulinemic vasculitis due to small-vessel deposition of circulating mixed cryoglobulins is the prototype of HCV-driven immune-mediated and lymphoproliferative disorders; interestingly, in some cases the disease may evolve to frank malignant lymphoma. In addition, HCV shows an oncogenic potential as suggested by several clinico-epidemiological and laboratory studies; in addition to hepatocellular carcinoma that represents the most frequent HCV-related malignancy, a causative role of HCV has been largely demonstrated in a significant percentage of patients with isolated B-cells non-Hodgkin’s lymphomas. The same virus may be also involved in the pathogenesis of papillary thyroid cancer, a rare neoplastic condition that may complicate HCV-related thyroid involvement. Patients with HCV infection are frequently asymptomatic or may develop only hepatic alteration, while a limited but clinically relevant number can develop one or more autoimmune and/or neoplastic disorders. Given the large variability of their prevalence among patients’ populations from different countries, it is possible to hypothesize a potential role of other co-factors, i.e., genetic and/or environmental, in the pathogenesis of HCV-related extra-hepatic diseases.
Core tip: The proposed definition of hepatitis C virus (HCV) syndrome encompasses the multiform complex of clinico-pathological conditions potentially correlated to chronic HCV infection. The natural history of HCV syndrome is the result of multifactorial and multistep pathogenetic process, which usually proceeds from mild, often isolated manifestations, to systemic immune-mediated disorders, and less frequently to overt malignancies. Here we analyze the clinical, epidemiological, and pathogenetic aspects of this multifaceted condition, including the updated results of the world literature.
- Citation: Ferri C, Sebastiani M, Giuggioli D, Colaci M, Fallahi P, Piluso A, Antonelli A, Zignego AL. Hepatitis C virus syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World J Hepatol 2015; 7(3): 327-343
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/327.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.327
Hepatitis C virus (HCV) has been isolated in 1989; it represents the main agent of the so-called nonA/nonB chronic hepatitis[1]; soon after the HCV discovery, several clinico-epidemiological, pathological, and laboratory studies definitely evidenced the role of this agent in the symptom complex associated to mixed cryoglobulinemia (MCs)[2-4]. Clinically, MCs is systemic autoimmune disorder, also termed cryoglobulinemic vasculitis (CV), characterized by cutaneous and visceral organ involvement, including chronic hepatitis present in a relevant number of patients[4-7]. The presence of liver involvement, rarely found in systemic vasculitis, suggested a role of hepatotropic viruses in CV since the seventies[4,6,7]; this hypothesis was ultimately confirmed by the high rate of HCV ongoing infection in large CV patients’ series[2-4,8]. On the other hands, MCs mimics some immune-mediated disorders and malignancies, especially B-cell non-Hodgkin’s lymphoma (B-NHL); this peculiar clinical feature suggested a possible role of HCV also in other immune-mediated and neoplastic conditions during the past two decades[4,6,7]. Besides liver involvement, a growing number of clinico-laboratory studies progressively evidenced a possible pathogenetic role of chronic HCV infection in various extrahepatic manifestations, among which the MCs represents the true prototype[4,6,7].
In this complex scenario, the demonstration of HCV lymphotropism represented a decisive advance in the knowledge of the pathogenesis of HCV-associated disorders[9,10]. The spectrum of these conditions includes both hepatic, organ-specific and systemic autoimmune disorders, and malignancies[4,6,7]; therefore we previously proposed the term “HCV syndrome” referring to this constellation of virus-driven clinical conditions[11]. The different clinical phenotypes of HCV-syndrome can be the results of genetic/environmental co-factors as suggested by the heterogeneous geographical prevalence of single HCV-associated manifestations[4,6,7].
The present review focuses on the state of the art of this multifaceted condition with particular emphasis to extra-hepatic manifestations.
Numerous studies regarding the biological aspects of HCV and the interactions between viral genome with the immune system of the infected subjects may explain the complex clinical spectrum of HCV-associated disorders[4,6,7,9-16]. Soon after HCV identification, the hepato- and lymphotropism of this virus has been clearly demonstrated[9,10]; in particular, the HCV infection of lymphoid tissue may represent a decisive step in the development of virus-driven autoimmune-lymphoproliferative diseases[4,11]. Epidemiological studies firstly suggested a possible pathogenetic role of HCV in MCs, a systemic disease sustained by indolent B-cell expansion[4-7]; moreover, cryoglobulinemia may be detected in a relevant percentage of individuals with HCV infection, frequently associated with circulating autoantibodies and/or mixed cryoglobulinemia, the hallmark of overt MCs[4-7,17].
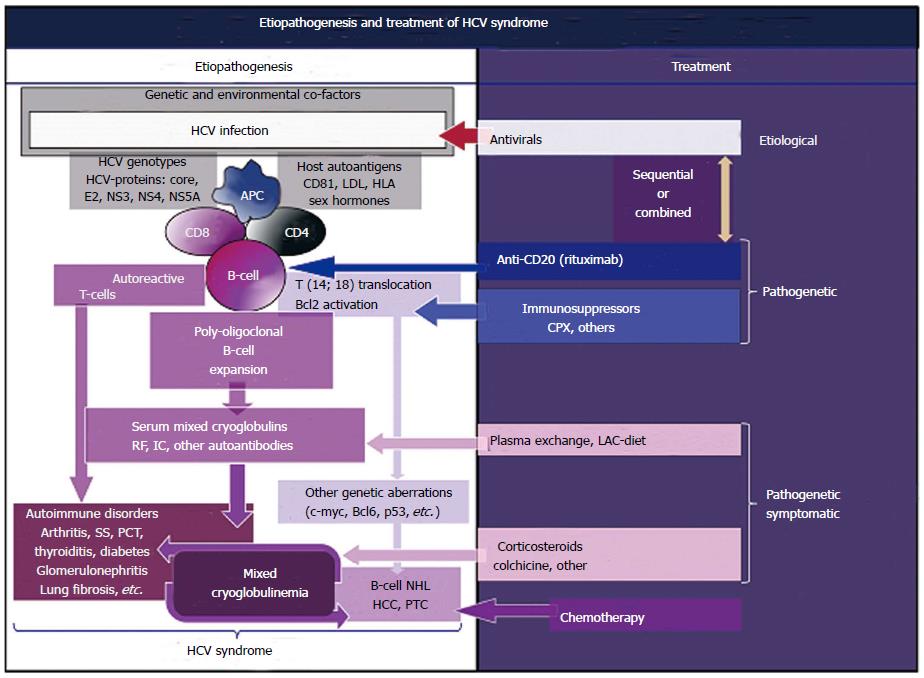
A positive, single-stranded RNA characterizes the HCV; given the absence of a DNA intermediate in the viral replication, HCV RNA sequences cannot incorporate into the genome of infected individuals; consequently, it may represent a chronic stimulus to the immune system, which through a multistep process may lead to clonal B-lymphocytes expansion[4,12-15]. In particular, a relevant number of patients with HCV infection show t(14;18) translocation, which in turn may lead to Bcl-2 proto-oncogene activation; more frequently HCV-associated MCs with mono-oligoclonal type II mixed cryoglobulins[4,6,7,18] (Figure 1). In addition, viral envelope E2 protein able to bind the molecule CD81, which is widely expressed on cell membrane of both hepatocytes and B-cells, might be decisive for HCV-associated autoimmune phenomena[4,6,7,19]. The interaction of HCV-E2 with CD81, part of cell-surface protein complex CD81-CD21-CD19-Leu 13 on the B-lymphocytes, may be able to reduce the threshold for the activation of B-cells by bridging complement recognition CD21-mediated and antigen-specific recognition (4, 6, 7, 13, 14, 19). The HCV-E2 and CD81 interaction in antigen-reactive B-lymphocytes may amplify the VDJ rearrangement frequency[13,14]; the t(14;18) translocation represents one of possible genetic aberration with consequent activation of bcl-2 proto-oncogene detectable in HCV-infected individuals[18]. The Bcl-2 over expression may prevent apoptosis and consequently may extend the survival B-lymphocytes[13,14,18]. The expansion of B-cells may lead to autoantibody production, including the anti-IgG rheumatoid factor constitutive of IgG-IgM immune-complexes, including mixed cryoglobulins[4,6,7,20].
While specific autoantibodies are typically found in patients with single-organ or systemic autoimmune disorders, cryoprecipitable and non-cryoprecipitable IgG-IgM immune-complexes are the serological hallmarks of MCs[4,6,7,20]. Moreover, a molecular mimicry mechanism that may involve specific HCV proteins and host autoantigens might produce B-cell activation with increased production of autoantibodies[4,6,7,20] (Figure 1). While the prolonged survival of B-lymphocytes can lead to additional genetic aberrations, such as c-myc, Bcl6, and p53 responsible to the development of malignant B-NHL in predisposed individuals[4,6,7,12-14,20] (Figure 1). The HCV oncogenic potential has been clearly identified in hepatocellular carcinoma and in a relevant number of patients with B-NHL[4,6,7,12-14,20-22] or thyroid cancer[23,24]. Comparably to the association between Helicobacter pylori infection and MALT lymphoma of the stomach, the same pathogenetic mechanism of chronic antigen stimulation producing malignant lymphoproliferation may be hypothesized for HCV-driven lymphomagenesis[4,6,7,12-14] (Figure 1). This important topic, in particular the possible role of HCV infection in B-NHL, was underlined in a recent review focusing on the proposed epidemiologic/virological guidelines to support a causative role for a given virus in human cancerogenesis[14].
The HCV-driven lymphomagenesis may be the result of various oncogenetic mechanisms that may be not mutually exclusive, through a multifactorial and multistep process[4,6,7,12-14]: chronic external stimulation by HCV antigens of B-cell receptors (CD19, CD21, CD81, B-cell receptors), in particular the high-affinity binding of HCV-E2 and CD81 may lead to bcl-2 activation; HCV replication in B-lymphocytes with oncogenic potential through viral proteins; the direct infection of B-lymphocytes by HCV may produce permanent genetic B-cell damage, the so-called mutator B-cell phenotype due to “hit and run” mechanism of cellular transformation. The potential role of viral penetration and replication in B-lymphocytes remains to be definitely elucidated; however, some studies supported the oncogenic role of HCV genome or viral proteins in B-lymphocytes[12-16].
More recently, a role of the B-cell-activating factor (BAFF or BLyS) in the pathogenesis of HCV-related lymphomagenesis has been suggested. BAFF is a specific cytokine of B-lymphocytes, which is essential for the development and survival of B-lymphocytes. A higher serum levels of BAFF are detectable in patients with HCV infection if compared to healthy controls, and more frequently in HCV-positive subjects developing lymphoproliferative disorders[15,16]; but the exact mechanisms of this enhanced concentration remain still to be deeply clarified. The evaluation of polymorphic variants of BAFF gene promoter suggested a possible explanation; namely, a particular allelic variant (-871T), possibly correlated with the BAFF gene increased transcriptional activity, was significantly more frequently found in HCV-positive subjects with than in those without MCs. As regards HCV-related NHL, increased levels of circulating osteopontin were associated with HCV-positive B-cell lymphoma. Moreover, the highest levels of osteoponin were observed among HCV-positive individuals with associated MC type II regardless the presence of B-NHL[15,16].
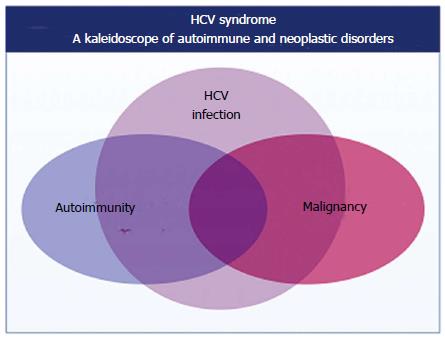
On the whole, HCV-infected patients represent one of the most useful models of study as regards the complex interactions between autoimmunity and oncogenesis in humans[4] (Figure 2).
The HCV biological activities, i.e., the hepato- and lymphotropism, are responsible for HCV syndrome, a mosaic of hepatic as well as organ-specific and systemic diseases[11] (Figures 1 and 3).
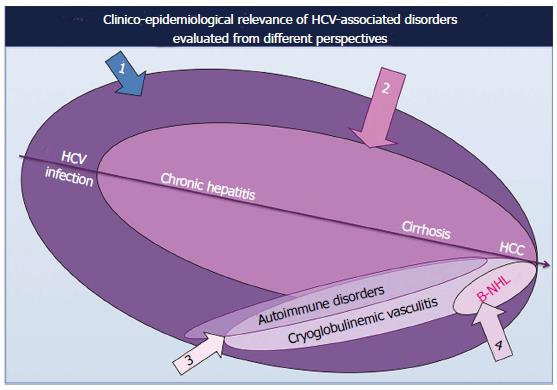
Several clinico-epidemiological studies published in the world literature demonstrated that the prevalence of HCV-related autoimmune/neoplastic disorders shows a manifest geographical heterogeneity[4,6,7,25]. This finding does not perfectly coincide with the varying prevalence of HCV infection in different parts of the world; therefore, we can hypothesize that HCV alone is not sufficient to produce the wide spectrum of diseases that may complicate the natural course of HCV infection. It is supposable that specific HCV genotypes, host genetic and/or environmental factors should cooperate in the pathogenesis of HCV syndrome[4,6,7,26,27]; even if the actual relevance of these putative co-factors should be fully elucidated.
Moreover, contrasting data as regards the prevalence of different HCV-associated disorders has been also reported among clinical studies on HCV-infected patients’ series from the same country, depending on specific specialization and/or investigative approach (sample sizes and choice of patients and/or control subjects) of different referring centers (Figure 3).
It is well known that the majority of individuals with HCV infection are asymptomatic, often for long-time periods, without consequences for their quality of life as well as the overall outcome; while a limited but relevant number of subjects may develop hepatic as well as extrahepatic diseases, often as late complication (Figure 1). The prolonged clinical follow-up of large series of HCV-positive patients suggests that the natural course of HCV infection may proceed through a multistage pathological process, usually from mild-moderate to clinically severe conditions, such as the MCs or malignant neoplasias[4,6,7] (Figures 1 and 3). However, it is possible that the slow progression of HCV syndrome in some individuals may be abruptly complicated by one of the most severe complications[4,28].
The symptom complex of HCV syndrome includes numerous autoimmune diseases and malignant neoplasias[11]. The statistical and clinical relevance of HCV association with different disorders is markedly variable; in this light, HCV-driven conditions can be classified in four groups according to the strength of association[4,11] (Table 1): level 1: the HCV is the etiological agent or it is present in a large percentage of patients; level 2: HCV is detectable in a statistically significant number of patients when compared to the general population, at least in some geographical areas, often demonstrated by clinical and laboratory investigations; level 3: a causative role of HCV has been suggested by some cohort studies or level 4: by isolated, anecdotal observations without definite pathogenetic link. The following paragraphs focuses on the main clinical and laboratory features of extra-hepatic manifestations of HCV syndrome.
| Strong association1 | Significant association2 | Possible association3 | Anecdotal association4 |
| Chonic hepatitis | B-cell NHL | Sicca syndrome/SS | PM/DM |
| Cirrhosis | Monoclonal gammopthies | Polyarthritis | PAN |
| HCC | Porphyria cutanea tarda | Pruritus | Behçet’s syndrome |
| Mixed cryoglobulinemia s./ | Glomerulonephritis | Osteosclerosis | Chronic urticaria |
| Cryoglobulinemic vasculitis | Autoimmune thyroiditis Papillary thyroid cancer Diabetes m. type 2 | Fibromyalgia Peripheral neuropathy Lung alveolitis Autoimmune hepatitis Cardiovascular inv. Lichen planus | Psorias Mooren corneal ulcer |
Extrahepatic manifestations of HCV syndrome are immune-mediated diseases[11]; MCs represents the most common condition, it represents a crossover between “benign” organ-specific and systemic autoimmune diseases, from one side, and malignant neoplastic disorders, from the other side[4,11] (Figure 1).
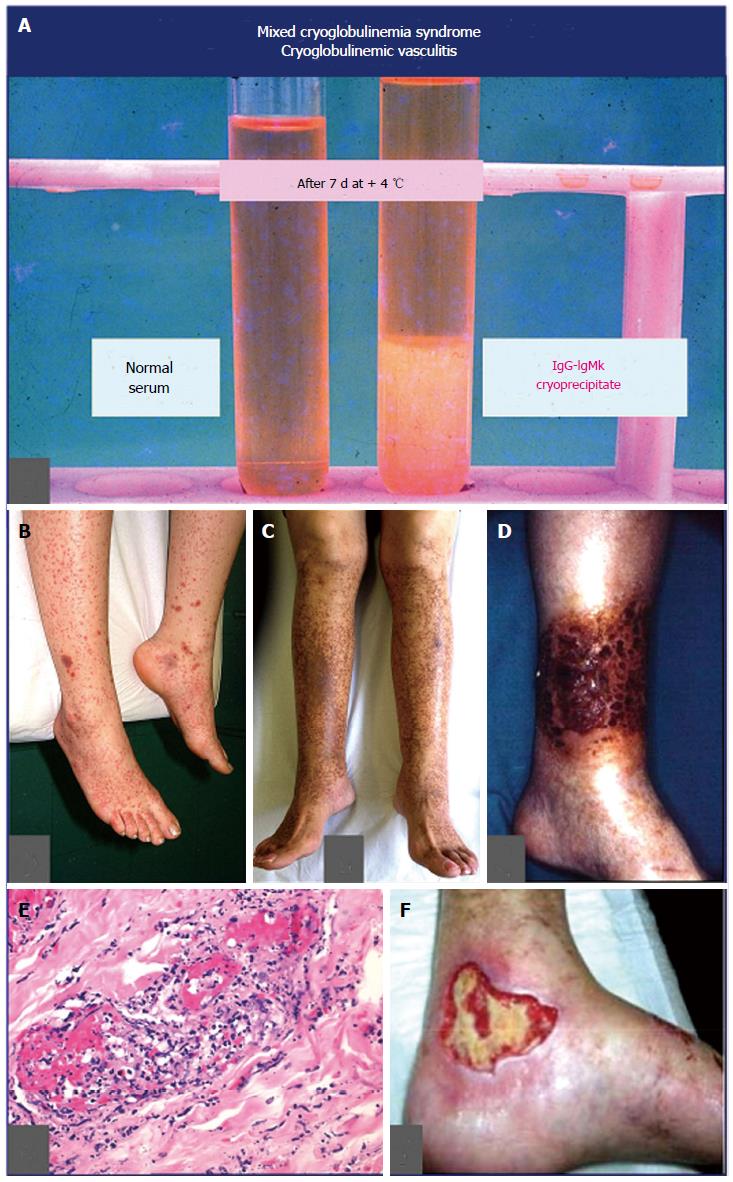
MCs, cryoglobulinemic vasculitis: MCs is commonly classified among small-vessel systemic vasculitides; thus, the terms MCs and CV should be referred to the same clinico-serological and pathological disorder[4-7,20]. The first one underlines the presence of the serological hallmark, i.e., mixed cryoglobulins, which characterize the disease, along with the “benign” B-cell lymphoproliferation, and the classical symptoms, namely purpura, weakness, and arthralgias[4-7,20]; while the term CV focuses on the pathological hallmark, i.e., the leucocytoclastic vasculitis, affecting small-medium sized vessels (Figure 4). Vascular damage is the consequence of the deposition of circulating cryo- and non-cryoprecipitable immune-complexes and complement; they produce both skin and internal organ damage[4-7,20] (Figures 1 and 4; Table 2 with MCs symptoms). CV represents a crossroads among immune-mediated disorders and malignancies (Figure 1); it is not rare to observe in a single patient during the natural course of the disease the entire spectrum of symptoms, from mild manifestations to overt vasculitic syndrome, and finally to most severe complications such as hepatic/renal failure, and/or cancer[4,28]. Compared to other systemic vasculitides, CV shows two distinctive symptoms, namely B-cell NHL and chronic hepatitis. While B-cell NHL affects a minority of patients (Table 2), generally as late complication, liver involvement is detectable in almost 3/4 of individuals[4-7,20]. The clinical course of chronic hepatitis is generally mild to moderate, and often asymptomatic for long time period; it may be complicated by cirrhosis and in some cases by hepatocellular carcinoma[4,28]. Renal involvement, usually a type I membranoproliferative glomerulonephritis, represents one of the most severe organ damage of CV[4,28,29]. Moreover, the rare, diffuse vasculitis is a life-threatening complication prognostically similar to classical systemic vasculitides[4,28]. Laboratory investigations are characterized by largely variable amounts of serum cryoglobulins and low complement with typically marked C4 reduction and normal C3; nonetheless, levels of cryocrit and complement are frequently not correlated with the severity/activity of CV[4]. Typically, B-lymphocyte expansion represents the substrate of CV, which in a number of patients may be complicated by overt lymphoma, usually a late disease manifestation[4,28,30]. The large majority of patients with CV show a chronic HCV infection; this association is particularly frequent in particular geographical areas, mainly Southern Europe[4]; generally in those countries where the presence of other HCV-associated extra-hepatic disorders are rather observed[4,25]. Conversely, other classical systemic vasculitides are significantly more frequent in other countries of Northern Europe and Northern America where HCV-associated CV is less frequently observed[4,31].
| Epidemiological features | |
| Mean age at disease onset ± SD, yr (range) | 55 ± 12 (29-72) |
| Mean disease duration ± SD, yr (range) | 12 ± 10 (1-40) |
| Female/male ratio | 3 |
| Clinical features | |
| Purpura | 98 |
| Weakness | 98 |
| Arthralgias | 91 |
| Arthritis (non-erosive) | 8 |
| Raynaud’s phenomenon | 32 |
| Sicca syndrome | 51 |
| Peripheral neuropathy | 81 |
| Renal involvement2 | 31 |
| Liver involvement | 73 |
| B cell non-Hodgkin’s lymphoma | 11 |
| Hepatocellular carcinoma | 3 |
| Serological and virological features | |
| Mean cryocrit (SD) | 4.4% (12) |
| Type II/type III mixed cryoglobulins | 2/1 |
| Mean C3 (SD) (nv 60-130 mg/dL) | 93 (30) |
| Mean C4 (SD) (nv 20-55 mg/dL) | 10 (12) |
| Anti-nuclear antibodies | 30 |
| Anti-mitochondrial antibodies | 9 |
| Anti-smooth muscle antibodies | 18 |
| Anti-extractable nuclear antigen antibodies | 8 |
| Anti-HCV antibodies ± HCV RNA | 92 |
| Anti-HBV antibodies | 32 |
| HBsAg | 1 |
Other systemic rheumatic diseases: CV is characterized by clinical polymorphism; therefore it is not rare to observe a clinical overlap between CV and other rheumatological disorders, mainly Sjögren’s syndrome or rheumatoid arthritis[4,28,31-35] (Table 1, Figure 1). Chronic arthritis, generally oligoarthritis, can develop during the natural course of HCV infection; the joint involvement commonly appears as non-erosive, less aggressive arthritis. HCV-associated MCs patients may develop mild oligoarthritis, on the contrary moderate-severe polyarthritis, comparable to classical rheumatoid arthritis, may be sporadically observed in HCV-infected patients treated with alpha-interferon[4,31,32]. On the other hand, given the relatively high prevalence of these conditions, we can observe a pure association of HCV infection with frank rheumatoid arthritis; the same association can be occasionally observed with primary Sjögren’s syndrome. While the possible involvement of HCV in the pathogenesis of Sjögren’s syndrome is still a controversial topic[4,31-35]. Differential diagnosis between CV and primary Sjögren’s syndrome may be very difficult in individual cases such patients with sicca syndrome, serum mixed cryoglobulins, HCV infection, and anti-RoSSA/LaSSB antibodies; these latter generally at low serum concentration[4,31-35]. This peculiar symptom complex that may satisfy classification criteria of both CV and primary Sjögren’s syndrome seems to identify a worse clinical variant, more frequently complicated by malignant lymphoma[31-35]. In these instances, it is preferable to consider these individuals as having Sjögren’s/MCs overlapping disease that should be treated according to individual clinical manifestations[31,32].
The presence in the clinical practice of overlapping MC/Sjögren’s suggests that HCV may trigger complex immunological alterations that, in genetically predisposed subjects, may cause various clinical phenotypes mimicking some well-known disorders, such as rheumatoid arthritis, Sjögren’s syndrome, and dermato-polymyositis[4,31,32] (Figure 3, Table 1).
Actually, the clinical value of other rheumatic disorders possibly triggered by HCV observed in limited patients’ series or anecdotal case reports deserve further investigations[4,31,32] (Table 1).
Endocrine disorders: Thyroiditis, diabetes type 2, and male gonadal dysfunction can be included among the most frequent endocrine alterations that may complicate HCV-positive individuals with and without MCs[4,31,36,37].
The same geographic heterogeneity that characterizes different disorders complicating HCV infection is reported for the prevalence of serum levels of anti-thyroid antibodies, which markedly varied from 2% to 48% of individuals in several cohort studies[36,38].
A possible explanation of this heterogeneity may be related to the variable contribution of environmental factors and host genetic predisposition among different patients’ populations, for example the iodine intake or the presence of other infectious factors[11,36,38].
In addition, hypothyroidism can be found in 2%-9% of subjects with HCV infection, generally as subclinical finding[36]; while various thyroid disorders and anti-thyroid antibodies are generally more frequently detected in hepatitis type C compared to hepatitis B or D[36]. In fact, the prevalence of these findings has been evaluated in a wide patients’ series with type C hepatitis compared to general population groups from geographical areas with variable iodine intake and subjects with hepatitis type B[36]. Hypothyroidism and autoimmune thyroiditis were found in patients with type C hepatitis in a statistically higher percentage if compared to control subjects; while comparable percentages of hyperthyroidism were detected. Comparable findings have been reported in another study evaluating thyroid alterations in HCV-associated MCs[36-38].
Overall, thyroid involvement can be considered as one of possible manifestations of MCs as well as of HCV syndrome[4,31,36-38].
Thyroid involvement should be periodically evaluated in HCV-infected patients to early diagnose the thyroid dysfunction and the possible malignant complications[36].
Diabetes type 2 can be considered another relevant endocrine disorder of the HCV syndrome, generally not correlated with presence/severity of hepatic involvement[36]. Preliminary, clinico-epidemiological studies reported an increased prevalence of diabetes type 2 in HCV-infected non-cirrhotic subjects compared with chronic hepatitis of different origin, a finding not confirmed in a subsequent report[36]. Successively, a case control study evaluated 564 Italian HCV-infected non-cirrhotic patients compared with 302 control individuals without history of drug or alcohol addiction, or positive for viral hepatitis serological markers, and 82 HBV-infected non-cirrhotic subjects[36]. Diabetes type 2 was significantly more frequent in HCV-infected non-cirrhotic patients compared to controls (12.6% vs 4.9% and 7%, respectively; P = 0.008). Of interest, prevalence of diabetes type 2 in HBV-infected non-cirrhotic subjects (7%) resulted within the age-adjusted range of prevalence rates for the Italian general population (4%)[36].
The clinical phenotypes of subjects with hepatitis and diabetes type 2 were quite different: individuals with non-cirrhotic HCV-positive diabetes type 2 were slightly older, with higher levels of triglycerides, blood pressure, and BMI, but lower concentrations of cholesterol HDL[36].
In addition, HCV-positive, non-cirrhotic subjects with diabetes type 2 had significantly lower BMI compared to control subjects with diabetes type 2 alone and significantly higher BMI (P < 0.05) compared to non-cirrhotic, non-diabetic HCV-infected subjects. Classical diabetes type 2 is characterized by the “metabolic syndrome” phenotype, i.e., overweight, older age, higher arterial pressure, and dyslipidemia. Conversely, non-cirrhotic, non-diabetic HCV-infected subjects resulted lean and with low levels of cholesterol LDL. These latter have been correlated with the hypobetalipoproteinemia induced by the binding competition of HCV with hepatocyte LDL receptors[36].
Patients with HCV chronic infection manifested a peculiar clinical variant different from the usual form of diabetes type 2. The classification of HCV-positive patients as diabetes type 2 is quite traditional; with the new pathogenetic information the boundaries between diabetes type 1, latent autoimmune diabetes, and diabetes type 2 are progressively weakening. In patients with HCV-associated MCs complicated by diabetes, an immune-mediated mechanism has been postulated for the endocrine disorder; a comparable autoimmune pathogenesis might be suggested also for diabetes observed in HCV-infected patients without MCs. This supposition is reinforced by the increasing observation of autoimmune alterations in patients with diabetes type 2[36,39-47]. HCV-related MCs patients showed abnormal serum levels of sex hormones[48]. Interestingly, erectile dysfunction was anecdotally observed in subjects with type C during antiviral treatment with interferon-alpha[49]. The putative relationship between HCV infection and gonadal dysfunction has been evaluated in 207 HCV-infected males (102 with MCs) in comparison with 207 age- and sex-matched individuals, selected randomly from 2010 subjects of Italian general population previously evaluated for the presence of erectile dysfunction[50]. The study adopted some important exclusion criteria, namely patients’ age over 55 years, recent treatment with interferon-alpha, presence of cardio-vascular and psychiatric disorders, diabetes, hypothyroidism, and renal failure.
HCV-positive patients showed a higher prevalence of erectile dysfunction compared to controls (P < 0.001). In addition, abnormally low testosterone plasma levels were detected in HCV-infected individuals with complicating erectile dysfunction. Of interest, erectile dysfunction as well as low serum testosterone was independent of the severity of hepatic damage. The alterations of sex hormones along with the frequent peripheral neuropathy might explain the erectile dysfunction[4,31,32].
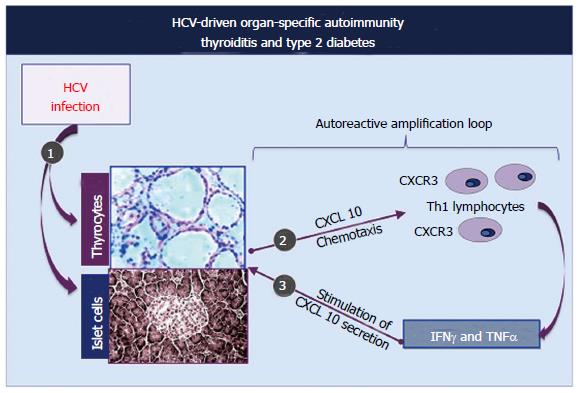
The above-mentioned findings are in keeping with the hypothesis of a possible role of patient’s hormonal status in immune mediated conditions triggered by HCV infection; we could hypothesize that low androgen levels may reduce endogenous depressive activity and consequently may amplify the proliferation of autoreactive B-lymphocytes triggered by HCV infection[4,31,32]. With regards the pathogenetic mechanisms responsible for HCV-related thyroid disorders, possibly HCV infection of thyrocytes may act by upregulating gene expression and secretion of CXCL10 (as previously shown in human hepatocytes) by recruiting Th1 lymphocytes, which secrete IFNγ and tumor necrosis factor alpha (TNF-α)[40,41].
In turn, these cytokines may induce the secretion of CXCL10 by thyrocytes, with consequent perpetuation of the immune cascade[36] (Figure 5).
The consequence may be the appearance of thyroid autoimmune diseases in subjects genetically predisposed. This pathogenetic hypothesis has been confirmed by a recent study that evaluated the serum levels of CXCL10 in patients with HCV-positive MCs, with and without autoimmune thyroid involvement[36,42], and also studying the association of thyroiditis with other autoimmune disorders[43-45].
Chronic immune-mediated inflammatory thyroid lesions may be responsible for the papillary thyroid cancer found in a significant number of HCV-infected individuals compared to controls[23,24,46].
Analogous pathogenetic mechanisms can be involved as consequence of HCV infection of pancreatic β-cells responsible for the up-regulation of CXCL10 gene expression and secretion[36] (Figure 5). The recruited Th1 lymphocytes, which secrete IFNγ and TNFα, amplify the secretion of CXCL10 by B-lymphocytes. The final result is the appearance of B-cell dysfunction, with the probable contribution of genetic predisposition. Both thyroid function abnormalities and diabetes type 2 should be considered among the manifestations of HCV syndrome[4,31,32,36].
It is opportune to evaluate these patients at regular intervals for the above endocrine disorders in order to identify subjects needing treatment and to early diagnose possible thyroid malignancies[47].
Porphyria cutanea tarda: Porphyria cutanea tarda (PCT) constitutes the most common clinical variant of porphyria; the disease is characterized by low activity of uroporphyrinogen decarboxylase (URO-D), the enzyme involved in the heme synthesis[51-55]. The URO-D deficiency is necessary but not sufficient for the clinical development of PCT, therefore possible pathogenetic co-factors have been proposed, including hepatotropic virus infection; this hypothesis was also suggested by the frequent chronic liver involvement in patients with PCT. Thus, the possible role of HCV has been investigated worldwide; numerous reports showed a broad range of prevalence of HCV infection in patients with PCT[51-55]. The HCV-associated PCT is particularly intriguing with regards the pathogenetic implications[52]. A direct role of HCV can be excluded considering the absence of alteration in porphyrine metabolism in HCV-positive patients without PCT[53]; while it is supposable that a cross-reactivity between host and HCV antigens and/or metabolic factors such as altered genes connected with iron metabolism[52] may contribute to a genetically-driven reactivity that may be relevant in individuals with PCT.
Renal involvement: Renal involvement represents a frequent complication of HCV-related MCs, which may seriously affect the overall outcome of these patients[4,28,29,31,32]. Glomerulonephritis is the result of immune complex glomerular deposition, mainly cryoglobulins. The actual pathogenetic relevance of HCV in the etiopathogenesis of glomerulonephritis remains not completely established; the detection of viral sequences in immune-complexes suggested an indirect role of HCV in the glomerular inflammation[4,28,29,31,32].
Moreover, a role of HCV has been suggested for tubulo-interstitial and glomerular renal damage in both transplanted and native kidney[56,57]. HCV-related glomerular injury may include different pathological patterns: mainly membranoproliferative glomerulonephritis (MPGN) in the presence/absence of cryoglobulinemia, while membranous nephropathy, rapidly progressive GN, immunotactoid and fibrillary GN, exudative-proliferative GN were less frequently observed[4,29]. Classical cryoglobulinemic type I MPGN is found in a significant proportion of HCV-associated MCs, while “primary” or clinically isolated MPGN is detected in less than one third of HCV-associated MPGN[56]. The latter variant has been observed mainly in Japan and United States[29,56,58]. Nevertheless, the actual prevalence of MPGN in HCV-positive patients without serum cryoglobulinemia is difficult to evaluate; possibly this condition may constitute a mild or subclinical variant of MCs, probably due to methodological difficulties in detecting circulating cryoglobulins[4]. It is not rare that MPGN is one of presenting symptoms of HCV-associated MCs, while the overt syndrome can appear as late manifestation of HCV infection[4,28]. Therefore, HCV-positive patients with apparently isolated GN should undergo to careful clinico-serological assessment in order to exclude possible hepatic and extrahepatic disorders, especially the MCs.
Miscellanea: Miscellanea of immune-mediated disorders may complicate HCV infection (Table 1); the association of lichen planus, in particular oral lesions, with HCV has been reported with a variable geographic prevalence[59,60]. Moreover, a wide number of mucocutaneous manifestations, generally as acute episode or chronic manifestation of well-known cutaneous diseases, are observed with variable prevalence in HCV-infected patients, often as limited series or anecdotal case reports[4,59,60] (Table 1). These symptoms may be the expressions of immune-mediated skin damage, triggered by viral proteins and possibly amplified by interferon-alpha treatment[4]. Peripheral nerve involvement is a frequent HCV-related manifestation, more often in patients with CV[4,61]; while involvement of central nervous system (CNS) is rarely observed. Vascular symptoms that may involve CNS may represent late manifestations of HCV syndrome, generally as comorbidities in subjects with particularly severe extra-hepatic symptoms treated with long-term corticosteroids. HCV-infected individuals may develop cardiovascular complications, which are not constantly confirmed by different studies[4,62,63]. Another controversial feature of HCV syndrome is the putative role of this virus in autoimmune hepatitis[4,31,32,64,65]. Circulating mixed cryoglobulins and some extrahepatic manifestations such as sicca syndrome, arthritis, and thyroiditis, can be observed in patients with autoimmune hepatitis; vice versa, HCV-infected patients may show serum non organ-specific autoantibodies[11,65]. Often, antigenic specificity of autoantibodies detectable in HCV-positive individuals presents only titer differences when compared to those found in “primary” autoimmune hepatitis[4,11]. Possibly, the heterogeneity of geographical distribution of HCV-associated autoimmune hepatitis[25] can be correlated to the variable cooperation of different causative agents, including HCV; therefore, this virus might be responsible for a distinct subset of AIH in infected individuals from specific geographical areas.
Following the discovery of HCV the oncogenic role of this virus has been established by several clinico-epidemiological and laboratory studies; besides hepatocellular carcinoma, HCV chronic infection may represent the trigger factor of papillary thyroid cancer and B-cell NHL.
Hepatocellular carcinoma: Hepatocellular carcinoma (HCC) is a primary hepatic cancer and occurs commonly as late complication of chronic hepatitis and cirrhosis[21,22]. Over the past two decades, the incidence of this malignancy is steeply increasing, analogously to the HCV prevalence worldwide[21,22]. In HCV-infected individuals HCC represents the most frequent malignancy[21,22]. The development and progression of HCC is a multistep process; a chronic insult, i.e., HCV, HBV, and alcohol, induces liver injury through oxygen species production, cellular DNA damage, endoplasmic reticulum stress, and necrosis of damaged hepatocytes. In this context, chronic inflammation and oxidative DNA damage favor the accumulation of mutations and epigenetic aberrations in hepatocytes or liver stem cells, which in turn may foster the development of dysplastic nodules and their malignant transformation to overt HCC[21,22]. This harmful complication can appear in the context of isolated type C hepatitis, as well as in patients with extrahepatic HCV-related manifestations[4,21,22]; even if the prevalence of HCC seems to be lower in HCV-associated MCs compared to the entire population of HCV-infected patients[4,28]. This intriguing topic needs to be confirmed by wider clinico-epidemiological investigations. Tentatively, it is possible that in patients with HCV-related extrahepatic diseases the rather frequent involvement of particular HCV genotypes such as the 2a/2b[4,26] and/or genetic background, as well as a different clinical course of the disease, including specific treatments such as immunomodulating drugs, may counteract the risk for developing this tumor.
Papillary thyroid cancer: Papillary thyroid cancer represents a rare neoplasia that may be associated to chronic HCV infection[23,24]. A significantly increased prevalence of thyroid cancer in type C hepatitis as well as in HCV-related MCs compared with controls was first noticed in 1999[23,46]; it was subsequently confirmed by a case control study reporting an increased prevalence of HCV infection in individuals with papillary thyroid cancer undergoing surgery[24]. In addition, high prevalence of papillary thyroid cancer in subjects with a past history of blood transfusions and/or liver disease seems to indirectly support the role of HCV in this malignancy[36]. However, a review of the literature shows discordant results, possibly owing to important epidemiological and methodological bias[36,66]. A recent study on large cohort of HCV-positive patients seem to confirm the increased prevalence of papillary thyroid cancer by excluding some possible biases such as iodine intake, gender and/or patients’ age[36]. In our studies, thyroid autoimmune alterations were more commonly found in patients developing thyroid papillary cancer irrespective of whether they had type C hepatitis alone or HCV-related MCs[23,24,46]. This observation suggests that immune-mediated thyroid alterations per se may be a predisposing condition for this malignancy. Although a possible causative role of HCV infection in papillary thyroid cancer is suggested by the above clinico-epidemiological studies, this association needs to be verified by further investigations.
B-cell NHL: Over the last two decades a putative role of HCV in B-lymphocyte lymphomagenesis has been progressively investigated considering the following observations: the lymphotropism of HCV was definitely demonstrated in individuals chronically infected, including those with HCV-related MCs[9,10]; in the same time, HCV revealed as the major etiological factor of MCs, a “benign” autoimmune-lymphoproliferative diseases that may be complicated by frank B-cell lymphomas[4,6,7,28,30,67,68]. Thus, the logical supposition was that the same virus might be also involved in the etiopathogenesis of ‘idiopathic’ B-cell NHL. In 1994, unexpected high rate of HCV ongoing infection in Italian series of patients with ‘idiopathic’ B-NHL was first reported[69]. Since this initial report numerous clinico-epidemiological and laboratory studies on different patients populations and in animal models, plainly established the causative role of this virus in a significantly higher percentage of patients with B-NHL compared to controls[69-103]. Once more, this association showed a geographical heterogeneity similarly to that observed for other HCV-associated diseases, especially the MCs; in fact, the association between HCV and B-cell lymphomas was not confirmed by other epidemiological studies[104-119]. This particular virus-related malignant lymphoproliferation may presents two major clinical variants: B-NHL as neoplastic manifestation of HCV-positive MCs, more often as late disease manifestation, or B-NHL complicating HCV infection without relevant extrahepatic manifestations[4,6,7,31,67,68]. The B-NHL of cryoglobulinemic patients can vary from extranodal, nodal or splenic marginal-zone lymphoma to diffuse large B-NHL or, less frequently, lymphoplasmacytic lymphoma/immunocytoma (LPL/Ic) and B-cell chronic lymphocytic leukemia (B-CLL)[4,120]. Lymphomas may be correlated to the expansion of peripheral B-lymphocytes and to lymphoid cell infiltrates frequently detectable in bone marrow and liver of patients with MCs[4,120]. These infiltrates containing lymphoid elements closely comparable to those characterizing B-CLL and LPL/Ic have been classified as “early lymphomas”[4,120]. However, different from overt malignant lymphomas, they usually remain stable for a long time and are followed by frank lymphatic malignancy in about 10% of individuals[4,120]. According to the above clinico-pathological features the term “monotypic lymphoproliferative disorder of undetermined significance (MLDUS)” has been proposed[4,120]. Interestingly, MLDUS observed in HCV-positive type II MC shows a significantly high incidence in those countries also characterized by frequent association between “idiopathic” B-NHL and HCV infection, as well as by rather high prevalence of genotype 2a/2c[4,120].
With regard to primary B-NHL a number of epidemiological studies (Table 3) confirmed the association of these malignancies with HCV in a significant proportion of patients; HCV-associated B-NHL showed the same geographical heterogeneity observed for other HCV-related extrahepatic disorders[69-119]. This epidemiological feature may reflect the multifactorial etiopathogenesis, including both genetic background and environmental cofactors, of this heterogeneous group of lymphatic malignancies[12-15]. Moreover, some discordant data among studies from the same country might be the consequence of recruitment bias (choice of patients and/or control subjects, sample sizes) at different referring centers as aforementioned (Figure 3). Whether an increased risk for all B-cell NHL is associated with HCV infection or only particular subtypes remains still open question[98]. Apart from the above epidemiological aspects, HCV can be included among other well known lymphotropic viruses, namely human T lymphotropic virus type I, Epstein Barr virus, human herpesvirus 8, and human immunodeficiency virus, responsible for a significant proportion of B-cell NHL[121]. Of interest, some meta-analysis studies of epidemiological investigations confirmed the strongly positive association between HCV infection and increased risk of B-NHL[90,98,122,123].
| Significant association1 | No association1 | ||||
| Ref. | Country | Prevalence2 | Ref. | Country | Prevalence2 |
| Ferri et al[69] | Italy | 34% (17/50) | Brind et al[104] | United Kingdom | 0% (0/63) |
| Luppi et al[70] | Italy | 42% (29/69) | Hanley et al[105] | United Kingdom | 0% (0/72) |
| Mazzaro et al[71] | Italy | 28% (56/199) | McColl et al[106] | Scotland | 0% (0/110) |
| Musolino et al[72] | Italy | 20.8% (5/24) | Thalen et al[107] | The Netherlands | 0% (0/115) |
| Silvestri et al[73] | Italy | 8.9% (42/470) | Ellenrieder et al[108] | Germany | 4.3% (3/69) |
| De Rosa et al[74] | Italy | 22.4 (59/263) | Timoraglu et al[109] | Turkey | 0% (0/48) |
| Zuckerman et al[75] | Mexico and United States | 22% (26/120) | Collier et al[110] | Canada | 0% (0/101) |
| Catassi et al[76] | Italy | 11.2% (16/143) | Shariff et al[111] | Canada | 2.3% (2/88) |
| Kashyap et al[77] | United States | 11.5% (36/312) | Udomsakdi-Auewarakul et al[112] | Thailand | 2.3% (3/130) |
| Luppi et al[78] | Italy | 22.3% (35/157) | Hausfater et al[113] | France | 1.83% (3/164) |
| Cucuianu et al[79] | Romania | 29.5% (20/68) | Isikdogan et al[114] | Turkey | 0% (0/119) |
| Vallisa et al[80] | Italy | 37.1% (65/175) | Giannoulis et al[115] | Greece | 1.9% (2/108) |
| Paydas et al[81] | Turkey | 11.4% (26/228) | Sonmez et al[116] | Turkey | 2.8% (3/109) |
| Harakati et al[82] | Saudi Arabia | 21.4% (12/56) | Okan et al[117] | Turkey | 3.1% (8/258) |
| Mizorogi et al[83] | Japan | 17% (17/100) | Park et al[118] | South Korea | 2.1% (5/235) |
| Zucca et al[84] | Switzerland | 9.4% (17/180) | Varma et al[119] | India | 1.75% (1/57) |
| 3Pioltelli et al[85] | Italy | 16% (48/300) | |||
| Sanchez Ruiz et al[86] | Spain | 11.7% (9/77) | |||
| Chindamo et al[87] | Brazil | 8.2% (9/109) | |||
| De Renzo et al[88] | Italy | 17.3% (39/227) | |||
| Imai et al[89] | Japan | 13.4% (21/156) | |||
| Gisbert et al[90] | Spain | 7% (7/99) | |||
| Mele et al[91] | Italy | 17.5% (70/400) | |||
| Yenice et al[92] | Turkey | 7.1% (6/84) | |||
| Iwata et al[93] | Japan | 11% (16/145) | |||
| Gisbert et al[94] | Spain | 5.8% (5/86) | |||
| Talamini et al[95] | Italy | 19.6% (44/225) | |||
| Cowgill et al[96] | Egypt | 42% (95/227) | |||
| Engels et al[97] | United States | 3.9% (32/813) | |||
| de Sanjose et al[98] | Spain | 3.9% (172/4784) | |||
| Spinelli et al[99] | Canada | 2.4% (19/795) | |||
| Chuang et al[100] | Taiwan | 11% (31/346) | |||
| Libra et al[101] | Italy | 19.7% (539/2736) | |||
| Kang et al[102] | South Korea | 2.8% (76/3932) | |||
| Nosotti et al[103] | Italy | 9.2% (19/207) | |||
| Range | 2.4%-42% | 0%-4.3% | |||
Finally, in HCV-infected patients with splenic marginal zone or indolent B-cell NHL, combined treatment with interferon-alpha and ribavirin may lead to HCV clearance and concomitant regression of lymphomas[124-126]. These observations clearly presuppose that the virus is the causative agent in at least some NHL subsets by directly driving the B-cell lymphoproliferation.
Considering the complexity of HCV syndrome because of its variable composition of clinical symptoms with specific pathogenetic, clinical, and prognostic characteristics, it is impossible to draw comprehensive therapeutical guidelines. In clinical practice, it can be useful to look at the therapeutical strategy developed for patients with MCs, which takes into account the different clinical variants of HCV syndrome[4,28,31,32]. This strategy is essentially based on three main levels of intervention (Figure 1): the etiological treatment by means of antiviral drugs directed at HCV eradication, the pathogenetic therapies with immunomodulating-antineoplastic drugs, and the pathogenetic/symptomatic therapies such as corticosteroids and plasmapheresis[4,28,31,32]. These three different therapeutic lines are not mutually exclusive; they are usefully employed in HCV-positive MCs, considering the composition and severity of clinical features observed in the single patient[4,28,31,32] (Figure 1). The etiological treatment, alone or in combination with immunosuppressors, may lead to HCV eradication and MCs remission[31,127-134]; as above mentioned the beneficial effect of HCV eradication is also observed in patients with B-cell NHL, as isolated condition or complicating the MCs[124-126]. In theory, antivirals should be regarded as the gold standard treatment in patients with overt HCV-associated clinical symptoms, considering the whole individual patient condition and the potential side effects or contraindications to these therapies[4,127-131]. The preemptive use of the novel direct antiviral drugs in HCV-infected individuals even in the absence of relevant clinical manifestations is a very critical issue, considering the necessary cost-benefit analysis.
On the other hand, clinico-biological parameters predictive of possible recovery of immune-system alterations after HCV eradication are still lacking. Combined pathogenetic and symptomatic therapies may be able to improve a single clinical manifestation of HCV syndrome; the clinical usefulness of these treatments has been largely reported in cryoglobulinemic patients, particularly for patients treated with anti-CD20 monoclonal antibody therapy[130,132-134]. In all cases, etiological, pathogenetic, and symptomatic treatments, in sequence or in combination, should be tailored on the single patient after a careful clinical evaluation[4,31,131-134]. Finally, long-term clinical monitoring of HCV-infected patients, including those with mild or asymptomatic clinical variants, is mandatory for a timely diagnosis and treatment of the most severe complications.
The hepato- and lymphotropism are the distinctive biological features of HCV responsible for a wide symptom complex including both hepatic and systemic autoimmune and neoplastic diseases. The strength of association largely varies among potentially HCV-driven disorders, a well as for a specific disease among patients’ series from different countries. Besides liver involvement, HCV represents the etiological agent of the majority patients with MCs, and may be implicated in a significant proportion of other autoimmune disorders and B-NHL.
A putative HCV-associated disease per se may constitute a clinical syndrome, characterized by a spectrum of clinico-serological variants; these latter can be regarded as the resulting phenotypes of a multifactorial and multistep process secondary to a variable combination of genetic, environmental, and infectious factors. In this context, HCV can be considered as one of possible causative agents producing distinct autoimmune or neoplastic disease subsets. Considering the frequent clinical overlap and the presence of multiple serum autoantibodies, it is frequent very difficult to make the differential diagnosis among “idiopathic” and HCV-associate autoimmune disorders.
The HCV syndrome is a multifaceted condition that encompasses the complex of HCV-related disorders. The syndrome may be considered as a continuum; this hypothesis is frequently suggested by the clinical history of individual patients that may develop most of HCV-driven immunological/neoplastic disorders over their clinical follow-up.
Future investigations should better define the boundaries of HCV syndrome, along with the actual etiopathogenetic role of this virus in different disorders and the involved, often unknown, co-factors, the effects of HCV eradication, and the correct therapeutic strategies for different HCV-related clinical symptoms.
Finally, considering the ongoing variations of the epidemiology of HCV infection and other possible co-factors, as well as the effects of the gradual improvement of therapeutical armamentarium, it is supposable that the spectrum of HCV syndrome, i.e., prevalence, clinical characteristics, and prognosis of different symptoms, might change over the time. The timely description of this evolving framework should be another intriguing issue of clinical investigations in the next future.
P- Reviewer: Kondo Y, Morizane R, Palazzi C S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4654] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 2. | Ferri C, Marzo E, Longombardo G, Lombardini F, Greco F, Bombardieri S. Alpha-interferon in the treatment of mixed cryoglobulinemia patients. International Cancer Update. Focus on Interferon Alfa 2b. Cannes, France. November 1-4, 1990. Eur J Cancer. 1991;27:81-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Pascual M, Perrin L, Giostra E, Schifferli JA. Hepatitis C virus in patients with cryoglobulinemia type II. J Infect Dis. 1990;162:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 203] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia--a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40:837-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 470] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Dammacco F, Sansonno D, Piccoli C, Racanelli V, D’Amore FP, Lauletta G. The lymphoid system in hepatitis C virus infection: autoimmunity, mixed cryoglobulinemia, and Overt B-cell malignancy. Semin Liver Dis. 2000;20:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995;88:115-126. [PubMed] |
| 8. | Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E, Mazzoni A, Pasero G, Bombardieri S, Highfield P. Association between hepatitis C virus and mixed cryoglobulinemia [see comment]. Clin Exp Rheumatol. 1991;9:621-624. [PubMed] |
| 9. | Zignego AL, Macchia D, Monti M, Thiers V, Mazzetti M, Foschi M, Maggi E, Romagnani S, Gentilini P, Bréchot C. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 320] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Ferri C, Monti M, La Civita L, Longombardo G, Greco F, Pasero G, Gentilini P, Bombardieri S, Zignego AL. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701-3704. [PubMed] |
| 11. | Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Pileri SA, Zignego AL. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig Liver Dis. 2007;39 Suppl 1:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Forghieri F, Luppi M, Barozzi P, Maffei R, Potenza L, Narni F, Marasca R. Pathogenetic mechanisms of hepatitis C virus-induced B-cell lymphomagenesis. Clin Dev Immunol. 2012;2012:807351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Hartridge-Lambert SK, Stein EM, Markowitz AJ, Portlock CS. Hepatitis C and non-Hodgkin lymphoma: the clinical perspective. Hepatology. 2012;55:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lunel F, Musset L, Cacoub P, Frangeul L, Cresta P, Perrin M, Grippon P, Hoang C, Valla D, Piette JC. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994;106:1291-1300. [PubMed] |
| 18. | Zignego AL, Ferri C, Giannelli F, Giannini C, Caini P, Monti M, Marrocchi ME, Di Pietro E, La Villa G, Laffi G. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G. Binding of hepatitis C virus to CD81. Science. 1998;282:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1551] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 20. | Gorevic PD. Rheumatoid factor, complement, and mixed cryoglobulinemia. Clin Dev Immunol. 2012;2012:439018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 585] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 22. | Flores A, Marrero JA. Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics. Clin Med Insights Oncol. 2014;8:71-76. [PubMed] |
| 23. | Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. JAMA. 1999;281:1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Barani L, Marchi S, Ferrannini E. Thyroid cancer in HCV-related chronic hepatitis patients: a case-control study. Thyroid. 2007;17:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Lenzi M, Johnson PJ, McFarlane IG, Ballardini G, Smith HM, McFarlane BM, Bridger C, Vergani D, Bianchi FB, Williams R. Antibodies to hepatitis C virus in autoimmune liver disease: evidence for geographical heterogeneity. Lancet. 1991;338:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Zignego AL, Ferri C, Giannini C, Monti M, La Civita L, Careccia G, Longombardo G, Lombardini F, Bombardieri S, Gentilini P. Hepatitis C virus genotype analysis in patients with type II mixed cryoglobulinemia. Ann Intern Med. 1996;124:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | De Re V, Caggiari L, Simula MP, De Vita S, Mazzaro C, Lenzi M, Massimo GM, Monti G, Ferri C, Zignego AL. Role of the HLA class II: HCV-related disorders. Ann N Y Acad Sci. 2007;1107:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Monti G, Pioltelli P, Saccardo F, Campanini M, Candela M, Cavallero G, De Vita S, Ferri C, Mazzaro C, Migliaresi S. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch Intern Med. 2005;165:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Ferri C, Sebastiani M, Saadoun D, Cacoub P. Cryoglobulinemia and hepatitis C virus. In, JWJ Bijsma, editor. EULAR compendium on rheumatic diseases, 2012. Chapter 42; London: BMJ Publishing Group Ltd 2012; 1042-1071. |
| 32. | Ferri C, Sebastiani M, Antonelli A, Colaci M, Manfredi A, Giuggioli D. Current treatment of hepatitis C-associated rheumatic diseases. Arthritis Res Ther. 2012;14:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Vitali C. Immunopathologic differences of Sjögren’s syndrome versus sicca syndrome in HCV and HIV infection. Arthritis Res Ther. 2011;13:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Ramos-Casals M, Loustaud-Ratti V, De Vita S, Zeher M, Bosch JA, Toussirot E, Medina F, Rosas J, Anaya JM, Font J. Sjögren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine (Baltimore). 2005;84:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002;46:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Antonelli A, Ferri C, Fallahi P, Giuggioli D, Nesti C, Longombardo G, Fadda P, Pampana A, Maccheroni M, Ferrannini E. Thyroid involvement in patients with overt HCV-related mixed cryoglobulinaemia. QJM. 2004;97:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Ferri C, Antonelli A, Mascia MT, Sebastiani M, Fallahi P, Ferrari D, Giunti M, Pileri SA, Zignego AL. B-cells and mixed cryoglobulinemia. Autoimmun Rev. 2007;7:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Antonelli A, Baj G, Marchetti P, Fallahi P, Surico N, Pupilli C, Malavasi F, Ferrannini E. Human anti-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes. 2001;50:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Antonelli A, Rotondi M, Fallahi P, Ferrari SM, Paolicchi A, Romagnani P, Serio M, Ferrannini E. Increase of CXC chemokine CXCL10 and CC chemokine CCL2 serum levels in normal ageing. Cytokine. 2006;34:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Santini E, Franceschini SS, Ferrannini E. Monokine induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma inducible T-cell alpha-chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-gamma agonists. J Clin Endocrinol Metab. 2009;94:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Antonelli A, Ferri C, Fallahi P, Ferrari SM, Sebastiani M, Ferrari D, Giunti M, Frascerra S, Tolari S, Franzoni F. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol. 2008;103:2488-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Antonelli A, Delle Sedie A, Fallahi P, Ferrari SM, Maccheroni M, Ferrannini E, Bombardieri S, Riente L. High prevalence of thyroid autoimmunity and hypothyroidism in patients with psoriatic arthritis. J Rheumatol. 2006;33:2026-2028. [PubMed] |
| 44. | Antonelli A, Fazzi P, Fallahi P, Ferrari SM, Ferrannini E. Prevalence of hypothyroidism and Graves disease in sarcoidosis. Chest. 2006;130:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Antonelli A, Ferri C, Fallahi P, Cazzato M, Ferrari SM, Sebastiani M, Ferrannini E. Clinical and subclinical autoimmune thyroid disorders in systemic sclerosis. Eur J Endocrinol. 2007;156:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Antonelli A, Ferri C, Fallahi P, Nesti C, Zignego AL, Maccheroni M. Thyroid cancer in HCV-related mixed cryoglobulinemia patients. Clin Exp Rheumatol. 2002;20:693-696. [PubMed] |
| 47. | Antonelli A, Bocci G, La Motta C, Ferrari SM, Fallahi P, Fioravanti A, Sartini S, Minuto M, Piaggi S, Corti A. Novel pyrazolopyrimidine derivatives as tyrosine kinase inhibitors with antitumoral activity in vitro and in vivo in papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:E288-E296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Ferri C, Cutolo M, Zignego AL, Longombardo G, Sulli A, Cavallaro D, Giusti M, Accardo S, Mazzocca A, Pasero G. Role of androgens in HCV-related mixed cryoglobulinemia. Arthritis Rheum. 1998;41:539A. |
| 49. | Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 322] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Ferri C, Bertozzi MA, Zignego AL. Erectile dysfunction and hepatitis C virus infection. JAMA. 2002;288:698-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Fargion S, Piperno A, Cappellini MD, Sampietro M, Fracanzani AL, Romano R, Caldarelli R, Marcelli R, Vecchi L, Fiorelli G. Hepatitis C virus and porphyria cutanea tarda: evidence of a strong association. Hepatology. 1992;16:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 205] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Ferri C, Baicchi U, la Civita L, Greco F, Longombardo G, Mazzoni A, Careccia G, Bombardieri S, Pasero G, Zignego AL. Hepatitis C virus-related autoimmunity in patients with porphyria cutanea tarda. Eur J Clin Invest. 1993;23:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | O’Reilly FM, Darby C, Fogarty J, O’Moore R, Courtney MG, O’Connor J, Kay EW, Leader M, Fielding JF, Murphy GM. Porphyrin metabolism in hepatitis C infection. Photodermatol Photoimmunol Photomed. 1996;12:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Cribier B, Chiaverini C, Dali-Youcef N, Schmitt M, Grima M, Hirth C, Lacour JP, Chosidow O. Porphyria cutanea tarda, hepatitis C, uroporphyrinogen decarboxylase and mutations of HFE gene. A case-control study. Dermatology. 2009;218:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | di Belgiojoso GB, Ferrario F, Landriani N. Virus-related glomerular diseases: histological and clinical aspects. J Nephrol. 2002;15:469-479. [PubMed] |
| 57. | Daghestani L, Pomeroy C. Renal manifestations of hepatitis C infection. Am J Med. 1999;106:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Uchiyama-Tanaka Y, Mori Y, Kishimoto N, Nose A, Kijima Y, Nagata T, Umeda Y, Masaki H, Matsubara H, Iwasaka T. Membranous glomerulonephritis associated with hepatitis C virus infection: case report and literature review. Clin Nephrol. 2004;61:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Nagao Y, Sata M. Hepatitis C virus and lichen planus. J Gastroenterol Hepatol. 2004;19:1101-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Halawani MR. Dermatological manifestations of hepatitis C virus infection in Saudi Arabia. Saudi Med J. 2014;35:531-537. [PubMed] |
| 61. | Ferri C, La Civita L, Cirafisi C, Siciliano G, Longombardo G, Bombardieri S, Rossi B. Peripheral neuropathy in mixed cryoglobulinemia: clinical and electrophysiologic investigations. J Rheumatol. 1992;19:889-895. [PubMed] |
| 62. | Ishizaka N, Ishizaka Y, Takahashi E, Tooda Ei, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 63. | Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | García-Buey L, García-Monzón C, Rodriguez S, Borque MJ, García-Sánchez A, Iglesias R, DeCastro M, Mateos FG, Vicario JL, Balas A. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Ferri C, Longombardo G, La Civita L, Greco F, Lombardini F, Cecchetti R, Cagianelli MA, Marchi S, Monti M, Zignego AL. Hepatitis C virus chronic infection as a common cause of mixed cryoglobulinaemia and autoimmune liver disease. J Intern Med. 1994;236:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Fallahi P, Ferrari SM, Giuggioli D, Manfredi A, Mancusi C, Fabiani S, Centanni M, Marchi S, Ferri C, Antonelli A. Thyroid involvement in hepatitis C - associated mixed cryoglobulinemia. Hormones (Athens. ). 2014;13:16-23. [PubMed] |
| 67. | Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sulfaro S, Franzin F, Tulissi P, Moretti M. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047-3053. [PubMed] |
| 68. | Ferri C, Monti M, La Civita L, Careccia G, Mazzaro C, Longombardo G, Lombardini F, Greco F, Pasero G, Bombardieri S. Hepatitis C virus infection in non-Hodgkin’s B-cell lymphoma complicating mixed cryoglobulinaemia. Eur J Clin Invest. 1994;24:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E, Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol. 1994;88:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Luppi M, Grazia Ferrari M, Bonaccorsi G, Longo G, Narni F, Barozzi P, Marasca R, Mussini C, Torelli G. Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia. 1996;10:351-355. [PubMed] |
| 71. | Mazzaro C, Zagonel V, Monfardini S, Tulissi P, Pussini E, Fanni M, Sorio R, Bortolus R, Crovatto M, Santini G. Hepatitis C virus and non-Hodgkin’s lymphomas. Br J Haematol. 1996;94:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Musolino C, Campo S, Pollicino T, Squadrito G, Spatari G, Raimondo G. Evaluation of hepatitis B and C virus infections in patients with non-Hodgkin’s lymphoma and without liver disease. Haematologica. 1996;81:162-164. [PubMed] |
| 73. | Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296-4301. [PubMed] |
| 74. | De Rosa G, Gobbo ML, De Renzo A, Notaro R, Garofalo S, Grimaldi M, Apuzzo A, Chiurazzi F, Picardi M, Matarazzo M. High prevalence of hepatitis C virus infection in patients with B-cell lymphoproliferative disorders in Italy. Am J Hematol. 1997;55:77-82. [PubMed] |
| 75. | Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 283] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Catassi C, Fabiani E, Coppa GV, Gabrielli A, Centurioni R, Leoni P, Barbato M, Viola F, Martelli M, De Renzo A. High prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma at the onset. Preliminary results of an Italian multicenter study. Recenti Prog Med. 1998;89:63-67. [PubMed] |
| 77. | Kashyap A, Nademanee A, Molina A. Hepatitis C and B-cell lymphoma. Ann Intern Med. 1998;128:695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Luppi M, Longo G, Ferrari MG, Barozzi P, Marasca R, Morselli M, Valenti C, Mascia T, Vandelli L, Vallisa D. Clinico-pathological characterization of hepatitis C virus-related B-cell non-Hodgkin’s lymphomas without symptomatic cryoglobulinemia. Ann Oncol. 1998;9:495-498. [PubMed] |
| 79. | Cucuianu A, Patiu M, Duma M, Basarab C, Soritau O, Bojan A, Vasilache A, Mates M, Petrov L. Hepatitis B and C virus infection in Romanian non-Hodgkin’s lymphoma patients. Br J Haematol. 1999;107:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Vallisa D, Bertè R, Rocca A, Civardi G, Giangregorio F, Ferrari B, Sbolli G, Cavanna L. Association between hepatitis C virus and non-Hodgkin’s lymphoma, and effects of viral infection on histologic subtype and clinical course. Am J Med. 1999;106:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Paydas S, Kiliç B, Sahin B, Buğdayci R. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders in Southern Turkey. Br J Cancer. 1999;80:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Harakati MS, Abualkhair OA, Al-Knawy BA. Hepatitis C Virus infection in Saudi Arab patients with B-cell non-Hodgkin’s lymphoma. Saudi Med J. 2000;21:755-758. [PubMed] |
| 83. | Mizorogi F, Hiramoto J, Nozato A, Takekuma Y, Nagayama K, Tanaka T, Takagi K. Hepatitis C virus infection in patients with B-cell non-Hodgkin’s lymphoma. Intern Med. 2000;39:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Zucca E, Roggero E, Maggi-Solcà N, Conconi A, Bertoni F, Reilly I, Castelli D, Pedrinis E, Piffaretti JC, Cavalli F. Prevalence of Helicobacter pylori and hepatitis C virus infections among non-Hodgkin’s lymphoma patients in Southern Switzerland. Haematologica. 2000;85:147-153. [PubMed] |
| 85. | Pioltelli P, Gargantini L, Cassi E, Santoleri L, Bellati G, Magliano EM, Morra E. Hepatitis C virus in non-Hodgkin’s lymphoma. A reappraisal after a prospective case-control study of 300 patients. Lombart Study Group of HCV-Lymphoma. Am J Hematol. 2000;64:95-100. [PubMed] |
| 86. | Sánchez Ruiz AC, Yebra Bango M, Portero F, Provencio Pulla M, Miralles Flores C, España Saz P. [Prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma]. Med Clin (Barc). 2001;116:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Chindamo MC, Spector N, Segadas JA, Pimenta G, Vanderborght B, Morais JC, Milito C, Moraes Coelho HS. Prevalence of hepatitis C infection in patients with non-Hodgkin’s lymphomas. Oncol Rep. 2002;9:657-659. [PubMed] |
| 88. | De Renzo A, Persico E, de Marino F, di Giacomo Russo G, Notaro R, di Grazia C, Picardi M, Santoro L, Torella R, Rotoli B. High prevalence of hepatitis G virus infection in Hodgkin’s disease and B-cell lymphoproliferative disorders: absence of correlation with hepatitis C virus infection. Haematologica. 2002;87:714-718; discussion 718. [PubMed] |
| 89. | Imai Y, Ohsawa M, Tanaka H, Tamura S, Sugawara H, Kuyama J, Fukuda K, Yonezawa T, Matsuzawa Y. High prevalence of HCV infection in patients with B-cell non-Hodgkin’s lymphoma: comparison with birth cohort- and sex-matched blood donors in a Japanese population. Hepatology. 2002;35:974-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Mele A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Liso V. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 92. | Yenice N, Güllük F, Arican N, Türkmen S. HCV prevalence in Hodgkin and non-Hodgkin lymphoma cases. Turk J Gastroenterol. 2003;14:173-176. [PubMed] |
| 93. | Iwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004;89:368-370. [PubMed] |
| 94. | Gisbert JP, García-Buey L, Arranz R, Blas C, Pinilla I, Khorrami S, Acevedo A, Borque MJ, Pajares JM, Fernández-Rañada JM. The prevalence of hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Eur J Gastroenterol Hepatol. 2004;16:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Talamini R, Montella M, Crovatto M, Dal Maso L, Crispo A, Negri E, Spina M, Pinto A, Carbone A, Franceschi S. Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Cowgill KD, Loffredo CA, Eissa SA, Mokhtar N, Abdel-Hamid M, Fahmy A, Strickland GT. Case-control study of non-Hodgkin’s lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol. 2004;33:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Engels EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, Whitby D, Colt JS, Hartge P. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer. 2004;111:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, Spinelli JJ, Zheng T, Zhang Y, Franceschi S. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 99. | Spinelli JJ, Lai AS, Krajden M, Andonov A, Gascoyne RD, Connors JM, Brooks-Wilson AR, Gallagher RP. Hepatitis C virus and risk of non-Hodgkin lymphoma in British Columbia, Canada. Int J Cancer. 2008;122:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Chuang SS, Liao YL, Chang ST, Hsieh YC, Kuo SY, Lu CL, Hwang WS, Lin IH, Tsao CJ, Huang WT. Hepatitis C virus infection is significantly associated with malignant lymphoma in Taiwan, particularly with nodal and splenic marginal zone lymphomas. J Clin Pathol. 2010;63:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Libra M, Polesel J, Russo AE, De Re V, Cinà D, Serraino D, Nicoletti F, Spandidos DA, Stivala F, Talamini R. Extrahepatic disorders of HCV infection: a distinct entity of B-cell neoplasia? Int J Oncol. 2010;36:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Kang J, Cho JH, Suh CW, Lee DH, Oh HB, Sohn YH, Chi HS, Park CJ, Jang SS, Lee KH. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Nosotti L, D’Andrea M, Pitidis A, Pimpinelli F, Dessanti ML, Pisani F, Vignally P, Petti MC. Hepatitis C virus infection prevalence and liver dysfunction in a cohort of B-cell non-Hodgkin’s lymphoma patients treated with immunochemotherapy. Scand J Infect Dis. 2012;44:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Brind AM, Watson JP, Burt A, Kestevan P, Wallis J, Proctor SJ, Bassendine MF. Non-Hodgkin’s lymphoma and hepatitis C virus infection. Leuk Lymphoma. 1996;21:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Hanley J, Jarvis L, Simmonds P, Parker A, Ludlam C. HCV and non-Hodgkin lymphoma. Lancet. 1996;347:1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | McColl MD, Singer IO, Tait RC, McNeil IR, Cumming RL, Hogg RB. The role of hepatitis C virus in the aetiology of non-Hodgkins lymphoma--a regional association? Leuk Lymphoma. 1997;26:127-130. [PubMed] |
| 107. | Thalen DJ, Raemaekers J, Galama J, Cooreman MP. Absence of hepatitis C virus infection in non-Hodgkin’s lymphoma. Br J Haematol. 1997;96:880-881. [PubMed] |
| 108. | Ellenrieder V, Weidenbach H, Frickhofen N, Michel D, Prümmer O, Klatt S, Bernas O, Mertens T, Adler G, Beckh K. HCV and HGV in B-cell non-Hodgkin’s lymphoma. J Hepatol. 1998;28:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Timurağlu A, Colak D, Oğünç D, Karadoğan I, Undar L. Hepatitis C virus association with non-Hodgkin’s lymphoma. Haematologia (Budap). 1999;29:301-304. [PubMed] |
| 110. | Collier JD, Zanke B, Moore M, Kessler G, Krajden M, Shepherd F, Heathcote J. No association between hepatitis C and B-cell lymphoma. Hepatology. 1999;29:1259-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Shariff S, Yoshida EM, Gascoyne RD, Le N, Connors JM, Middleton PJ, Shenkier TN. Hepatitis C infection and B-cell non-Hodgkin’s lymphoma in British Columbia: a cross-sectional analysis. Ann Oncol. 1999;10:961-964. [PubMed] |
| 112. | Udomsakdi-Auewarakul C, Auewarakul P, Sukpanichnant S, Muangsup W. Hepatitis C virus infection in patients with non-Hodgkin lymphoma in Thailand. Blood. 2000;95:3640-3641. [PubMed] |
| 113. | Hausfater P, Cacoub P, Sterkers Y, Thibault V, Amoura Z, Nguyen L, Ghillani P, Leblond V, Piette JC. Hepatitis C virus infection and lymphoproliferative diseases: prospective study on 1,576 patients in France. Am J Hematol. 2001;67:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Isikdogan A, Ayyildiz O, Dursun M, Tiftik N, Batun S, Muftuoglu E. Hepatitis C virus in patients with non-Hodgkin’s lymphoma in southeastern Anatolia region of Turkey: a prospective case-control study of 119 patients. Leuk Lymphoma. 2003;44:1745-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Giannoulis E, Economopoulos T, Mandraveli K, Giannoulis K, Nikolaides C, Zervou E, Papageorgiou E, Zoulas D, Tourkantonis A, Giannopoulos G. The prevalence of hepatitis C and hepatitis G virus infection in patients with B cell non-Hodgkin lymphomas in Greece: a Hellenic Cooperative Oncology Group Study. Acta Haematol. 2004;112:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Sonmez M, Bektas O, Yilmaz M, Durmus A, Akdogan E, Topbas M, Erturk M, Ovali E, Omay SB. The relation of lymphoma and hepatitis B virus/hepatitis C virus infections in the region of East Black Sea, Turkey. Tumori. 2007;93:536-539. [PubMed] |
| 117. | Okan V, Yilmaz M, Bayram A, Kis C, Cifci S, Buyukhatipoglu H, Pehlivan M. Prevalence of hepatitis B and C viruses in patients with lymphoproliferative disorders. Int J Hematol. 2008;88:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Park SC, Jeong SH, Kim J, Han CJ, Kim YC, Choi KS, Cho JH, Lee M, Jung HH, Ki SS. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Varma S, Menon MC, Garg A, Malhotra P, Sharma A, Chawla YK, Dhiman RK. Hepatitis C virus infection among patients with non-Hodgkin’s lymphoma in northern India. Hepatol Int. 2011;5:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 120. | Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 121. | Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 122. | Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 123. | Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 124. | Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 560] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 125. | Mazzaro C, De Re V, Spina M, Dal Maso L, Festini G, Comar C, Tirelli U, Pozzato G. Pegylated-interferon plus ribavirin for HCV-positive indolent non-Hodgkin lymphomas. Br J Haematol. 2009;145:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 126. | Arcaini L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJ, Bernuzzi P, Merli M, Varettoni M, Chiappella A, Ambrosetti A. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol. 2014;25:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 127. | Cacoub P, Lidove O, Maisonobe T, Duhaut P, Thibault V, Ghillani P, Myers RP, Leger JM, Servan J, Piette JC. Interferon-alpha and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2002;46:3317-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 128. | Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus--associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54:3696-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 129. | Mazzaro C, Zorat F, Caizzi M, Donada C, Di Gennaro G, Maso LD, Carniello G, Virgolini L, Tirelli U, Pozzato G. Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study. J Hepatol. 2005;42:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Pietrogrande M, De Vita S, Zignego AL, Pioltelli P, Sansonno D, Sollima S, Atzeni F, Saccardo F, Quartuccio L, Bruno S. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev. 2011;10:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 131. | Gragnani L, Fabbrizzi A, Triboli E, Urraro T, Boldrini B, Fognani E, Piluso A, Caini P, Ranieri J, Monti M. Triple antiviral therapy in hepatitis C virus infection with or without mixed cryoglobulinaemia: a prospective, controlled pilot study. Dig Liver Dis. 2014;46:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 132. | Ferri C, Cacoub P, Mazzaro C, Roccatello D, Scaini P, Sebastiani M, Tavoni A, Zignego AL, De Vita S. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun Rev. 2011;11:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 133. | De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 134. | Dammacco F, Tucci FA, Lauletta G, Gatti P, De Re V, Conteduca V, Sansonno S, Russi S, Mariggiò MA, Chironna M. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood. 2010;116:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |