Published online Sep 21, 2019. doi: 10.3748/wjg.v25.i35.5334
Peer-review started: May 8, 2019
First decision: July 22, 2019
Revised: August 8, 2019
Accepted: August 19, 2019
Article in press: August 19, 2019
Published online: September 21, 2019
Although pathological response is a common endpoint used to assess the efficacy of neoadjuvant chemotherapy (NAC) for gastric cancer, the problem of a low rate of concordance from evaluations among pathologists remains unresolved. Moreover, there is no globally accepted consensus regarding the optimal evaluation. A previous study based on a clinical trial suggested that pathological response measured using digitally captured virtual microscopic slides predicted patients’ survival well. However, the pathological concordance rate of this approach and its usefulness in clinical practice were unknown.
To investigate the prognostic utility of pathological response measured using digital microscopic slides in clinical practice.
We retrospectively evaluated pathological specimens of gastric cancer patients who underwent NAC followed by surgery and achieved R0 resection between March 2009 and May 2015. Residual tumor area and primary tumor beds were measured in one captured image slide, which contained the largest diameter of the resected specimens. We classified patients with < 10% residual tumor relative to the primary tumorous area as responders, and the rest as non-responders; we then compared overall survival (OS) and relapse-free survival (RFS) between these two groups. Next, we compared the prognostic utility of this method using conventional Japanese criteria.
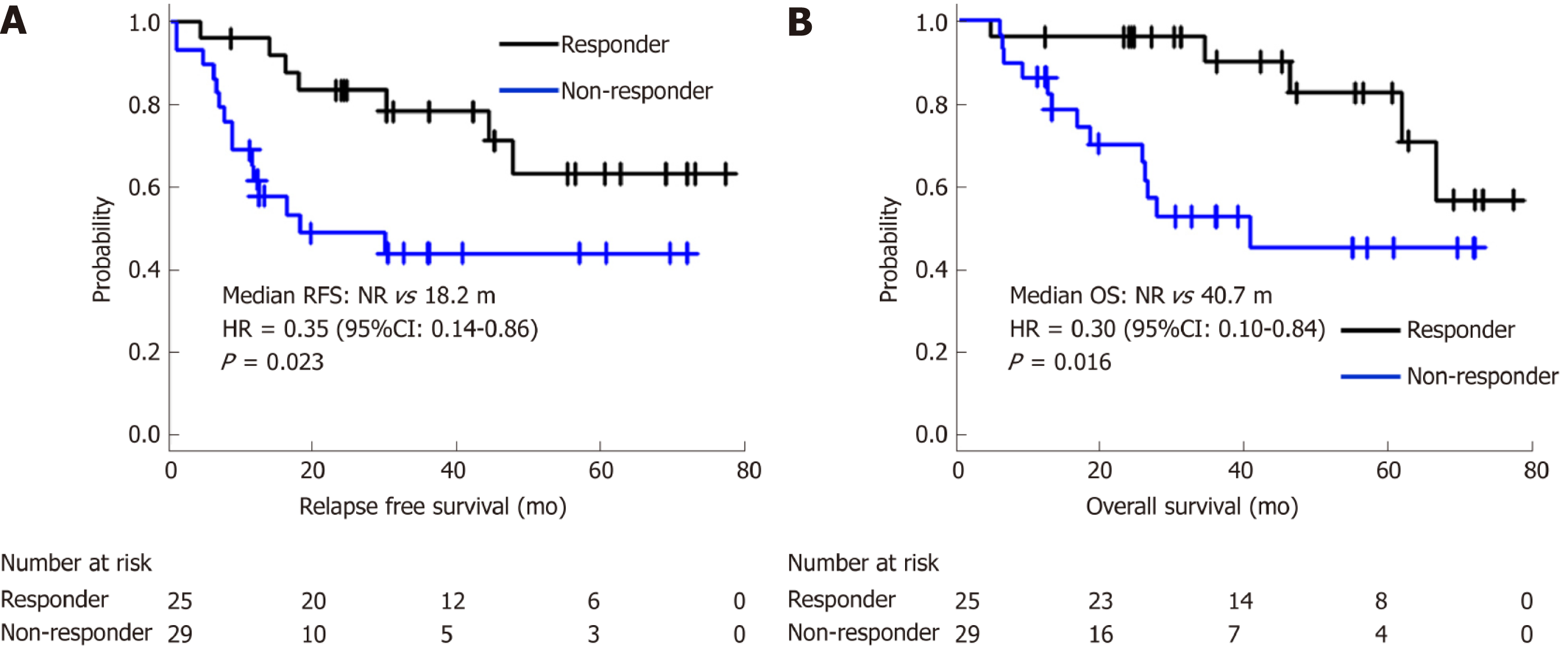
Fifty-four patients were evaluated. The concordance rate between two evaluators was 96.2%. Median RFS of 25 responders and 29 non-responders was not reached (NR) vs 18.2 mo [hazard ratio (HR) = 0.35, P = 0.023], and median OS was NR vs 40.7 mo (HR = 0.3, P = 0.016), respectively. This prognostic value was statistically significant even after adjustment for age, eastern cooperative oncology group performance status, macroscopic type, reason for NAC, and T- and N-classification (HR = 0.23, P = 0.018). This result was also observed even in subgroup analyses for different macroscopic types (Borrmann type 4/non-type 4) and histological types (differentiated/undifferentiated). Moreover, the adjusted HR for OS between responders and non-responders was lower in this method than that in the conventional histological evaluation of Japanese Classification of Gastric Carcinoma criteria (0.23 vs 0.39, respectively).
The measurement of pathological response using digitally captured virtual microscopic slides may be useful in clinical practice.
Core tips: Although pathological response is commonly evaluated to assess the efficacy of neoadjuvant chemotherapy for gastric cancer in clinical practice, the results evaluated by pathologists are sometimes discordant. A previous study suggested that pathological evaluation using digital virtual microscopic slides might be useful. However, the application of this approach to clinical practice was not investigated. We thus assessed the utility of this method in clinical practice and the concordance rate between evaluators, and compared the usefulness of this method with conventional histological evaluation using Japanese Classification of Gastric Carcinoma criteria.
- Citation: Kawai S, Shimoda T, Nakajima T, Terashima M, Omae K, Machida N, Yasui H. Pathological response measured using virtual microscopic slides for gastric cancer patients who underwent neoadjuvant chemotherapy. World J Gastroenterol 2019; 25(35): 5334-5343
- URL: https://www.wjgnet.com/1007-9327/full/v25/i35/5334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i35.5334
Gastric cancer is common, being the third leading cause of cancer-related death globally. Its incidence was estimated to be 1033701 new cases and 782685 deaths due to it occurred in 2018[1]. Of these, 49% were from East Asia, including China, Japan, and South Korea. Although standard treatments for locally advanced gastric cancer differ from one country to the next, surgery has a key role for curative treatment. Despite the application of multimodal treatments, the relapse rate is still high, resulting in a poor prognosis. Type 4 tumor, large type 3 tumor, and extended nodal metastasis, such as bulky lymph nodes or positive para-aortic lymph nodes, in particular were associated with high recurrence rates, even if curative resection was achieved[2,3]. To improve survival, several perioperative treatment strategies have been investigated. Whereas neoadjuvant chemotherapy (NAC) is considered as a standard therapy in the Western world, it is still exploratory and normally investigated in clinical trials in Japan[4-6].
Although progression-free survival (PFS) or overall survival (OS) is often used as an endpoint in clinical trials of gastric cancer treated with NAC followed by surgery, a long period is required to confirm these outcomes. As an alternative, the response rate based on the Response Evaluation Criteria in Solid Tumor (RECIST) guidelines is one of the short-term endpoints[7], but is not applicable to gastric cancer without measurable lesions. Moreover, there has been some debate about its prognostic utility[8].
Pathological response, which is commonly used for a surrogate marker for PFS and OS after surgery globally, is evaluated microscopically in resected specimens of the stomach and estimated based on the percentage of the residual tumor area relative to the primary tumor bed. Although many clinical trials have used pathological response as an endpoint, no globally accepted consensus has been reached regarding the optimal cut-off percentage to classify individuals as responders. Various definitions regarding the cut-off percentage, such as 10%[9,10], 40%[11], 50%[12], and 67%[13], were used in previous clinical trials. Moreover, this subjective evaluation by pathologists was shown to have limited reproducibility.
Nakamura et al[14] retrospectively evaluated pathological specimens digitally captured on virtual microscopic slides from four clinical trials of NAC for gastric cancer (JCOG0001[6], JCOG0002[2], JCOG0210[4], and JCOG0405[5]), and concluded that pathological response using a 10% cut-off on virtual microscopic slides was the best prognostic marker in this method for patients who achieved R0 resection, rather than a cut-off of 33%, 50%, or 67%. However, this study included only patients with type 4 tumor, large type 3 tumor, or extended nodal metastasis. In clinical practice, gastric cancer patients with esophageal or other organic invasion sometimes receive NAC before surgery to avoid an extended operation. Moreover, this study did not report the rate of concordance between pathologists and did not compare the prognostic utility of this cut-off with the histological evaluation criteria of the 14th Japanese Classification of Gastric Carcinoma (JCGC)[15] generally used in Japanese clinical practice. Kurokawa et al[8] reported that the OS of patients who achieved a histological response of JCGC of grade 1b, 2, or 3 was significantly longer than that of those who did not.
Here, we examine whether the 10% cut-off for pathological response evaluated using virtual microscopic slides is useful as a prognostic marker even in clinical practice and compare the usefulness of this approach with conventional JCGC criteria.
Sixty-one patients with gastric or esophagogastric junction adenocarcinoma who received NAC followed by surgery at Shizuoka Cancer Center between March 2009 and May 2015 were retrospectively identified from medical records. Of these, seven patients in whom R0 resection was not achieved were excluded. This study was approved by the Institutional Review Committee of Shizuoka Cancer Center (Shizuoka, Japan) and met the standards set forth in the Declaration of Helsinki.
Histological evaluations of JCGC criteria were performed as described previously[15] and were classified into five categories according to the proportion of the residual tumor relative to the primary tumorous area: grade 3, no viable tumor cells remain; grade 2, viable tumor cells remain in less than one-third of the primary tumorous area; grade 1b, viable tumor cells remain in more than one-third but less than two-thirds of the tumorous area; grade 1a, viable tumor cells occupy more than two-thirds of the tumorous area; and grade 0, no evidence of a treatment effect. The primary tumor area was defined by microscopic findings such as necrosis, foamy macrophage accumulation, and interstitial fibrosis below the submucosal layer.
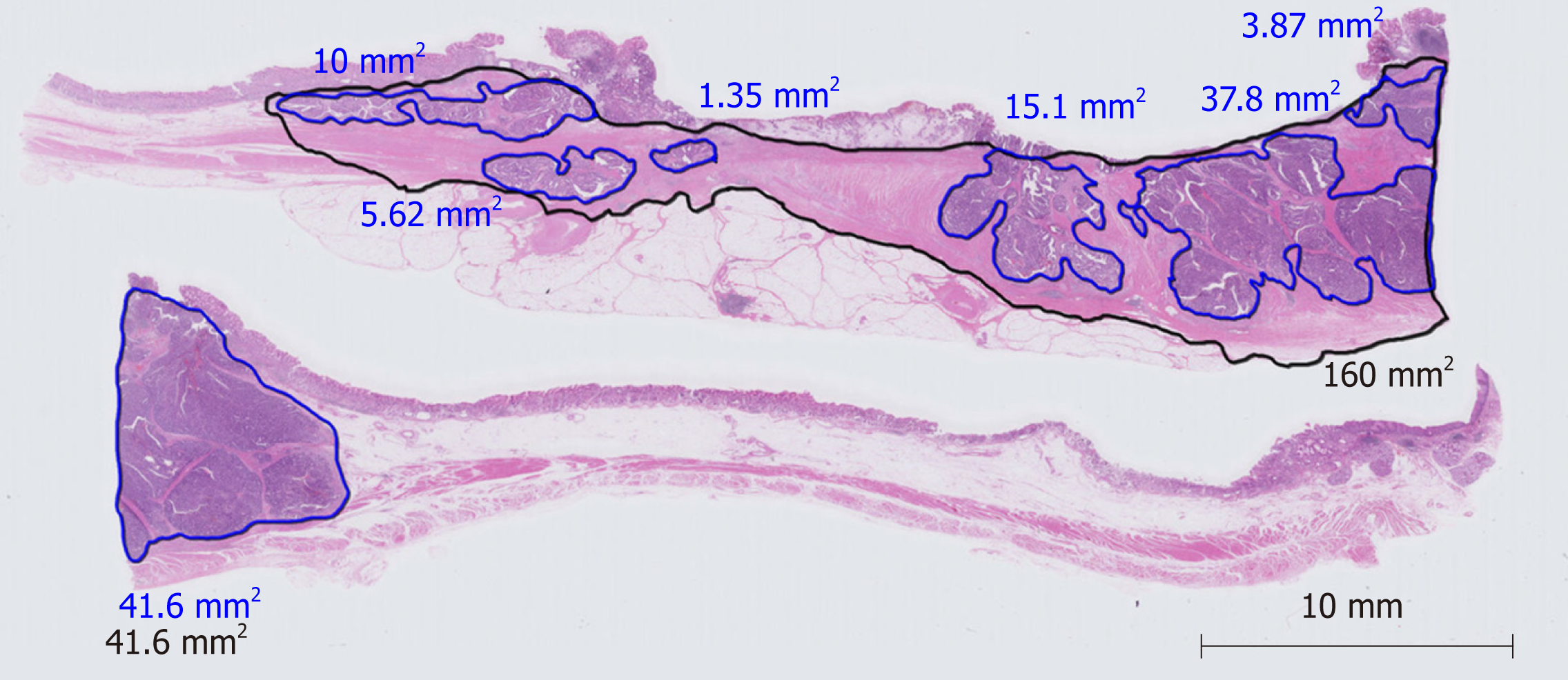
Pathological evaluation of a therapeutic effect using digitally captured virtual microscopic slides was performed by the same method as previously reported by Nakamura et al[14]. Briefly, hematoxylin-eosin-stained pathological specimens including the largest tumor diameter were digitally captured on a virtual microscopic slide. The largest tumor diameter was determined by macroscopic findings and by reference to preoperative imaging findings. The square measures of residual tumor and primary tumorous area were calculated using software for virtual microscopic diagnosis (NanoZoomer Virtual Microscopy System, Hamamatsu Photonics). If tumor cells were sparsely distributed in the specimen, for example, when the density of tumor cells was less than 10%, the square measure multiplied by 0.1 was added to the sum of the residual tumor area. Pathological response was calculated as the percentage of the residual tumor area relative to the primary tumorous area. A representative example a virtual microscopic slide and pathological diagnosis is shown in Figure 1. Histological type (differentiated or undifferentiated) and TNM staging were assigned according to the 14th JCGC. Pathological specimens were prepared and evaluated using JCGC criteria by pathologists of Shizuoka Cancer Center. Two authors (SK and TS) captured the hematoxylin-eosin-stained pathological slides including the largest tumor diameter in digital microscopic images and independently evaluated them. If the opinions of the two authors differed, they discussed the case in order to reach a consensus.
Based on the 10% cut-off evaluated using virtual microscopic slides, patients were classified into a responder group, in which the residual tumor area was less than 10%, and a non-responder group comprising the rest. OS was defined as the time from the date of operation to the date of death from any cause. Relapse-free survival (RFS) was defined as the time from the date of operation to the date of confirming recurrence or death from any cause, whichever came first. Survival rates were estimated using the Kaplan–Meier method. The hazard ratio (HR) of responders to non-responders in OS was calculated by Cox regression analysis. HRs adjusted by the multivariate stratified Cox model were also estimated, including age, eastern cooperative oncology group performance status (ECOG PS), macroscopic type, reason for NAC, T-classification, and N-classification as covariates. All statistical tests were two-sided, and P < 0.05 was considered significant. Statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[16].
The clinicopathological characteristics of the 54 eligible patients in this study are shown in Table 1. The median age of the patients was 64 years (range 19–83) and all of them had ECOG PS of 0 to 1. Half of the patients had histologically undifferentiated-type adenocarcinoma and 18.5% had type 4 tumors. Ninety percent of patients received a platinum doublet regimen and the remaining 10% received a triplet regimen as NAC. The main reasons for NAC were as follows: 53% of patients had extended nodal metastasis; 13% had large type 3 tumor; 17% had type 4 tumor; and 17% had other reasons, including esophageal or other organ invasion, or solitary liver metastasis. Follow-up was performed until May 2016. The median follow-up time was 37.7 mo.
| Background | n (%) | Background | n (%) |
| Age | Median 64 | M-classification | |
| Range 39–77 | |||
| < 65 yr | 28 (51.9) | 0 | 51 (94.4) |
| ≥ 65 yr | 26 (48.1) | 1 | 3 (5.6) |
| Gender | Tumor histology | ||
| Male | 40 (74.1) | Differentiated | 26 (48.1) |
| Female | 14 (25.9) | Undifferentiated | 28 (51.9) |
| ECOG PS | Reason for NAC | ||
| 0 | 46 (85.2) | Clinical SE or T4b | 14 (25.9) |
| 1 | 8 (14.8) | Extended LN | 24 (44.4) |
| Tumor type | Large type 3 | 5 (9.3) | |
| Non-type 4 | 44 (81.5) | Type 4 | 4 (7.4) |
| Type 4 | 10 (18.5) | Others | 7 (13.0) |
| Primary site | HER2 overexpression | ||
| Stomach | 51 (94.4) | Negative or unknown | 48 (88.9) |
| Esophagogastric junction | 3 (5.6) | Positive | 6 (11.1) |
| T-classification | NAC regimen | ||
| 1 | 1 (1.9) | SP or SOX (+ trastuzumab) | 49 (90.7) |
| 2 | 2 (3.7) | DCS | 5 (9.3) |
| 3 | 13 (24.1) | Adjuvant treatment | |
| 4 | 38 (70.4) | S-1 | 52 (96.3) |
| N-classification | Others or no treatment | 2 (3.7) | |
| 0 | 6 (11.1) | Participant of clinical trials | |
| 1 | 10 (18.5) | Yes | 17 (31.5) |
| 2 | 22 (40.7) | No | 37 (68.5) |
| 3 | 15 (27.8) | ||
| Not evaluable | 1 (1.9) |
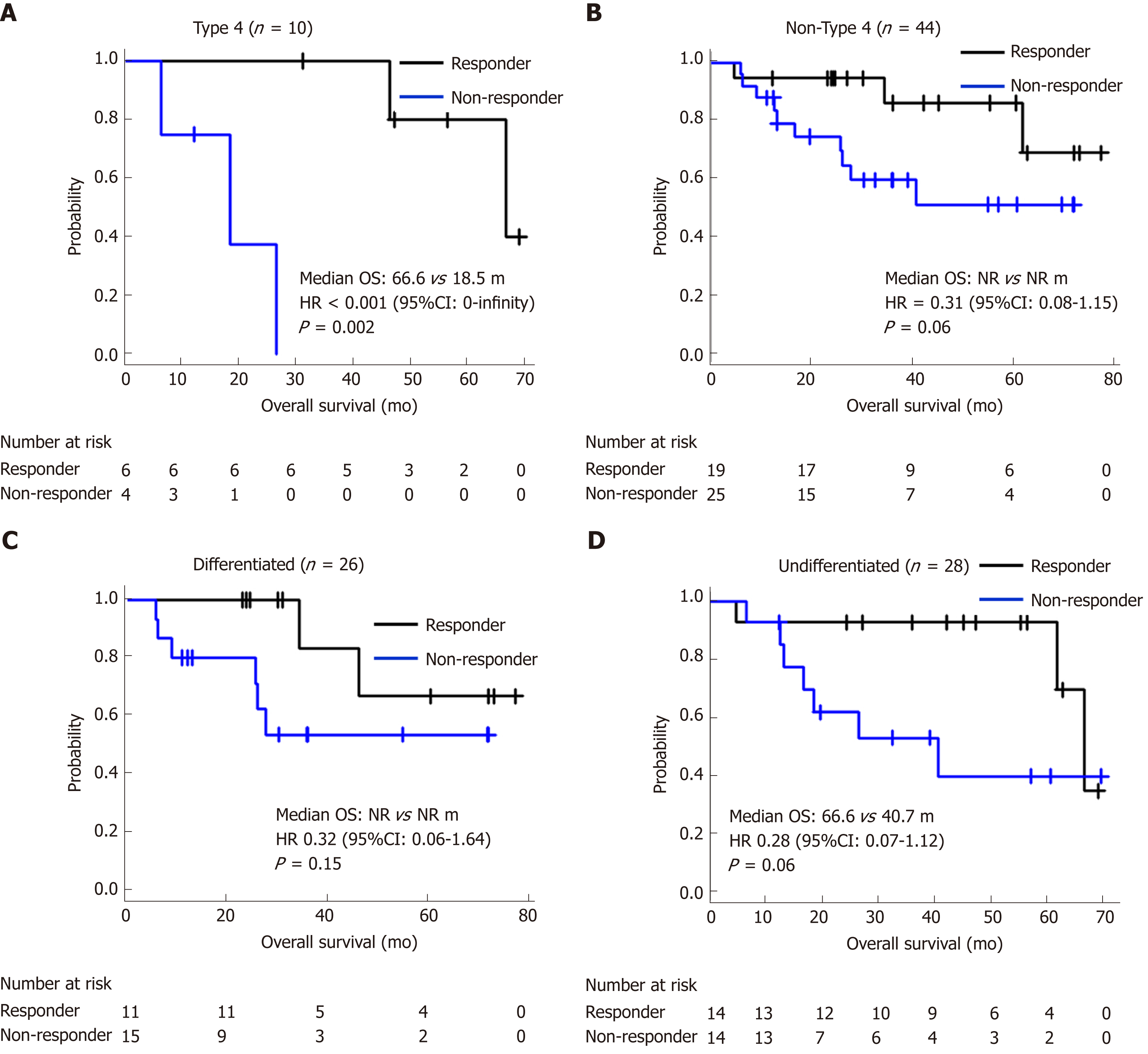
Twenty-five (46.3%) patients were classified into the responder group and 29 (53.7%) into the non-responder group. The concordance rate between two evaluators was 96.2%. Eighteen patients died within the observational period and among them, 16 (88.9%) died of recurrent gastric cancer. The survival curves of responders and non-responders are shown in Figure 2. Median RFS of responders and non-responders was not reached (NR) and 18.2 mo, respectively [HR = 0.35, 95% confidence interval (CI): 0.14–0.86, P = 0.023]. Median OS (mOS) was NR and 40.7 mo, respectively (HR = 0.30, 95%CI: 0.10–0.84, P = 0.016). Even after adjustment for age, ECOG PS, macroscopic type, reason for NAC, T-stage, and N-stage, the OS of responders was significantly better than that of non-responders (HR = 0.23, 95%CI: 0.07–0.78, P = 0.018). There were no other statistically significant prognostic factors in the clinicopathological background (Table 2). This tendency was also observed in subgroup analyses for macroscopic types (type 4/non-type 4) and histological types (differentiated/undifferentiated) shown in Figure 3.
| Background | HR (95%CI) | P value | |
| Age | ≥ 65 yr | 0.57 (0.21–1.51) | 0.26 |
| Gender | Male | 1.09 (0.38–3.07) | 0.87 |
| ECOG PS | 0 | 0.37 (0.04–2.84) | 0.34 |
| Macroscopic type | Type 4 | 1.51 (0.53–4.25) | 0.43 |
| Primary site | Stomach | 0.27 (0.05–1.24) | 0.09 |
| Tumor histology | Undifferentiated | 1.14 (0.44–2.90) | 0.78 |
| T-classification | ≥ 4a | 4.73 (0.62–35.7) | 0.13 |
| N-classification | ≥ 3a | 1.63 (0.57–4.61) | 0.35 |
| M-classification | 1 | 0.96 (0.22–4.19) | 0.95 |
| HER2 | Positive | < 0.001 (0–infinity) | 0.14 |
| Main reason for NAC | Extended LN | 1 (reference) | |
| Large type 3 | 1.22 (0.32–4.70) | 0.76 | |
| Type 4 | 1.49 (0.48–4.60) | 0.48 | |
| Others | 0.62 (0.13–3.02) | 0.58 | |
| NAC regimen | SP or SOX | 1.85 (0.24–14.2) | 0.55 |
| Adjuvant treatment | Yes | 0.43 (0.05–3.39) | 0.42 |
| Participant of clinical trials | Yes | 0.79 (0.29–2.14) | 0.65 |
Next, to compare the prognostic utility of the 10% cut-off evaluated using virtual microscopic slides with that of conventional JCGC criteria, the adjusted HR of each cut-off was calculated. Histological evaluations according to the JCGC criteria revealed that the numbers of patients who had grades 0, 1a, 1b, 2, and 3 were 4 (7.4%), 13 (24.0%), 11 (20.3%), 22 (40.7%), and 4 (7.4%), respectively. When using the 10% cut-off on virtual microscopic slides, the adjusted HR for RFS was 0.29 (95%CI: 0.10-0.82, P = 0.019) and that for OS was 0.23 (95%CI: 0.07-0.78, P = 0.018). These HRs were the lowest upon comparing the HRs for each cut-off according to the JCGC criteria (Table 3). Moreover, in this study, there was no statistically significant difference in OS of the two groups according to each cut-off of the JCGC criteria.
| Histological criteria of JCGC | RFS | OS | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Grade 0 vs 1a–3 | 0.52 (0.15–1.80) | 0.304 | 2.27 (0.27–19.0) | 0.449 |
| Grade 0–1a vs 1b–3 | 0.63 (0.26–1.55) | 0.321 | 0.72 (0.26–1.95) | 0.52 |
| Grade 0–1b vs 2–3 | 0.33 (0.12–0.87) | 0.026 | 0.39 (0.13–1.15) | 0.089 |
| Grade 0–2 vs 3 | 0.69 (0.08–5.34) | 0.722 | 0.62 (0.07–5.34) | 0.665 |
| Our method | ||||
| Responder vs non-responder | 0.29 (0.10–0.82) | 0.019 | 0.23 (0.07–0.78) | 0.018 |
The prognostic utility of pathological response, especially pathological complete response (pCR), has been indicated in many studies[13,17-19]. However, patients who achieve pCR are rare. Indeed, in this study, only 7.4% of patients achieved pCR. Therefore, a more common endpoint is needed. Nakamura et al[14] suggested that a 10% cut-off evaluated using virtual microscopic slides could be the global standard cut-off of residual tumor. Our analysis supports that result and indicates that this approach may be applicable to clinical practice. Our results suggest that the method may be useful for a prognostic marker regardless of the reason for undergoing NAC, which has not been examined in clinical trials but is sometimes considered in clinical practice, including esophageal or adjacent organ invasion, or a metastatic lesion.
In addition, in this study, we compared the prognostic utility of this method with that of using the conventional JCGC criteria. Kurokawa et al[8] reported the prognostic utility of JCGC criteria and demonstrated that histological responders who achieved a response of grade 1b or more had significantly longer survival than non-responders. Compared with this, in our study, the HRs of PFS and OS were the lowest when responders were defined as patients who achieved a response of grade 2 or more. This difference appeared to be caused by the differences in the patients included in each study. The study by Kurokawa et al[8] also included patients who underwent R1 or R2 resection. Although our data were limited to patients who underwent R0 resection, the HRs for RFS and OS with the 10% cut-off evaluated using virtual microscopic slides were lower than those for the other cut-offs according to the JCGC criteria. Therefore, our method might at least be as useful as the JCGC criteria. We consider that it would be worthwhile confirming this result in future prospective studies.
The advantage of histological evaluation with virtual microscopic slides compared with conventional histological evaluation is its objectivity. Smyth et al[20] reported that there was poor inter-observer agreement of pathological response independently evaluated by two pathologists (kappa = 0.64) in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy trial. Therefore, to improve the rate of concordance among pathologists and standardize pathological evaluations, more objective and simpler methods were needed. Our method used only stained pathological specimens of the largest tumor diameter and its concordance rate was high (96.2%). Thus, we consider that this approach is more applicable for evaluating pathological response.
Evaluating the area of type 4 tumors is sometimes difficult. Although Nakamura et al[14] reported that a 10% cut-off evaluated using virtual microscopic slides in type 4 tumors was less useful than that in non-type 4 tumors, our results suggest that this cut-off s useful in both of these groups. This discrepancy appears to be caused by the small sample size of our study (n = 10) and the subjectivity of the evaluation for the area in which tumor cells were sparsely distributed. Further improvement of the evaluation method is thus needed.
Our study had some limitations. First, the analysis was performed in a single center and the sample size was relatively small. To evaluate the application of the cut-off widely in clinical practice and to examine the concordance rate of pathological response precisely, a multi-center study is desirable. Second, patients included in this analysis were those seen from March 2009 until May 2015. However, in 2011, trastuzumab was approved for HER2-positive gastric cancer in Japan. mOS of patients with recurrence after surgery might thus have been prolonged after 2011. Third, this analysis was limited to patients who underwent R0 resection. Therefore, to evaluate whether the cut-off is useful for patients who have undergone R1 or R2 resection, further studies will be needed. Fourth, this was a retrospective study. It could thus have been influenced by confounding factors such as demographic factors or patient comorbidities.
In conclusion, a 10% cut-off of pathological response evaluated using virtual microscopic slides might be a useful prognostic marker for gastric cancer patients who have undergone NAC followed by R0 resection. The utility of this method needs to be evaluated in further prospective studies.
Although pathological response is a common endpoint used to assess the efficacy of neoadjuvant chemotherapy (NAC) for gastric cancer, the problem of a low rate of concordance from evaluations among pathologists remains unresolved. Moreover, there is no globally accepted consensus regarding the optimal evaluation.
Pathological response is commonly used for a surrogate marker for progression-free survival and overall survival (OS) after surgery. However, no globally accepted consensus has been reached regarding the optimal cut-off, and the reproducibility between pathologists was limited.
To examine the clinical utility of 10% cut-off for pathological response evaluated using virtual microscopic slides as a prognostic marker.
We retrospectively evaluated pathological specimens of gastric cancer patients who underwent NAC followed by surgery and achieved R0 resection between March 2009 and May 2015. Residual tumor area and primary tumor beds were measured in one captured image slide. We classified patients with < 10% residual tumor relative to the primary tumorous area as responders, and the rest as non-responders; we then compared OS and relapse-free survival (RFS) between these two groups.
Fifty-four patients were evaluated. The concordance rate between two evaluators was 96.2%. Median RFS of 25 responders and 29 non-responders was not reached (NR) vs 18.2 mo [hazard ratio (HR) = 0.35, P = 0.023], and median OS was NR vs 40.7 mo (HR = 0.3, P = 0.016), respectively. This result was also observed even in subgroup analyses for different macroscopic types (Borrmann type 4/non-type 4) and histological types (differentiated/undifferentiated).
The measurement of pathological response using digitally captured virtual microscopic slides may be useful in clinical practice because of its objectivity and its high concordance rate. We consider that this approach is more applicable for evaluating pathological response.
Out results indicated that the measurement of pathological response using digitally captured virtual microscopic slides might be as useful as conventional criteria. However, further studies is needed to evaluate the utility of the method especially for patients who have undergone R1 or R2 resection, and type 4 tumor.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park WS S-Editor: Tang JZ L-Editor:A E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51172] [Article Influence: 8528.7] [Reference Citation Analysis (122)] |
| 2. | Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuburaya A, Miyashiro I, Kaji M, Ninomiya M. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer. 2009;12:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Keighley MR, Moore J, Roginski C, Powell J, Thompson H. Incidence and prognosis of N4 node involvement in gastric cancer. Br J Surg. 1984;71:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 219] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 6. | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 19118] [Article Influence: 1274.5] [Reference Citation Analysis (1)] |
| 8. | Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, Fukuda H. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Reim D, Gertler R, Novotny A, Becker K, zum Büschenfelde CM, Ebert M, Dobritz M, Langer R, Hoefler H, Friess H, Schumacher C. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol. 2012;19:2108-2118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Valenti V, Hernandez-Lizoaín JL, Beorlegui MC, Diaz-Gozalez JA, Regueira FM, Rodriguez JJ, Viudez A, Sola I, Cienfuegos JA. Morbidity, mortality, and pathological response in patients with gastric cancer preoperatively treated with chemotherapy or chemoradiotherapy. J Surg Oncol. 2011;104:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Brenner B, Shah MA, Karpeh MS, Gonen M, Brennan MF, Coit DG, Klimstra DS, Tang LH, Kelsen DP. A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locally advanced gastric cancer. Ann Oncol. 2006;17:1404-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Jia Y, Dong B, Tang L, Liu Y, Du H, Yuan P, Wu A, Ji J. Apoptosis index correlates with chemotherapy efficacy and predicts the survival of patients with gastric cancer. Tumour Biol. 2012;33:1151-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Nakamura K, Kuwata T, Shimoda T, Mizusawa J, Katayama H, Kushima R, Taniguchi H, Sano T, Sasako M, Fukuda H. Determination of the optimal cutoff percentage of residual tumors to define the pathological response rate for gastric cancer treated with preoperative therapy (JCOG1004-A). Gastric Cancer. 2015;18:597-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 16. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9275] [Cited by in F6Publishing: 10758] [Article Influence: 978.0] [Reference Citation Analysis (0)] |
| 17. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 18. | Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC, Nivers R, Morris J, Pisters PW. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M, Langley R, Ghidini M, Braconi C, Wotherspoon A, Grabsch HI, Valeri N. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721-2727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |