Published online Oct 14, 2018. doi: 10.3748/wjg.v24.i38.4356
Peer-review started: July 16, 2018
First decision: August 1, 2018
Revised: August 3, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: October 14, 2018
To investigate the potential effect of inhibitors of phosphodiesterase-5 (PDE-5) for therapy of portal hypertension in liver cirrhosis.
In the rat model of thioacetamide-induced liver fibrosis/cirrhosis the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway was investigated. Expression and localization of PDE-5, the enzyme that converts vasodilating cGMP into inactive 5’-GMP, was in the focus of the study. Hepatic gene expression of key components of the NO-cGMP pathway was determined by qRT-PCR: Endothelial NO synthase (eNOS), inducible NO synthase (iNOS), soluble guanylate cyclase subunits α1 and β1 (sGCa1, sGCb1), and PDE-5. Hepatic PDE-5 protein expression and localization were detected by immunohistochemistry. Serum cGMP concentrations were measured using ELISA. Acute effects of the PDE-5 inhibitor Sildenafil (0.1 mg/kg or 1.0 mg/kg) on portal and systemic hemodynamics were investigated using pressure transducers.
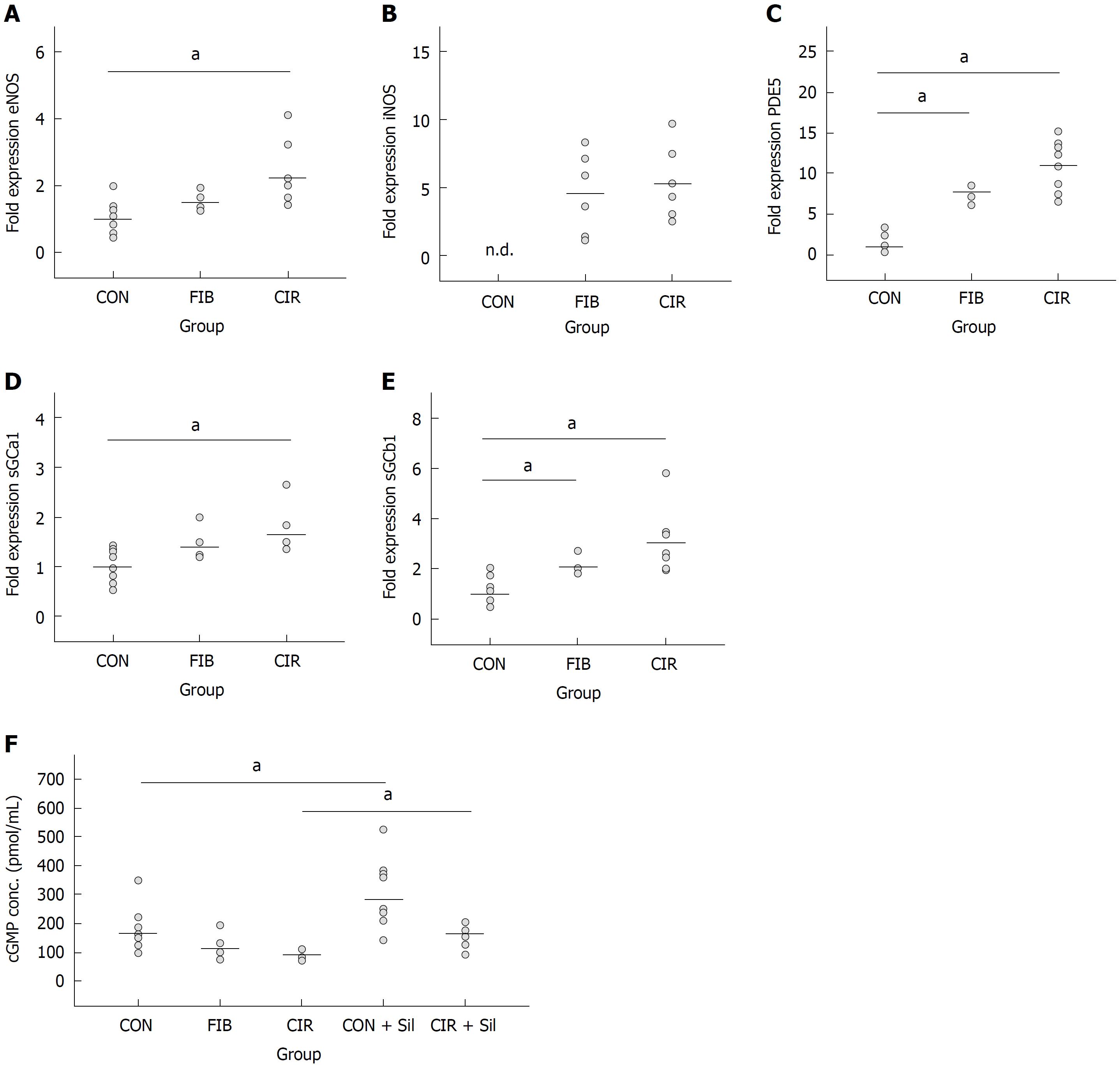
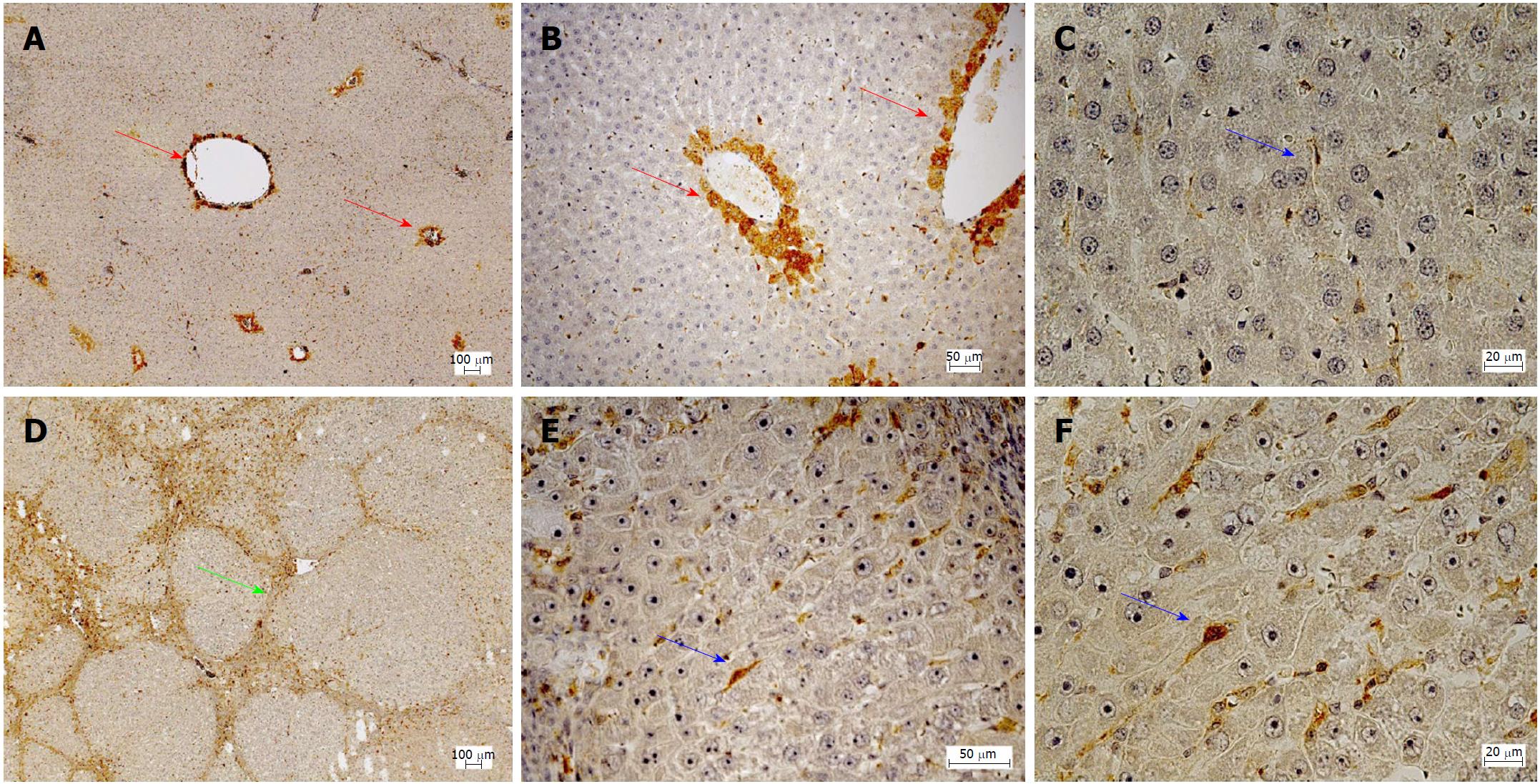
Hepatic gene expression of eNOS (2.2-fold; P = 0.003), sGCa1 (1.7-fold; P = 0.003), sGCb1 (3.0-fold; P = 0.003), and PDE-5 (11-fold; P = 0.003) was increased in cirrhotic livers compared to healthy livers. Overexpression of PDE-5 (7.7-fold; P = 0.006) was less pronounced in fibrotic livers. iNOS expression was only detected in fibrotic and cirrhotic livers. In healthy liver, PDE-5 protein was localized primarily in zone 3 hepatocytes and to a lesser extent in perisinusoidal cells. This zonation was disturbed in cirrhosis: PDE-5 protein expression in perisinusoidal cells was induced approximately 8-fold. In addition, PDE-5-expressing cells were also found in fibrous septa. Serum cGMP concentrations were reduced in rats with cirrhotic livers by approximately 40%. Inhibition of PDE-5 by Sildenafil caused a significant increase in serum cGMP concentrations [+ 64% in healthy rats (P = 0.024), + 85% in cirrhotic rats (P = 0.018)]. Concomitantly, the portal venous pressure was reduced by 19% in rats with liver cirrhosis.
Overexpression and abrogated zonation of PDE-5 likely contribute to the pathogenesis of cirrhotic portal hypertension. PDE-5 inhibition may therefore be a reasonable therapeutic approach for portal hypertension.
Core tip: A constriction of sinusoids plays an important role in the pathogenesis of cirrhotic portal hypertension, wherein the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway plays a pivotal role. In a rat model of liver cirrhosis phosphodiesterase-5 (PDE-5) was markedly overexpressed both on the mRNA and the protein level. PDE-5 converts the vasodilating cGMP to inactive 5’-GMP. In healthy liver a zonation of PDE-5 was found which is abrogated in cirrhosis. Serum cGMP was reduced in cirrhosis. Inhibition of PDE-5 by Sildenafil normalized serum cGMP levels and lowered portal venous pressure. Hence, the inhibition of PDE-5 may be a promising adjunct in portal hypertension therapy.
- Citation: Schaffner D, Lazaro A, Deibert P, Hasselblatt P, Stoll P, Fauth L, Baumstark MW, Merfort I, Schmitt-Graeff A, Kreisel W. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J Gastroenterol 2018; 24(38): 4356-4368
- URL: https://www.wjgnet.com/1007-9327/full/v24/i38/4356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i38.4356
Portal hypertension is among the most important complications of liver cirrhosis[1]. Several factors contribute to its pathogenesis[2,3]. Disturbed liver architecture resulting from fibrosis, scarring and nodule formation, angiogenesis, and vascular occlusion increase intrahepatic flow resistance. Furthermore, morphological, molecular, and functional changes within the hepatic sinusoids also substantially contribute to the increased intrahepatic flow resistance and consequently to pathogenesis of portal hypertension[3,4]. Generally, intrahepatic flow resistance is controlled by sinusoidal tone with liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs) being the two central cellular elements within the sinusoids which are involved[4]. During cirrhosis development fenestrations of LSECs are lost, which is associated with an abnormal deposition of a basement membrane matrix, the so-called capillarization. Moreover, quiescent HSCs are activated and transformed into myofibroblasts, which are characterized by contractile elements, production of extracellular matrix, and loss of stored vitamin A.
The sinusoidal tone is regulated by the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) system[5]. NO is synthesized in LSECs by endothelial NO synthase (eNOS), diffuses to HCSs where it activates soluble guanylate cyclase (sGC) which in turn catalyzes the formation of cGMP. The latter mediates a signal transduction cascade leading to relaxation of HSCs. The action of cGMP is terminated by phosphodiesterase-5 (PDE-5) which catalyzes its conversion to inactive 5’-GMP.
In cirrhotic liver, NO formation in liver sinusoids is reduced, which results in sinusoidal constriction and increased intrahepatic flow resistance[6]. Activated HSCs exhibit increased contractility and impaired responsiveness to the vasodilator NO, while responsiveness to the vasoconstrictor endothelin (ET-1) is increased, both resulting in increased intrahepatic flow resistance[7]. In contrast, NO formation in the splanchnic system is increased which increases blood flow towards the liver, a process which further aggravates the increase of intrahepatic flow resistance[6,8].
The current mainstay of medical therapy of portal hypertension is non-selective beta-blockers (NSBB)[9,10]. β1 receptor blockade reduces portal flow by decreasing cardiac output while β2 blockade allows unopposed α1-adrenergic activity resulting in splanchnic vasoconstriction and decreased portal inflow. However, their therapeutic efficacy is limited by frequent side effects such as circulatory dysregulation, which prevent sufficient dosing in many cases, particularly in decompensated cirrhosis[11-13]. Therapy of portal hypertension by transjugular portosystemic shunts is effective, but may cause exacerbations of hepatic encephalopathy, at least in patients with advanced cirrhosis[9]. Organic nitrates deliver NO and reduce portal venous pressure. However, their action is unspecific and leads to arterial hypotension. Therefore, there is a need of novel medical therapies to treat portal hypertension.
Many approaches have been proposed to specifically target the reduced NO availability in diseased liver. Endothelial NOS gene transfer[14,15], neuronal NOS gene transfer[16], Akt (a kinase involved in NOS activation) gene transfer[17] and administration of tetrahydrobiopterin (BH4, a cofactor of NOS)[18], have not gone beyond preclinical testing. Liver specific NO delivering drugs (e.g., NCX-1000) yielded disappointing results[19,20]. Statins lead to enhanced activity of endothelial NO synthase, mediated by an effect on the Rho/Rho-kinase-/Akt protein phosphorylation pathway[21]. Although statins reduced portal hypertension in a clinical setting[22], the effects on clinical outcome were modest[23]. Preclinical and clinical studies on inhibitors of PDE-5, restricting the inactivation of cGMP, yielded promising but variable results[24-30].
The aim of the current study was to determine liver disease-induced alterations in hepatic gene expression of eNOS, iNOS, sGC, and PDE-5, in hepatic protein expression of PDE-5, as well as in serum cGMP concentrations. Moreover, acute effects of the PDE-5 inhibitor Sildenafil on portal and systemic hemodynamics were tested. The model of thioacetamide-induced liver fibrosis/cirrhosis in rats was used.
The animal research protocol was approved by the local institutional animal care and use committee (Regierungspräsidium Freiburg, Germany, ref. No. G-13/89). Animal care was performed in accordance to the rules of the German animal protection law and the animal care guidelines of the European community (2010/63/EU). A total of 141 male rats (Charles River, Sulzfeld, Germany) were studied. All were clearly recognizable from their permanent and unique identifiers. Rats were housed in individually ventilated cages in a laboratory animal facility and received daily human care. All had free access to food and water and were exposed to a 12:12-h light-dark cycle at an ambient temperature of 22 °C to 25 °C. Before starting any experiments, the rats were allowed to acclimatize to the ambient conditions for one week.
For the biochemical investigations Wistar rats were used only. Hemodynamic measurements in healthy livers were performed in Sprague Dawley rats. Corresponding experiments in rats with with fibrotic or cirrhotic livers were performed in Wistar rats since the presence of cholangiocellular carcinomas in association with thioacetamide in Wistar rats is much lower than in Sprague Dawley rats.
Among the current models of induction of liver disease in laboratory animals[31] the model of thioacetamide (TAA)-induced liver fibrosis/cirrhosis was chosen. Therefore, the protocol described previously by Li et al[32] was used but TAA exposure time was prolonged to 16 wk.
The median lobe of each rat’s liver was excised, fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were stained with hematoxylin-eosin, sirius red, and periodic acid-schiff diastase, and stained for reticulin and iron. Fibrosis was evaluated semiquantitatively by a blinded pathologist according to the Desmet score[33].
In order to detect PDE-5 protein expression and localization immunohistochemical staining of liver sections was performed with a rabbit polyclonal anti-PDE-5A-antibody (ab64179, Abcam, Cambridge, United Kingdom) at a 1:500 dilution. Antibody binding was detected by Dako REAL EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse (K5007). Quantification of PDE-5 protein along the sinusoids was performed in sections of respectively 4 healthy and cirrhotic livers.
Using Zeiss Axioplan microscope the number of stained perisinusoidal cells was counted in 20 random high power-fields (HPF) (400 × magnification) for each sample. PDE-5 staining around the central vein in healthy livers and in fibrous septa in cirrhotic livers was not considered.
The left lateral lobe of each rat’s liver was excised, cut into pieces, snap frozen in liquid nitrogen, and stored at -80 °C until used for qRT-PCR. Hepatic total mRNA was extracted using the RNeasy® Mini Kit mRNA extraction kit (Qiagen, Hilden, Germany). Complementary DNA synthesis was performed using the First Strand cDNA synthesis kit (Thermo Fisher Scientific, MA, United States). qRT-PCR was performed with SYBR Green (Invitrogen, Karlsruhe, Germany) and 10% dimethylsulfoxide (Sigma-Aldrich, Schnelldorf, Germany) on a thermocycler (LightCyler® 480, Roche, Basel, Switzerland; 40 cycles: 30 s 95 °C; 30 s 60 °C; 40 s 72 °C). Specificity of the PCR products was assessed by melting curve analysis. All primers (Table 1) were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast) and synthesized by Microsynth, Balgach, Switzerland.
| Gene | Forward primer (5´-3´) | Reverse primer (5´-3´) | Product length (bp) |
| eNOS | 5´-AAGTGGGCAGCATCACCTAC-3´ | 5´-GCCTGGGAACCACTCCTTTT-3´ | 211 |
| iNOS | 5´-CTCACTGGGACTGCACAGAA-3´ | 5´-TGTTGAAGGGTGTCGTGAAA-3´ | 128 |
| PDE-5 | 5´-GCGGAGGAAGAAACAAGGGA-3´ | 5´-ATCGGCAAAGAACCTCGTGT-3´ | 196 |
| sGCa1 | 5´-GCCCCACGACATACAGGTTA-3´ | 5´-GCGGCTCACTAATCTACCCC-3´ | 229 |
| sGCb1 | 5´-AATTACGGTCCCGAGGTGTG-3´ | 5´-ACCAGCATTGAGGTTGAGGAC-3´ | 147 |
| 18sRNA (reference) | 5´-GTAACCCGTTGAACCCCATT-3´ | 5´-CCATCCAATCGGTAGTAGCG-3´ | 151 |
| srsf4 (reference) | 5´-GGTTCTGGACGCAGTGGATA-3´ | 5´-CTCCTTCGTTTTTGCGTCCC-3´ | 193 |
At the end of the invasive hemodynamic measurements blood samples were taken via the left carotid artery and stored at -80 °C until used for the quantification of serum cGMP concentrations by ELISA (ab133052, Abcam, Cambridge, United Kingdom).
Before the invasive hemodynamic measurements were started rats were fasted for 1.5 h to avoid prandial effects on portal flow parameters. Anesthesia was initiated in an animal induction chamber using a mixture of 3% isoflurane and 97% oxygen. It was maintained by an intraperitoneally injected bolus of 0.3-0.4 mL pentobarbital (125 mg/mL). Rats were fixed on a homeothermic controlled operating table which kept body temperature stable at 37 °C ± 0.5 °C. Vital parameters were monitored. Tracheotomy was performed and a tracheal cannula was inserted. Rats were mechanically ventilated (50 breaths/min) and muscle relaxation was induced by intraperitoneal injection of 0.5 mL pancuronium (0.4 mg/mL).
To monitor the central venous pressure (CVP) the right external jugular vein was cannulated with PE-10, which was positioned near the right atrium. A second PE-10 tubing was inserted and used for continuous infusion of isotone electrolyte solution (1 mL/h). The electrolyte solution was enriched with pentobarbital (15 mg/mL) to ensure continuous anesthesia. To monitor mean arterial pressure (MAP) the left carotid artery was cannulated with PE-50 tubing.
Median laparotomy was performed and the portal vein was exposed. To monitor portal venous pressure (PVP) a peripheral venous catheter was inserted into the portal vein. After a stabilization period of 10-15 min, basal values of all parameters were obtained and the intervention was administered through the second CVP-tubing. Rats were randomly allocated in one of three intervention groups: NaCl (0.9%), Sildenafil (Revatio®, Pfizer, Berlin, Germany) 0.1 mg/kg (Sil 0.1 mg/kg), and Sildenafil 1.0 mg/kg (Sil 1.0 mg/kg). The intervention was applied in a standardized volume of 0.6 mL.
Results were expressed as median ± interquartile range (IQR). Only results of the qRT-PCR experiments were expressed as mean ± standard deviation (SD) to enable the quantification of gene expression with the comparative Ct method[34].
To evaluate the effect of Sildenafil on hemodynamic parameters, absolute values were normalized (PVPnorm, MAPnorm, HRnorm). Hereby time point “10 min” was taken as baseline value and set to 100% since the administration of 0.6 mL liquid volume into the right atrium caused parameter variations for the next few minutes before they reached a new steady state. For all 9 groups the relative median of differences (RMD) was calculated to determine the change in parameters at time point “60 min” to baseline (“10 min”).
To determine differences among groups the non-parametric Kruskal-Wallis test was used. Post-hoc pairwise comparisons between groups[35] were corrected for multiple comparisons according to Bonferroni. For reasons of consistency the non-parametric Kruskal-Wallis test and post-hoc pairwise comparisons with Bonferroni correction were also used for the qRT-PCR experiments. A two-tailed P-value of < 0.05 was considered as statistically significant.
For statistical analyses SPSS® software 23.0 (IBM Corp., Armonk, NY, United States) was used.
Increased hepatic gene expression of key enzymes of the NO-cGMP pathway and decreased serum cGMP concentrations in liver fibrosis/cirrhosis. Inhibition of PDE-5 by Sildenafil leads to renormalization of cGMP concentrations
In order to determine alterations in the key parameters of the NO-cGMP pathway and serum cGMP concentrations induced by liver fibrosis/cirrhosis different biochemical analyses were conducted. Fifty-three rats were included in these studies. However, the data sets of the 6 rats having no fibrosis after 16 wk of TAA exposure were excluded. For the statistical analysis of hepatic gene expression of eNOS, iNOS, PDE5, sGCa1 and sGCb1, and serum cGMP concentrations, the remaining 47 rats were classified depending on their histologically assessed degree of liver fibrosis: CON (healthy control, n = 11), FIB (fibrosis, n = 6), and CIR (cirrhosis, n = 8).
Moreover, to evaluate the effect of Sildenafil on serum cGMP concentrations two additional groups were analyzed: rats with healthy livers (CON + Sil, n = 12) and cirrhotic livers (CIR + Sil, n = 10) which have undergone the hemodynamic measurement with Sildenafil (1.0 mg/kg) as intervention.
qRT-PCR results showed that gene expression became the higher the more the rats became diseased (CON < FIB < CIR) (Figure 1 and Table 2). iNOS expression was detected in diseased rats only. In fibrotic livers gene expression analysis revealed significantly increased expression of PDE-5 (7.7-fold; P = 0.006), and sGCb1 (2.1-fold; P = 0.018) compared with healthy livers, whereas eNOS expression was significantly increased in non-adjusted pairwise comparisons only (Table 3).
| CON (n = 11) Mean ± SD | FIB (n = 6) Mean ± SD | CIR (n = 8) Mean ± SD | |
| eNOS (fold exp.) | 1.0 ± 0.4 | 1.5 ± 0.3' | 2.2 ± 1.0 |
| iNOS (fold exp.) | n.d.1 | 4.6 ± 3.0 | 5.3 ± 2.3 |
| PDE5 (fold exp.) | 1.0 ± 1.0 | 7.7 ± 0.9 | 11.0 ± 3.1 |
| sGCa1 (fold exp.) | 1.0 ± 0.3 | 1.4 ± 0.3 | 1.7 ± 0.4 |
| sGCb1 (fold exp.) | 1.0 ± 0.5 | 2.1 ± 0.3 | 3.0 ± 1.3 |
| CON (n = 11)Median ± IQR | FIB (n = 6)Median ± IQR | CIR (n = 8)Median ± IQR | CON + Sil (n = 12)Median ± IQR | CIR + Sil (n = 10)Median ± IQR | |
| cGMP (pmol/mL) | 152 ± 86 | 100 ± 68 | 91 ± 22 | 249 ± 153 | 168 ± 52 |
In contrast, in cirrhotic livers a significantly increased expression of eNOS (2.2-fold; P = 0.003), PDE-5 (11-fold; P = 0.003), sGCa1 (1.7-fold; P = 0.003) and sGCb1 (3-fold; P = 0.003) was measured when compared with healthy livers.
No significant differences between fibrotic and cirrhotic livers were detected (P ≥ 0.05).
Serum cGMP concentrations showed a nonsignificant decrease of 34% (P = 0.453) in rats with fibrotic livers and of 40% (P = 0.054) in rats with cirrhotic livers (Figure 1 and Table 4). Again, no differences between fibrotic and cirrhotic livers were observed (P = 1.000). Administration of Sildenafil led to a significant increase in serum cGMP concentrations of 64% (P = 0.024) in rats with healthy livers and of 85% (P = 0.018) in rats with cirrhotic livers.
| eNOS | iNOS | PDE5 | sGCa1 | sGCb1 | cGMP | ||
| CON vs FIB | sig. (pairwise) | 0.0241 | - | 0.0021 | 0.065 | 0.0061 | 0.151 |
| sig. (adjusted) | 0.072 | - | 0.0061 | 0.195 | 0.0181 | 0.453 | |
| CON vs CIR | sig. (pairwise) | 0.0011 | - | 0.0011 | 0.0011 | 0.0011 | 0.0181 |
| sig. (adjusted) | 0.0031 | - | 0.0031 | 0.0031 | 0.0031 | 0.054 | |
| FIB vs CIR | sig. (pairwise) | 0.280 | 0.732 | 0.201 | 0.194 | 0.267 | 0.495 |
| sig. (adjusted) | 0.840 | 1.000 | 0.603 | 0.582 | 0.801 | 1.000 | |
| CON vs CON + Sil | sig. (pairwise) | n.m.2 | n.m.2 | n.m.2 | n.m.2 | n.m.2 | 0.0121 |
| sig. (adjusted) | n.m.2 | n.m.2 | n.m.2 | n.m.2 | n.m.2 | 0.0241 | |
| CIR vs CIR + Sil | sig. (pairwise) | n.m.2 | n.m.2 | n.m.2 | n.m.2 | n.m.2 | 0.0091 |
| sig. (adjusted) | n.m.2 | n.m.2 | n.m.2 | n.m.2 | n.m.2 | 0.0181 |
In addition, the effect of the hemodynamic measurements and particularly the associated operative procedure on hepatic gene expression and serum cGMP concentration was analyzed. Only for eNOS a significant decrease was determined (data not shown).
Immunohistochemistry was performed to analyze hepatic protein expression of PDE-5 in healthy and cirrhotic livers (Figure 2). In healthy livers, PDE-5 protein was predominantly expressed by perivenular hepatocytes (zone 3) and to a lesser extent by perisinusodial cells. In contrast, in cirrhotic livers hepatic zoning of PDE-5 was abrogated and bands of fibrous septa were formed. PDE-5 protein expression by perisinusoidal cells was increased, but positive cells were also present in fibrous septa.
Subsequent quantitative analysis of positive perisinusoidal cells revealed 3 stained cells per HPF in healthy livers and 23 stained cells per HPF in cirrhotic livers, what corresponds to a 7.7-fold increase.
To evaluate the effect of Sildenafil on portal and systemic blood pressure, hemodynamic measurements were performed. One hundred and ten rats were included in this study and sorted by their histological degree of liver fibrosis. However, the data sets of the 2 rats having no fibrosis after 16 wk of TAA exposure were excluded. For the statistical analysis of the hemodynamic parameters, the remaining 108 rats were classified into three groups depending on their histologically assessed degree of liver fibrosis: CON (healthy control, n = 55), FIB (fibrosis, n = 29), and CIR (cirrhosis, n = 24). Each of those had 3 subgroups which are categorized based on intervention in form of NaCl, Sildenafil 0.1 mg/kg (Sil 0.1 mg/kg) or Sildenafil 1 mg/kg (Sil 1.0 mg/kg), which was applied in a standardized volume of 0.6 mL. Absolute values of the parameters are listed (Table 5).
| CON | FIB | CIR | |||||||
| NaCl (n = 19) | Sil 0.1 mg/kg (n = 18) | Sil 1.0 mg/kg (n = 18) | NaCl (n = 7) | Sil 0.1 mg/kg (n = 15) | Sil 1.0 mg/kg (n = 7) | NaCl (n = 7) | Sil 0.1 mg/kg (n = 7) | Sil 1.0 mg/kg (n = 10) | |
| Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | Median ± IQR | |
| Body weight (g) | 375 ± 15 | 370 ± 28 | 380 ± 27 | 359 ± 35 | 363 ± 28 | 361 ± 24 | 336 ± 40 | 350 ± 46 | 337 ± 15 |
| PVP_0 (mmHG) | 6.4 ± 0.7 | 6.6 ± 0.4 | 6.3 ± 0.7 | 6.0 ± 0.9 | 6.2 ± 1.3 | 6.2 ± 0.9 | 6.8 ± 1.6 | 7.5 ± 1.7 | 7.7 ± 1.1 |
| PVP_10 (mmHG) | 6.0 ± 0.9 | 6.6 ± 0.6 | 6.6 ± 0.9 | 5.7 ± 1.2 | 5.5 ± 1.3 | 5.1 ± 1.4 | 6.8 ± 1.4 | 7.1 ± 1.6 | 7.1 ± 2.2 |
| PVP_30 (mmHG) | 5.9 ± 0.7 | 6.3 ± 0.7 | 6.4 ± 0.8 | 5.3 ± 1.4 | 5.4 ± 1.3 | 4.8 ± 1.4 | 6.5 ± 1.2 | 6.6 ± 1.0 | 6.3 ± 1.3 |
| PVP_60 (mmHG) | 5.9 ± 0.7 | 6.3 ± 0.8 | 6.4 ± 1.1 | 5.1 ± 1.2 | 5.2 ± 1.2 | 4.8 ± 1.5 | 6.6 ± 2.0 | 6.3 ± 1.0 | 5.8 ± 1.2 |
| MAP_0 (mmHG) | 89 ± 12 | 106 ± 14 | 101 ± 28 | 65 ± 30 | 59 ± 21 | 58 ± 28 | 49 ± 19 | 47 ± 15 | 60 ± 19 |
| MAP_10 (mmHG) | 83 ± 20 | 94 ± 14 | 85 ± 27 | 55 ± 25 | 45 ± 10 | 42 ± 8 | 42 ± 11 | 41 ± 5 | 38 ± 16 |
| MAP_30 (mmHG) | 79 ± 21 | 88 ± 17 | 77 ± 21 | 50 ± 14 | 43 ± 12 | 37 ± 6 | 41 ± 12 | 35 ± 8 | 36 ± 8 |
| MAP_60 (mmHG) | 76 ± 21 | 82 ± 23 | 74 ± 28 | 44 ± 11 | 40 ± 8 | 37 ± 7 | 38 ± 3 | 34 ± 5 | 34 ± 7 |
| HR_0 (bpm) | 373 ± 40 | 377 ± 47 | 368 ± 31 | 320 ± 30 | 335 ± 30 | 314 ± 22 | 307 ± 38 | 308 ± 76 | 365 ± 28 |
| HR_10 (bpm) | 362 ± 55 | 383 ± 44 | 380 ± 50 | 313 ± 23 | 339 ± 41 | 351 ± 29 | 286 ± 33 | 316 ± 71 | 374 ± 22 |
| HR_30 (bpm) | 356 ± 57 | 366 ± 52 | 373 ± 41 | 291 ± 20 | 310 ± 43 | 310 ± 21 | 268 ± 40 | 313 ± 57 | 350 ± 36 |
| HR_60 (bpm) | 347 ± 39 | 359 ± 43 | 362 ± 49 | 284 ± 26 | 304 ± 41 | 295 ± 33 | 254 ± 36 | 302 ± 47 | 324 ± 30 |
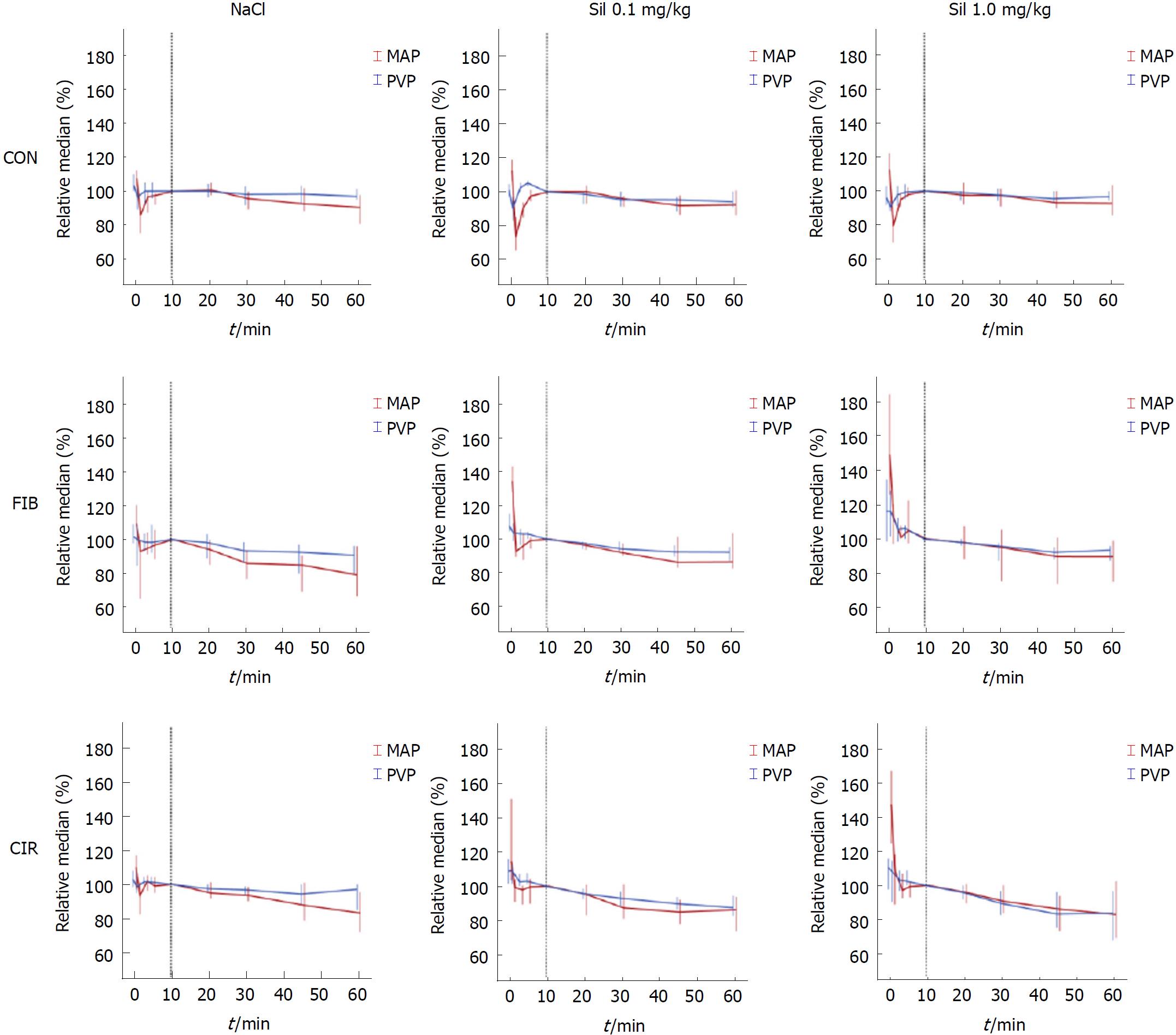
All parameters of interest were normalized (PVPnorm, MAPnorm, and HRnorm) to compensate differences in absolute values between rats with healthy and diseased livers, the time point “10 min” being taken as baseline and set to 100% (see methods/statistical analysis).
Regarding the course of hemodynamic parameters, a decrease in parameter values was observed for all groups regardless of intervention (Figure 3). In rats with diseased livers the decrease in PVPnorm (%) (blue curve) compared to the decrease in MAPnorm (%) (red curve) became more pronounced with Sildenafil and increasing dosage. CVPnorm, respiration ratenorm, oxygen saturationnorm and body temperaturenorm, remained unchanged in all groups (data not shown).
The effect of Sildenafil was evaluated by comparing the change in parameter values at time point “60 min” to baseline (“10 min”) (Table 6). All parameters were normalized (PVPnorm, MAPnorm, HRnorm,) before differences were calculated for the interval 10 min to 60 min post-intervention with time point “10 min” being set to 100%. Significant differences (P < 0.05) between Sil 0.1 mg/kg and CON or Sil 1.0 mg/kg and CON are marked by an “1”. No significant differences between Sil 0.1 mg/kg and Sil 1.0 mg/kg were observed.
| CON | FIB | CIR | |||||||
| NaCl (n = 19) | Sil 0.1 mg/kg (n = 18) | Sil 1.0 mg/kg (n = 18) | NaCl (n = 7) | Sil 0.1 mg/kg (n = 15) | Sil 1.0 mg/kg (n = 7) | NaCl (n = 7) | Sil 0.1 mg/kg (n = 7) | Sil 1.0 mg/kg (n = 10) | |
| RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | RMD (%) ± IQR | |
| PVPnorm | - 3 ± 7 | - 6 ± 10 | - 3 ± 6 | - 9 ± 11 | - 8 ± 8 | - 7 ± 6 | - 3 ± 7 | - 13 ± 7 | - 19 ± 26 |
| MAPnorm | - 10 ± 17 | - 8 ± 16 | - 7 ± 19 | - 21 ± 24 | - 14 ± 21 | - 10 ± 10 | - 17 ± 16 | - 14 ± 11 | - 17 ± 23 |
| HRnorm | - 4 ± 6 | - 4 ± 5 | - 4 ± 5 | - 8 ± 5 | - 8 ± 14 | - 12 ± 13 | - 6 ± 6 | - 8 ± 11 | - 14 ± 101 |
In rats with healthy livers intragroup comparisons showed nonsignificant effects of Sildenafil on PVPnorm (P = 0.399), MAPnorm (P = 0.867), and HRnorm (P = 0.664). Also in rats with fibrotic livers intragroup comparisons revealed nonsignificant effects of Sildenafil on PVPnorm (P = 0.320), MAPnorm (P = 0.272), and HRnorm (P = 0.311). In contrast, the administration of Sildenafil caused a trend towards a lower PVPnorm (P = 0.088) in rats with cirrhotic livers. Moreover, Sildenafil showed nonsignificant effects on MAPnorm (P = 0.915) but for HRnorm a significant decrease of 8% (RMD) in Sil 1.0 mg/kg was found compared to NaCl (P = 0.024).
This study aimed to determine the alterations of key components in NO-cGMP pathway in the rat model of TAA-induced liver fibrosis/cirrhosis. Hemodynamic measurements were performed to further elucidate the potential of PDE-5 inhibitors in portal hypertension therapy. An increased hepatic gene expression of key enzymes of the NO-cGMP pathway in diseased rats was demonstrated wherein a significant overexpression of PDE-5 was the most notable finding. Enhanced levels of PDE-5 protein expression were confirmed by immunohistochemical staining which revealed that PDE-5 zonation found in healthy livers was abrogated in diseased livers. With the increased PDE-5 expression, serum concentrations of cGMP, the most potent vasodilating compound in hepatic sinusoids, were reduced in rats with diseased livers. Administration of the PDE-5 inhibitor Sildenafil led to a significant increase of cGMP concentrations with accompanying reduction in portal venous pressure in rats with cirrhosis.
In healthy liver eNOS is constitutively expressed and uniformly distributed among the hepatic lobules (i.e., LSECs, hepatocytes, endothelium of hepatic arteries, terminal venules, biliary epithelium). High concentrations of eNOS-derived NO attenuate HSC activity and have a protective role in liver function[36]. Activity of eNOS is reduced in cirrhosis contributing to decreased sinusoidal dilation[4,36]. The amount of eNOS is not lowered but the translational modification through Akt or interactions with caveolin prevent full functional capacity[37]. Present results revealed a 2.2-fold higher mRNA expression of eNOS in cirrhotic livers. However, this does not necessarily translate to a higher NO production.
In contrast, iNOS expression was observed only in fibrotic and cirrhotic livers. This is consistent with the hypothesis that multiple pathogenic factors like LPS, toxins, or viruses are accompanied by overexpression of iNOS[38,39]. NO derived from iNOS activity may have pathogenic effect (probably mediated by peroxynitrite). It is assumed that iNOS competes with eNOS for the cofactor BH4, so that the effect of eNOS-derived NO, which maintains HSCs in the quiescent state, is abrogated[18,40]. Thus, the present results suggest that in the TAA model HSCs were activated and transformed into myofibroblasts.
A previous study from Theilig et al[41] showed that in healthy livers nearly all HSCs were immunostained for sGC in the periphery of the hepatic lobule, while the intensity decreased in the direction of the central vein. Around the central vein there was nearly no sGC detectable. A further preclinical study from Davies et al[42] in the model of bile duct ligation-induced cirrhosis demonstrated decreased hepatic sGC activity but the addition of NO donors increased its activity. These authors did not consider a zonation of sGC. Therefore, the lower sGC activity may indicate the loss of zonation in cirrhosis. cGMP concentrations were not measured in the study but the authors speculate that addition of substrates for eNOS and application of an phosphodiesterase inhibitor could be beneficial on vascular dysfunction in cirrhosis. Loureiro-Silva et al[43] used Western blotting and found 1.6-fold overexpression of the b1-subunit of sGC in the model of CCl4-induced cirrhosis. This corresponds to the present data of increased mRNA expression of both sGC subunits [a1 (1.7-fold) and b1 (3-fold)] in TAA-induced cirrhosis. A possible zonation of sGC was not investigated in the present study.
Likewise, Loureiro-Silva et al[43] demonstrated an overexpression of PDE-5. Using immunofluorescence a cellular mapping of the enzyme was not possible in this study. Moreover, Lee et al[25] found a markedly increased protein expression of PDE-5 and a slight overexpression of sGC. One week administration of Sildenafil induced a further overexpression of sGC and a reduction of PDE-5. This effect was accompanied by a reduction of portal venous pressure and portal perfusion pressure. Finally, Angeli et al[44] found lowered cGMP content and increased PDE-5 activity in the cirrhotic liver but also in the kidney. The present study showed an 11-fold overexpression of PDE-5 on the mRNA level and an approximately 8-fold overexpression on the protein level in perisinusoidal cells (most probably HSCs) in cirrhosis. In healthy livers, a marked zonation was observed, low frequency of positive cells in perisinusoidal cells (most probably HCSs) and a marked expression of PDE-5 in perivenular hepatocytes (zone 3). In cirrhosis, the zonation was lost and a markedly increased staining was observed in perisinusoidal cells. The fibrous septa in cirrhosis also exhibited high staining, but a clear attribution to distinct cell types was not yet possible.
Regarding cGMP, Lee et al[26] found that in patients with liver cirrhosis after administration of Sildenafil cGMP in liver veins increases and intrahepatic flow resistance decreases. Levels of cGMP in humans with healthy liver were not determined. Other groups found elevated serum cGMP levels in animals and humans with advanced cirrhosis[45-47]. The increase of serum cGMP levels after administration of Sildenafil as demonstrated in the present study reflects the anticipated effect that Sildenafil reduces the PDE-5-induced inactivation of cGMP.
The opposing zonation of sGC and PDE-5 in the liver lobule of healthy rats suggests a physiological role in maintaining cGMP in the sinusoids at the appropriate level: Near the portal triad high sGC activity catalyzes the formation of high levels of cGMP which is not inactivated by the low activity of PDE-5. Near the terminal venule cGMP production is low and high PDE-5 activity inactivates cGMP before entering the systemic circulation. For many important metabolic processes in the liver lobule a zonation has been already found[48,49]. Generally, in cirrhosis the zonation of enzymes and metabolic functions is lost along with disturbed architecture of residual liver lobules. Using immunohistochemistry the present study showed that in cirrhosis the zonation of PDE-5 was lost and the number of PDE-5 positive perisinusoidal cells was largely increased. These data suggest that not only an altered expression but also a disturbed zonation of key components of the NO-cGMP system in cirrhosis may play an important role in the pathophysiology of portal hypertension.
To investigate whether pharmaceutical inhibition of PDE-5 might be a favorable measure in order to at least partly correct the dysregulation of the NO-cGMP pathway, hemodynamic measurements were performed in the next step to evaluate the acute effects of administration of either NaCl or Sildenafil (Sil 0.1 mg/kg or Sil 1.0 mg/kg). The parameters portal venous pressure (PVP), mean arterial pressure (MAP), and heart rate (HR) were monitored over 60 min in the same rat pool than before, i.e., in rats with healthy, fibrotic and cirrhotic livers.
At the start of the measurements MAP was relatively low in diseased rats compared to healthy rats. However, it is known that advanced human liver cirrhosis is accompanied by a low MAP in the hyperdynamic circulatory state[50] and the patients are highly vulnerable to fluid loss and anesthesia, what might also explain the different baseline values in the present study. Consequently, PVP in diseased rats was relatively low in the present study. The most noticeable change was observed after high-dose Sildenafil (1.0 mg/kg) in rats with cirrhosis. This led to a trend towards decreased PVP by 19% and a nonsignificant lowering of MAP. Interestingly, Sildenafil showed no effect on PVP in rats with healthy livers and a physiological PDE-5 expression, supporting the hypothesis that the presence of high PDE-5 expression in a particular tissue reflects the effect of a PDE-5 inhibitor[51]. No statistical significance was observed in the present study, what might be attributed to the high variation of the decrease of PVP. Nevertheless, the present preclinical results support previous clinical findings[30] and provide additional arguments about the benefits of PDE-5 inhibitors in therapy of portal hypertension.
qRT-PCR data did not reflect the enzymatic activity. cGMP concentrations were measured from the carotid arterial instead of the portal venous serum.
The administration of 0.6 mL liquid into the right atrium caused parameter variations for some minutes due to volume overload. During the course of 60 min, a decrease in parameter values (i.e., PVP, MAP, and HR) even in the NaCl groups could be observed, indicating that the anesthesia, as well as the operative conditions, could have added more variability and uncertainty to the measurements. The procedure itself - the operative procedure of the rats and the hemodynamic measurement - lasted about 2.5 to 3 h per rat. The use of different rat strains (Sprague Dawley for healthy liver, Wistar for diseased liver), as well as the fact that some of the diseased rats had CCCs could have influenced the results of the hemodynamic measurements.
This study provided further evidence that liver cirrhosis is associated with dysregulations in the NO - cGMP pathway. The most remarkable findings in the context of liver cirrhosis were hepatic overexpression and abrogated zonation of PDE-5 which might contribute to sinusoidal constriction and portal hypertension. Hence, the inhibition of PDE-5 may be a promising adjunct in portal hypertension therapy.
Cirrhotic portal hypertension is partly caused by sinusoidal constriction, wherein the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway plays a pivotal role. Whereas in systemic circulation there is excess NO formation, NO availability is reduced inside the liver (“NO-paradox”). Currently non-selective beta-blockers (NSBB) are mainly used as medical therapy for portal hypertension. β1 receptor blockade reduces portal flow by decreasing cardiac output while β2 blockade allows unopposed α1-adrenergic activity resulting in splanchnic vasoconstriction and decreased portal inflow. However, their therapeutic efficacy is limited by frequent side effects such as circulatory dysregulation, which often prevent sufficient dosing, particularly in decompensated cirrhosis.
Many approaches have been proposed to specifically target the reduced NO availability in diseased liver. Liver specific NO delivering drugs (e.g., NCX-1000) yielded disappointing results. Statins lead to enhanced activity of endothelial NO synthase. Although statins reduced portal hypertension in a clinical setting, the effects on clinical outcome were modest. As vasodilating cGMP is converted to inactive 5’-GMP by phosphodiesterase-5 (PDE-5), PDE-5 inhibitors could exert a beneficial effect in portal hypertension therapy. However, preclinical and clinical studies on PDE-5 inhibition yielded promising but variable results.
Novel medical therapies of cirrhotic portal hypertension are clearly needed, because NSBB have only limited efficacy. Targeting the altered NO-cGMP pathway in liver cirrhosis may be an intriguing approach to solve this problem. The imbalance between vasodilating (cGMP) and vasoconstricting (e.g., endothelins) compounds may be positively influenced by inhibiting the conversion of cGMP to inactive 5’-GMP by PDE-5 inhibitors. In this preclinical study, liver disease-induced alterations of biochemical components of the NO-cGMP pathway, in which cGMP represents the most potent vasodilating compound, were investigated. The current study aimed to demonstrate a potential rationale for therapy of cirrhotic portal hypertension using PDE-5 inhibitors.
The study investigated alterations of the NO-cGMP in an animal model of liver fibrosis/cirrhosis. The aim was to show whether or not the data yielded a rationale for therapy of cirrhotic portal hypertension using inhibitors of PDE-5.
Gene expression of key components of the NO-cGMP pathway was investigated using PCR. Expression and localization of PDE-5 in healthy and cirrhotic livers was demonstrated by immunohistochemistry. The vasodilating compound cGMP was measured by ELISA. Systemic and portal hemodynamic parameters were monitored after application of NaCl, low-dose Sildenafil, and high-dose Sildenafil.
Key enzymes of the NO-cGMP system were overexpressed in cirrhosis: endothelial NO synthase (2.2-fold), soluble guanylate cyclase subunit α1 (1.7-fold) and soluble guanylate cyclase subunit β1 (3.0-fold), and PDE-5 (11-fold). Inducible NO synthase was expressed only in diseased livers. Immunohistochemistry showed a zonation of PDE-5 protein in healthy livers: Pronounced expression in zone 3 hepatocytes and slight expression in perisinusoidal cells (probably hepatic stellate cells). This zonation of PDE-5 was abrogated in cirrhotic livers. The enzyme was overexpressed in perisinusoidal cells. The vasodilating compound cGMP was reduced in liver cirrhosis. Inhibition of PDE-5 by Sildenafil could at least partly correct these disturbances: It led to a significant increase of cGMP and a reduction of portal venous pressure.
The present study provided evidence that alterations of the NO-cGMP pathway may play an important role in the pathogenesis of cirrhotic portal hypertension. In healthy liver a zonation of PDE-5 was found, which may have a significant function in maintaining an appropriate level of the vasodilating compound cGMP in the sinusoids. This zonation was lost in cirrhosis and PDE-5 was overexpressed in perisinusoidal cells resulting in decreased cGMP serum levels. This disturbance was at least partly counteracted by inhibition of PDE-5. Sildenafil induced a normalization of cGMP levels and a reduction of portal venous pressure.
The present study provides the rationale for the use of inhibitors of PDE-5 in the therapy of cirrhotic portal hypertension. Clinical studies are needed to test whether patients with portal hypertension in liver cirrhosis will benefit from application of PDE-5 inhibitors in terms of clinical endpoints such as prevention of (re)-bleeding from esophageal varices or prolonged survival.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garbuzenko DV S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1187] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 2. | García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121-S130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 141] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 4. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Mónica FZ, Bian K, Murad F. The Endothelium-Dependent Nitric Oxide-cGMP Pathway. Adv Pharmacol. 2016;77:1-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Rockey DC. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis. 2001;21:337-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2011] [Cited by in F6Publishing: 2037] [Article Influence: 226.3] [Reference Citation Analysis (2)] |
| 10. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 11. | Ge PS, Runyon BA. The changing role of beta-blocker therapy in patients with cirrhosis. J Hepatol. 2014;60:643-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 13. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-90.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | Van de Casteele M, Omasta A, Janssens S, Roskams T, Desmet V, Nevens F, Fevery J. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut. 2002;51:440-445. [PubMed] [Cited in This Article: ] |
| 15. | Shah V, Chen AF, Cao S, Hendrickson H, Weiler D, Smith L, Yao J, Katusic ZS. Gene transfer of recombinant endothelial nitric oxide synthase to liver in vivo and in vitro. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1023-G1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105:741-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Morales-Ruiz M, Cejudo-Martín P, Fernández-Varo G, Tugues S, Ros J, Angeli P, Rivera F, Arroyo V, Rodés J, Sessa WC. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003;125:522-531. [PubMed] [Cited in This Article: ] |
| 18. | Matei V, Rodríguez-Vilarrupla A, Deulofeu R, Colomer D, Fernández M, Bosch J, Garcia-Pagán JC. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Berzigotti A, Bellot P, De Gottardi A, Garcia-Pagan JC, Gagnon C, Spénard J, Bosch J. NCX-1000, a nitric oxide-releasing derivative of UDCA, does not decrease portal pressure in patients with cirrhosis: results of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010;105:1094-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Fiorucci S, Antonelli E, Tocchetti P, Morelli A. Treatment of portal hypertension with NCX-1000, a liver-specific NO donor. A review of its current status. Cardiovasc Drug Rev. 2004;22:135-146. [PubMed] [Cited in This Article: ] |
| 21. | Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 223] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 22. | Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, Marchiori RC, Rezende GF. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160-1170.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 24. | Clemmesen JO, Giraldi A, Ott P, Dalhoff K, Hansen BA, Larsen FS. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol. 2008;14:6208-6212. [PubMed] [Cited in This Article: ] |
| 25. | Lee KC, Yang YY, Huang YT, Lee FY, Hou MC, Lin HC, Lee SD. Administration of a low dose of sildenafil for 1 week decreases intrahepatic resistance in rats with biliary cirrhosis: the role of NO bioavailability. Clin Sci (Lond). 2010;119:45-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Lee KC, Yang YY, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol Res. 2008;38:1186-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rössle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006;23:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Halverscheid L, Deibert P, Schmidt R, Blum HE, Dunkern T, Pannen BH, Kreisel W. Phosphodiesterase-5 inhibitors have distinct effects on the hemodynamics of the liver. BMC Gastroenterol. 2009;9:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Tandon P, Inayat I, Tal M, Spector M, Shea M, Groszmann RJ, Garcia-Tsao G. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:546-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, Neagu M, Rössle M, Zipprich A, Caca K. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Wallace MC, Hamesch K, Lunova M, Kim Y, Weiskirchen R, Strnad P, Friedman SL. Standard operating procedures in experimental liver research: thioacetamide model in mice and rats. Lab Anim. 2015;49:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol. 2002;36:488-493. [PubMed] [Cited in This Article: ] |
| 33. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] [Cited in This Article: ] |
| 34. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [PubMed] [Cited in This Article: ] |
| 35. | Dunn OJ. Multiple Comparisons Using Rank Sums. Technometrics. 1964;6:241-252. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2025] [Cited by in F6Publishing: 1306] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 36. | Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, Vogt A, Dienes HP, Lammert F, Reichen J. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 38. | Iwakiri Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin Mol Hepatol. 2015;21:319-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Hasselblatt P, Rath M, Komnenovic V, Zatloukal K, Wagner EF. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:17105-17110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Dai Y, Cui J, Cun Y, Shi A. Tetrahydrobiopterin ameliorates hepatic ischemia-reperfusion Injury by coupling with eNOS in mice. J Surg Res. 2012;176:e65-e71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Theilig F, Bostanjoglo M, Pavenstädt H, Grupp C, Holland G, Slosarek I, Gressner AM, Russwurm M, Koesling D, Bachmann S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J Am Soc Nephrol. 2001;12:2209-2220. [PubMed] [Cited in This Article: ] |
| 42. | Davies NA, Hodges SJ, Pitsillides AA, Mookerjee RP, Jalan R, Mehdizadeh S. Hepatic guanylate cyclase activity is decreased in a model of cirrhosis: a quantitative cytochemistry study. FEBS Lett. 2006;580:2123-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Loureiro-Silva MR, Iwakiri Y, Abraldes JG, Haq O, Groszmann RJ. Increased phosphodiesterase-5 expression is involved in the decreased vasodilator response to nitric oxide in cirrhotic rat livers. J Hepatol. 2006;44:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Angeli P, Jiménez W, Veggian R, Fasolato S, Volpin R, MacHenzie HS, Craighero R, Libera VD, Sticca A, Arroyo V. Increased activity of guanosine 3’-5’-cyclic monophosphate phosphodiesterase in the renal tissue of cirrhotic rats with ascites. Hepatology. 2000;31:304-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Montoliu C, Kosenko E, Del Olmo JA, Serra MA, Rodrigo JM, Felipo V. Correlation of nitric oxide and atrial natriuretic peptide changes with altered cGMP homeostasis in liver cirrhosis. Liver Int. 2005;25:787-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Bezinover D, Kadry Z, Uemura T, Sharghi M, Mastro AM, Sosnoski DM, Dalal P, Janicki PK. Association between plasma cyclic guanosine monophosphate levels and hemodynamic instability during liver transplantation. Liver Transpl. 2013;19:191-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Kirstetter P, Moreau R, Vachiery F, Gadano A, Soupison T, Pilette C, Pussard E, Cailmail S, Takahashi H, Lebrec D. Plasma concentrations of cyclic 3’, 5’-guanosine monophosphate in patients with cirrhosis: relationship with atrial natriuretic peptide and haemodynamics. J Gastroenterol Hepatol. 1997;12:233-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Soto-Gutierrez A, Gough A, Vernetti LA, Taylor DL, Monga SP. Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems. Exp Biol Med (Maywood). 2017;242:1605-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Jungermann K. Metabolic zonation of liver parenchyma. Semin Liver Dis. 1988;8:329-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 107] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 133] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 51. | Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334:930-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |