Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.870
Peer-review started: December 12, 2017
First decision: December 27, 2017
Revised: January 2, 2018
Accepted: January 16, 2018
Article in press: January 16, 2018
Published online: February 21, 2018
Hepatocholangiocarcinoma (cHCC-ICC) is a rare primary hepatic tumor defined by the presence of histological features of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). Its prevalence ranges from 1%-5% of all primary liver cancers. We report the case of a 55-year-old cirrhotic male patient admitted to our university hospital for dysphagia, revealing a 10 cm lower-third esophageal metastasis of an unresectable cHCC-ICC with stem-cell features. Computed tomography and abdominal magnetic resonance imaging scans revealed multiple hepatic lesions combining features of both HCC and ICC, associated with synchronous bone metastasis. Histological and immunohistochemical analyses of biopsies from the esophageal lesion and the hepatic tumor confirmed the diagnosis of cHCC-ICC with a stem cell-subtype, according to the World Health Organization classification. After a multidisciplinary meeting, the patient was treated with chemotherapy. He received two cycles of a gemcitabine plus cisplatin regimen before bone progression, and he died 3 mo after the initial diagnosis.
Core tip: Hepatocholangiocarcinoma (cHCC-ICC) represents less than 5% of all hepatic tumors and remains an uncommon cancer, with no guidelines concerning its management. Esophageal metastasis is a rare presentation of hepatic tumors. To our knowledge, this case report is the first to describe an esophageal lesion revealing a metastatic stem cell-subtype cHCC-ICC.
- Citation: Salimon M, Chapelle N, Matysiak-Budnik T, Mosnier JF, Frampas E, Touchefeu Y. Esophageal metastasis of stem cell-subtype hepatocholangiocarcinoma: Rare presentation of a rare tumor. World J Gastroenterol 2018; 24(7): 870-875
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.870
Primary liver cancer is the sixth most common cancer worldwide[1]. The majority of intrahepatic cancers are hepatocellular carcinomas (HCCs) or intrahepatic cholangiocarcinomas (ICCs). The prevalence of hepatocholangiocarcinoma (cHCC-ICC), combining histological features of HCC and ICC, ranges from 1% to 5% of primary hepatic cancers[2]. In 1949, Allen and Lisa[3] were the first to describe and classify cHCC-ICC into three subtypes (A, B and C). The classification subsequently evolved until the latest World Health Organization classification, proposed in 2010 (Table 1)[4].
| World Health Organization 2010[4] |
| cHCC-ICC classical: Typical HCC and typical ICC |
| cHCC-ICC-SC |
| cHCC-ICC-SC-typical: Nests of mature-looking hepatocytes with peripheral clusters of small cells that have a high nucleus:cytoplasm ratio and hyperchromatic nuclei. |
| cHCC-ICC SC-int: Tumor cells show features intermediate between hepatocytes and cholangiocytes. These tumor cells show strands, solid nests and/or trabeculae of small, uniform cells with scant cytoplasm and hyperchromatic nuclei. |
| cHCC-ICC-SC-CLC: Admixtures of small monotonous glands, antler-like anastomosing patterns. Each tumor cell is cuboidal, smaller in size than normal hepatocytes, with a high nucleus: cytoplasm ratio, and distinct nucleoli. |
Here we report the case of a patient diagnosed with a cHCC-ICC of stem cell-subtype, presenting with dysphagia and revealing an esophageal metastasis.
A 55-year-old male was admitted to the University Hospital of Nantes, France, in January 2017 for investigation of a recent and elective dysphagia to solids associated with an alteration in general status (ECOG score 2) and weight loss of 14 kg.
The patient had a medical history of schizophrenia, alcoholic cirrhosis with Child-Pugh score A and a daily alcohol intake of 30 g, Barrett’s esophagus C1M6, and heavy cigarette smoking.
The first biological analyses showed isolated thrombocytopenia of 107 G/L, and normal renal and hepatic functions. The C-reactive protein level was 9.9 mg/L.
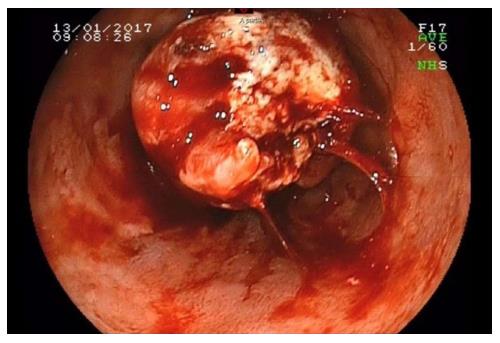
Esophageal endoscopy (Figure 1) revealed a significant, quasiobstructive lesion of the lower third of the esophagus. Histological analysis confirmed an esophageal localization of an undifferentiated carcinoma, with immunohistochemical analysis indicating HCC with positive hepatocyte antigen.
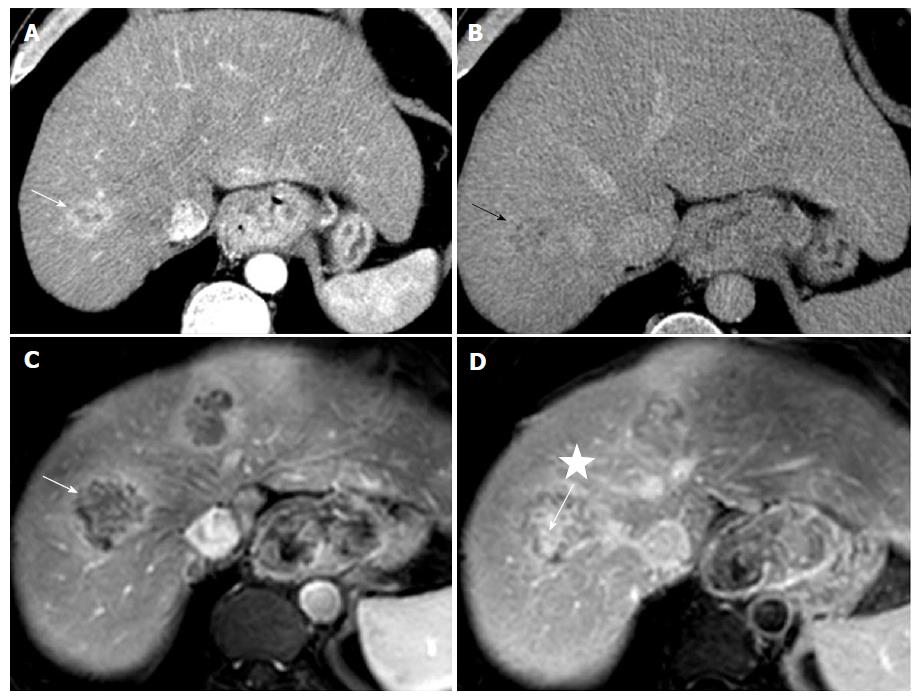
Thoraco-abdomino-pelvic computed tomography (CT) and abdominal magnetic resonance imaging (MRI) scans were performed. CT scans were performed before and after injection of contrast media, including arterial, portal and delayed phase at 5 min. MRI scan included T1-weighted sequence with fat suppression before and after injection of gadolinium chelates at the same phases. CT and MRI scan analyses (Figure 2) revealed the esophageal lesion and multiple hepatic nodules, mainly located in the right liver. Hepatic tumors exhibited atypical imaging features for classic HCC but showed combined imaging features of both HCC with peripheral arterial enhancement and delayed wash out, and ICC with delayed central fibrous enhancement. The tumors more closely resembled ICC. Metastases were present in adrenal glands (33 mm on the right adrenal gland and 17 mm on the left adrenal gland) and lymph nodes of the celiac region, associated with a bony lesion of the right iliac branch invading the pubic symphysis.
All tumor markers were normal: alpha fetoprotein (αFP): 1.8 ng/mL (normal range: 0.8-8.8 ng/mL); carbohydrate antigen 19-9 (CA19-9): 4.5 U/mL (normal range: < 37 U/mL); and, carcinoembryonic antigen: 2.4 μg/L (normal range: < 5 μg/L).
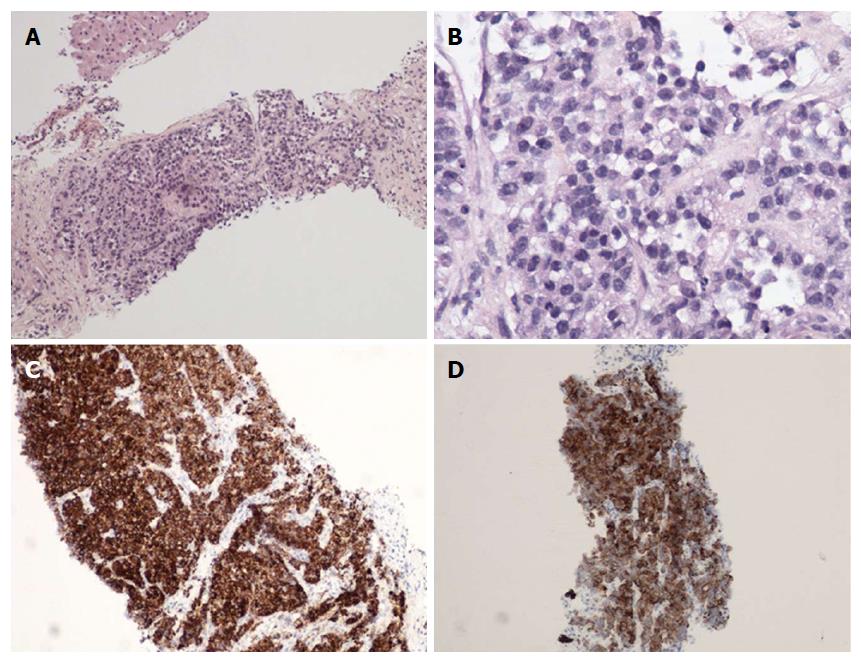
A liver biopsy was performed. Histological and immunohistochemical analyses showed cHCC-ICC with stem cell features (small cells) and an intermediate cell subtype, as described in Table 1. The tumor consisted of small cuboidal cells arranged in a ductal pattern at the borders of nodules, in continuity with a trabecular pattern at the center. Tumor cells concomitantly expressed hepatocyte antigen HepPar1 and cytokeratin 19, normally expressed by biliary cells (Figure 3). The tumor cells appeared to be growing within and replacing regenerative nodules of cirrhosis.
The patient was treated with systemic intravenous gemcitabine and cisplatin combined chemotherapy (gemcitabine 1000 mg/m2 and cisplatin 25 mg/m² every week for 2 wk, with 1 wk of rest before a new cycle). He received two cycles of chemotherapy. During the follow-up, the patient showed a progressive alteration in general condition, with an ECOG score of 3, associated with the appearance of diffuse bone pain. A bone scintigraphy was performed 2 mo after the beginning of chemotherapy, and revealed a multifocal metastatic spread over the entire axial and peripheral skeleton with right ilio-pubic and voluminous right humeral lesions. The patient was then managed with best supportive care and died 87 d after the beginning of treatment.
To our knowledge, this is the first report of an esophageal metastasis of a cHCC-ICC. Esophageal metastases are uncommon. In HCC, the incidence of metastatic esophageal tumors is low, accounting for less than 0.4%[5]. Few case reports have described the presence of esophageal metastases from HCC or ICC[6-14]. These metastases could develop by the spread of tumor cells infiltrating the portal system[15]. The dissemination by hepatofugal portal flow to the esophagus seems to be one possible route for esophageal metastasis[8].
There are no current guidelines for the treatment of unresectable, locally advanced or metastatic cHCC-ICC. We recently described the first series of patients with unresectable cHCC-ICC treated with gemcitabine plus platinum-based chemotherapy. In that retrospective study, including 30 patients, according to RECIST criteria, the partial response rate was 28.6%, stable disease rate 50% and progressive disease rate 21.4%. Median progression-free survival and overall survival were 9.0 mo and 16.2 mo, respectively[16].
The diagnosis of cHCC-ICC is challenging. The radiological diagnosis is difficult due to the high frequency of cHCC-ICC mimicking HCC, from 30% to 50% when considering the major features of HCC (arterial phase hyperenhancement, wash out and capsule appearance)[17,18]. However, the addition of non-HCC features in the radiologic assessment could improve the diagnostic accuracy[18]. Histological diagnosis is the gold standard, but is difficult to obtain in the absence of surgical specimens. The conduct of liver biopsies to sample both components of cHCC-ICC is infrequent. In a series of 23 resected cHCC-ICC, all of the tumors were misdiagnosed at preoperative histology, with 20 considered to be HCC and three classed as ICC[19].
As in the case presented here, criteria for identifying cHCC-ICC have been proposed previously[20]. The combination of elevated serum tumor markers and enhancement patterns on imaging should strongly suggest the diagnosis of cHCC-ICC in the following circumstances: imaging features of both ICC and HCC, regardless of marker levels; elevation of both αFP and CA19-9, regardless of imaging appearance; or discordance between imaging and tumor marker elevation (typical HCC enhancement pattern with elevated CA19-9 or typical ICC enhancement pattern with elevated αFP)[20].
The combination of histological, radiological and biological criteria is important to identify cHCC-ICC patients. Even in patients with cirrhosis and liver tumor with typical enhancement patterns of HCC, biopsies should be advocated in the presence of biological or imaging features suggesting a cHCC-ICC, as the prognosis and the management of these tumors could be different[21].
A 55-year-old cirrhotic male patient was admitted for dysphagia.
Elective dysphagia for solids associated with an alteration in general status and a weight loss of 14 kg.
Primary esophageal cancer.
Normal renal and hepatic functions. Normal carbohydrate antigen 19-9, carcinoembryonic antigen and alpha fetoprotein.
Thoraco-abdomino-pelvic computed tomography and abdominal magnetic resonance imaging scans were performed and revealed hepatic lesions combining imaging features of both hepatocellular carcinoma, and intrahepatic cholangiocarcinoma.
Stem cell-subtype hepatocholangiocarcinoma.
Chemotherapy: gemcitabine - cisplatin.
Hepatocholangiocarcinoma is a primary hepatic tumor representing less than 5% of all hepatic tumors. This is the first report of an esophageal metastasis of a hepatocholangiocarcinoma.
The diagnosis of hepatocholangiocarcinoma is challenging. The addition of non-HCC features in the radiologic assessment could improve the diagnostic accuracy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Goral V, Lee CL, Tarnawski AS S- Editor: Wang JL L- Editor: Filipodia E- Editor: Huang Y
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19781] [Article Influence: 2197.9] [Reference Citation Analysis (17)] |
| 2. | Bergquist JR, Groeschl RT, Ivanics T, Shubert CR, Habermann EB, Kendrick ML, Farnell MB, Nagorney DM, Truty MJ, Smoot RL. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford). 2016;18:886-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] [Cited in This Article: ] |
| 4. | Theise ND, Nakashima O, Park YN. Combined hepatocellular-cholangiocarcinoma. Bosman FT, Carneiro F, Hruban RH, Theise ND, (Eds), WHO classification of tumors of the digestive system. Lyon: IARC 2010; 225-227. [Cited in This Article: ] |
| 5. | Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277-287. [PubMed] [Cited in This Article: ] |
| 6. | Sohara N, Takagi H, Yamada T, Ichikawa T, Abe T, Itoh H, Mori M. Esophageal metastasis of hepatocellular carcinoma. Gastrointest Endosc. 2000;51:739-741. [PubMed] [Cited in This Article: ] |
| 7. | Kume K, Murata I, Yoshikawa I, Kanagawa K, Otsuki M. Polypoid metastatic hepatocellular carcinoma of the esophagus occurring after endoscopic variceal band ligation. Endoscopy. 2000;32:419-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Cho A, Ryu M, Yoshinaga Y, Ishikawa Y, Miyazawa Y, Okazumi S, Ochiai T. Hepatocellular carcinoma with unusual metastasis to the esophagus. Hepatogastroenterology. 2003;50:1143-1145. [PubMed] [Cited in This Article: ] |
| 9. | Tsubouchi E, Hirasaki S, Kataoka J, Hidaka S, Kajiwara T, Yamauchi Y, Masumoto T, Hyodo I. Unusual metastasis of hepatocellular carcinoma to the esophagus. Intern Med. 2005;44:444-447. [PubMed] [Cited in This Article: ] |
| 10. | Yan SL, Hung YH, Yang TH. Metastatic hepatocellular carcinoma of the esophagus: an unusual cause of upper gastrointestinal bleeding. Endoscopy. 2007;39 Suppl 1:E257-E258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Choi CS, Kim HC, Kim TH, Seo GS, Kim KH, Cho EY, Seo SO, Oh HJ, Choi SC. Does the endoscopic finding of esophageal metastatic hepatocellular carcinoma progress from submucosal mass to polypoid shape? Gastrointest Endosc. 2008;68:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Xie LY, Fan M, Fan J, Wang J, Xu XL, Jiang GL. Metastatic hepatocellular carcinoma in the esophagus following liver transplantation. Liver Transpl. 2008;14:1680-1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Sato T, Krier M, Kaltenbach T, Soetikno R. Cholangiocarcinoma metastasis to the esophagus. Endoscopy. 2010;42 Suppl 2:E250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Boonnuch W, Akaraviputh T, Nino C, Yiengpruksawan A, Christiano AA. Successful treatment of esophageal metastasis from hepatocellular carcinoma using the da Vinci robotic surgical system. World J Gastrointest Surg. 2011;3:82-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Arakawa M, Kage M, Matsumoto S, Akagi Y, Noda T, Fukuda K, Nakashima T, Okuda K. Frequency and significance of tumor thrombi in esophageal varices in hepatocellular carcinoma associated with cirrhosis. Hepatology. 1986;6:419-422. [PubMed] [Cited in This Article: ] |
| 16. | Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, Matysiak-Budnik T, Bennouna J, Tiako Meyo M, Lecomte T. Br J Cancer 2017. . [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013;201:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Potretzke TA, Tan BR, Doyle MB, Brunt EM, Heiken JP, Fowler KJ. Imaging Features of Biphenotypic Primary Liver Carcinoma (Hepatocholangiocarcinoma) and the Potential to Mimic Hepatocellular Carcinoma: LI-RADS Analysis of CT and MRI Features in 61 Cases. AJR Am J Roentgenol. 2016;207:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996;11:758-764. [PubMed] [Cited in This Article: ] |
| 20. | Maximin S, Ganeshan DM, Shanbhogue AK, Dighe MK, Yeh MM, Kolokythas O, Bhargava P, Lalwani N. Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open. 2014;1:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Serra V, Tarantino G, Guidetti C, Aldrovandi S, Cuoghi M, Olivieri T, Assirati G, De Ruvo N, Magistri P, Ballarin R. Incidental Intra-Hepatic Cholangiocarcinoma and Hepatocholangiocarcinoma in Liver Transplantation: A Single-Center Experience. Transplant Proc. 2016;48:366-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |