Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16268
Revised: May 2, 2014
Accepted: July 16, 2014
Published online: November 21, 2014
AIM: To explore the potential correlation between insulin-like growth factor receptor-1 (IGF-1R) expression and rectal cancer radiosensitivity.
METHODS: Eighty-seven rectal cancer patients (cTNM I-III) treated in our department between January 2011 and December 2012 were enrolled. All subjects were treated with preoperative radiotherapy and radical resection of rectal carcinoma. Immunohistochemistry and reverse transcription polymerase chain reaction (RT-PCR) were performed to detect IGF-1R expression in pre-treatment and postoperative colorectal cancer specimens. Radiosensitivity for rectal cancer specimens was evaluated by observing rectal carcinoma mass regression combined with fibrosis on HE staining, degree of necrosis and quantity of remaining tumor cells. The relative IGF-1R expression was evaluated for association with tumor radiosensitivity.
RESULTS: Immunohistochemistry showed diffuse IGF-1R staining on rectal cancer cells with various degrees of signal density. IGF-1R expression was significantly correlated with cTNM staging (P = 0.012) while no significant association was observed with age, sex, tumor size and degree of differentiation (P = 0.424, 0.969, 0.604, 0.642). According to the Rectal Cancer Regression Grades (RCRG), there were 31 cases of RCRG1 (radiation sensitive), 28 cases of RCRG2 and 28 cases of RCRG3 (radiation resistance) in 87 rectal cancer subjects. IGF-1R protein hyper-expression was significantly correlated with a poor response to radiotherapy (P < 0.001, r = 0.401). RT-PCR results from pre-radiation biopsy specimens also showed that IGF-1R mRNA negative group exhibited a higher radiation sensitivity (P < 0.001, r = 0.497). Compared with the pre-radiation biopsy specimens, the paired post-operative specimens showed a significantly increased IGF-1R protein and mRNA expression in the residual cancer cells (P < 0.001, respectively).
CONCLUSION: IGF-1R expression level may serve as a predictive biomarker for radiosensitivity of rectal cancer before preoperative radiotherapy.
Core tip: This was the first report examining the relation between insulin-like growth factor receptor-1 (IGF-1R) expression and radiosensitivity in rectal cancer. Results showed that IGF-1R hyperexpression is significantly associated with poor response to radiotherapy. Compared with the pre-radiation biopsy specimens, the paired post-operative specimens showed a significantly increased IGF-1R expression in the residual cancer cells.
- Citation: Wu XY, Wu ZF, Cao QH, Chen C, Chen ZW, Xu Z, Li WS, Liu FK, Yao XQ, Li G. Insulin-like growth factor receptor-1 overexpression is associated with poor response of rectal cancers to radiotherapy. World J Gastroenterol 2014; 20(43): 16268-16274
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16268
Radiotherapy stands as one of the major supplementary treatments for rectal carcinoma. Preoperative radiotherapy effectively eliminates the peri-tumor subclinical loci, shrinks the tumor size, lowers the stage, maximizes the chance of anus reservation, decreases local recurrence and maximizes the survival rate[1-3]. However, not all rectal tumors respond well to radiation. Radiotherapy resistance stands as a major challenge in real world practice[4]. Prediction of sensitivity to radiotherapy is therefore of clinical significance in selecting patients suitable for preoperative treatment.
Conditions with increased insulin-like growth factor-1 (IGF-1) levels, such as acromegaly, are associated with an increased risk of malignancy, especially in colorectal cancer[5,6]. The expression of IGF-1 and IGF receptor-1 (IGF-1R) is also found in the majority of colorectal carcinoma specimens[7], which implies that IGF-1/IGF-1R may play an important role in the pathogenesis of colorectal carcinoma. Studies on IGF/IGF-1R signaling revealed that the binding of IGF-1 to its receptor activates a series of signal transduction events related to DNA damage repair, which is considered part of the radiation protection mechanism[8]. Based on these preliminary data, the current study sought to examine the relative expression of IGF-1R in biopsies collected before and after radiotherapy, which may provide exploratory evidence on the relationship between IGF-1R expression and sensitivity to radiotherapy.
Eighty-seven rectal cancer patients (cTNM I-III) treated in our department between January 2011 and December 2012 were enrolled. All subjects were treated with preoperative radiotherapy and radical resection of rectal carcinoma. Biopsies before radiotherapy were obtained via rectoscopy while biopsies after radiotherapy were collected during surgery. Chest X-ray, computed tomography (CT) and ultrasound examinations were performed to rule out metastases to lung, liver and peri-aortic lymph nodes. Varian 23EX linear accelerator was used for preoperative radiotherapy. Intensity-modulated radiation therapy (total dosage 50 Gy, 2 Gy/time, 25 times during a 5 wk period) were used for the treatment. Surgical operations were performed 4-6 wk after radiotherapy.
Reagents: Mouse anti-human IGF-1R monoclonal antibody was purchased from Novocastra (United Kingdom). Methodology: Tissue specimens were formalin-fixed, paraffin-embedded and consecutively sliced into 4-μm thick sections for immunohistochemistry. The sections were prepared with classic citric acid-SP immunohistochemistry methodology. The slides were routinely de-waxed and dehydrated followed by antigen repair under high temperature and high pressure (0.01 mmol/L citric acid buffer, 121 °C, 2 min). Routine immunohistochemistry staining was performed thereafter as follows: DAB color development, haematoxylin nucleus re-staining, ethanol gradient dehydration, transparentizing with dimethylbenzene and resin mounting. Results: IGF-1R staining shows yellow, yellowish brown or brown signal in cytoplasm. By analyzing the signal strength, non-staining is considered negative (score 0), yellow: weak positive (score 1), yellowish brown: medium positive (score 2) and dark brown is considered strong positive (score 3). Also, results are categorized by positive cell percentage as follows: 0% (score 0), 1%-10% (score 1), 10%-50% (score 2), 50%-100% (score 3). Final result is defined as the product of two independent scores. Score 0 is considered negative, score 1-3 is defined as +, score 4-6 is defined as ++, score 9 is defined +++, respectively. Five typical resolution fields were selected from each slide and total cell count is no less than 1000.
Preoperative sensitivity for rectal cancer specimens was evaluated by observing rectal carcinoma mass regression combined with observation of fibrosis in HE staining, degree of necrosis and quantity of remaining tumor cells. According to the Rectal Cancer Regression Grades (RCRG) proposed by Wheeler et al[9], RCRG 1: Sterilization or only microscopic foci of adenocarcinoma remaining, with marked fibrosis; 2: Marked fibrosis but presentation of macroscopic disease; 3: Little or no fibrosis with abundant macroscopic disease. The RCRG was assessed by two pathologists who were completely blinded to this study.
Reagents: Tissue RNA extraction kit and reverse transcription polymerase chain reaction (RT-PCR) kit were purchased from Promega (United States). IGF-1R primer sequence: Forward: 5’-TCT TCA AGT GCG GCT CCC T-3’, Reverse: 5’-TCA CGC TCT GCT TCA CCA C-3’. Beta-actin primer sequence: Forward 5’-CTT CCT GGG CAT GGA GTC-3’, Reverse 5’-GCC GAT CCA CAC GGA GTA-3’. All necrotic specimens were removed and no less than 90% tumor cell was confirmed with pathology before RT-PCR analysis. Fresh frozen tissue (10 mg) was pulverized and total RNA was extracted following the manufacturer’s instructions. RNA quality was confirmed with A260/280 ratio between 1.8-2.0 with an ultraviolet spectrophotometer. One step RT-PCR reaction (50 μL) was used for the current study. Reaction settings were as follows: 50 °C 30 min for reverse transcription, 95 °C 2 min followed by 95 °C denaturing for 50 s, 58 °C annealing for 30 s and 72 °C extension for 40 s. Twenty-eight cycles of reaction were performed before a final step of extension (72 °C) for 7 min. PCR products were separated with electrophoresis and analyzed with a Scion imaging analysis system. Relative IGF-1R expression was normalized by beta-actin internal control. Triplicates were used in the analysis and final results were calculated as the mean value of triplicates.
SPSS 10.0 software was used for statistical analysis. χ2 analysis was used for ratio comparison and Spearman test was used in correlation analysis. P < 0.05 was considered a statistically significant difference.
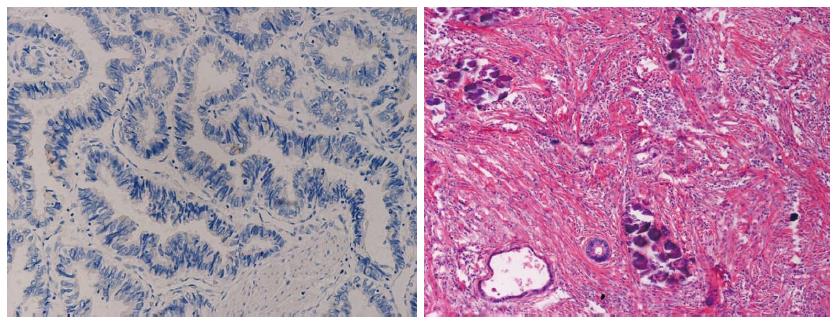
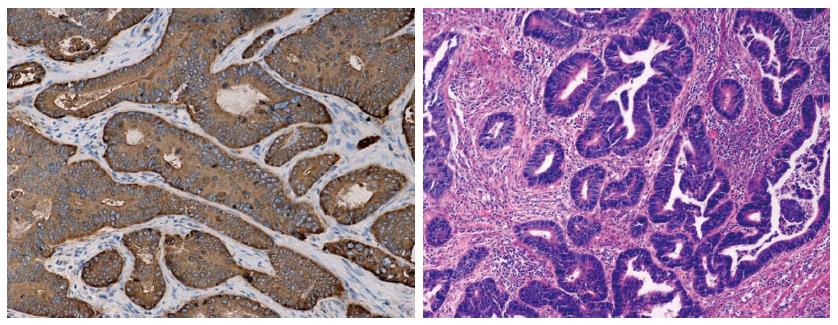
Immunohistochemistry showed diffuse IGF-1R staining on rectal cancer cells with various degrees of signal density (Figures 1 and 2). With 1:120 antibody concentration, very rare positive staining was observed in surrounding stromal cells. There was strong IGF-1R expression (++ to +++ in 54 out of 87 subjects) in rectal carcinoma specimens before radiotherapy. IGF-1R expression is significantly correlated with cTNM staging (P = 0.012) while no significant association was observed with age, sex, tumor size and degree of differentiation (P = 0.424, 0.969, 0.604, 0.642 respectively, Table 1)
| Clinico-pathological parameters | Cases | IGF-1R | P value | |
| 0-+ | ++-+++ | |||
| Age | ||||
| < 55 | 43 | 14 | 29 | |
| ≥ 55 | 44 | 19 | 25 | 0.424 |
| Gender | ||||
| Male | 49 | 18 | 31 | |
| Female | 38 | 15 | 23 | 0.969 |
| Stage | ||||
| I-II | 47 | 24 | 23 | |
| III | 40 | 9 | 31 | 0.012a |
| Differentiation | ||||
| Well, moderately | 51 | 21 | 30 | |
| Poorly | 36 | 12 | 24 | 0.604 |
| Size (cm) | ||||
| < 4 | 46 | 19 | 27 | |
| ≥ 4 | 41 | 14 | 27 | 0.642 |
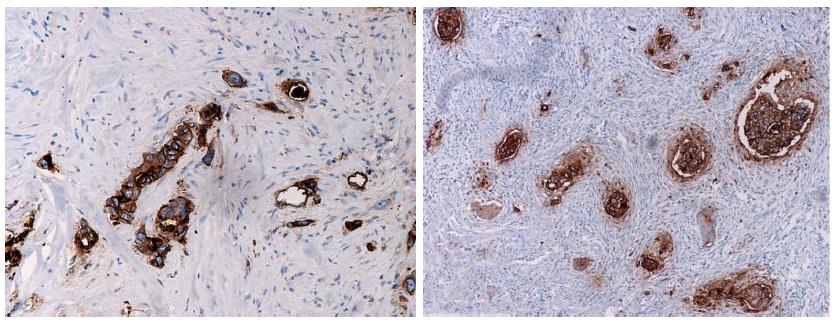
Compared with preoperative biopsy specimens, the remaining tumor cells in the corresponding 83 surgical specimens obtained after radiotherapy showed stronger IGF-1R expression (P < 0.001, Figure 3). Four subjects had no remaining tumor cells after radiotherapy. Most of the specimens that underwent radiotherapy showed strong IGF-1R expression (+++, 47/83, 56.6% or ++ 19/83, 22.9%). There were another 13 subjects with negative IGF-1R expression before radiotherapy who showed IGF-1R protein expression afterwards.
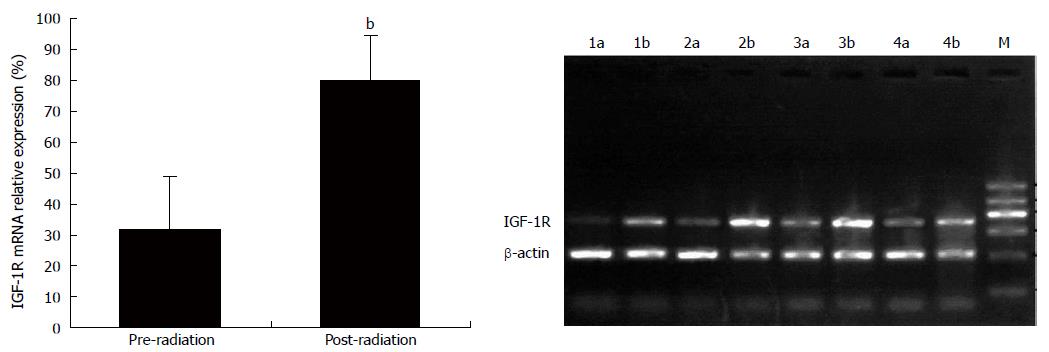
The product of RT-PCR is a 427 bp fragment. Seventy-seven out of 87 rectal carcinoma specimens from patients who underwent radiotherapy showed IGF-1R mRNA expression, which is consistent with immunohistochemistry data (P > 0.05). Part of the surgical specimen, especially those with a Stage 1 reaction to radiotherapy, did not meet the standards for RT-PCR analysis due to fibrosis and necrosis in most of the tumor tissues. Fifty-eight subjects met the standards for RT-PCR (tumor cell > 90%). Compared with the corresponding specimens that underwent radiotherapy, IGF-1R mRNA expression was robustly up-regulated (Figure 4), which was consistent with IGF-1R protein expression as shown in immunohistochemistry results.
In 87 rectal cancer subjects, there were 31 cases of RCRG1, 28 cases of RCRG2 and 28 cases of RCRG3. In 4 RCRG1 cases, rectal tumor cells were completely eliminated as manifested by reactive fibrosis, necrosis and calcification after radiotherapy on HE slides.
The association between IGF-1R expression in rectal carcinoma specimens before radiotherapy and sensitivity to radiotherapy is summarized in Table 2. Subjects with low IGF-1R protein expression (0-+) in immunohistochemistry showed improved sensitivity to radiotherapy (P < 0.001, r = 0.401). RT-PCR data revealed that subjects with negative IGF-1R mRNA expression showed higher sensitivity to radiotherapy (P < 0.001, r = 0.497). Out of 10 cases with negative IGF-1R before radiotherapy, 8 cases showed RCRG1 reactions and 2 cases showed RCRG2 reactions, with noRCRG3 reactions. Typical cases were shown in Figures 1 and 2.
| IGF-1R expression | Cases | RCRG | P value | r | ||
| 1 | 2 | 3 | ||||
| IHC | ||||||
| 0-+ | 33 | 18 | 9 | 6 | ||
| ++-+++ | 54 | 13 | 19 | 22 | < 0.001 | 0.401 |
| RT-PCR | ||||||
| - | 10 | 8 | 2 | 0 | ||
| + | 77 | 23 | 26 | 28 | < 0.001 | 0.497 |
The outcome of rectal cancer radiotherapy varies dramatically in different patients. The sensitivity to radiotherapy remains a major concern for clinicians. It has been documented that radiation-mediated clonogenic cell death in solid tumors stands as the major underlying mechanism by which radiation exerts its therapeutic benefits[10,11]. With the advancement of research on cell cycle regulation apoptosis and DNA repair, scientists endeavored to identify molecular biomarkers for radiotherapy sensitivity. Data showed that mutations of anti-tumor gene p53, expression of Ku-70 (an important DNA protease involved in DNA damage repair), expression of cell proliferation marker Ki-67, and up-regulation of PCNA are all considered to be negatively associated with sensitivity to radiotherapy[12,13]. Even though there is a rapid accumulation of related literature, the significance of those molecular biomarkers in clinical contexts remains controversial, which warrants further investigations on by-path biomarkers for radiotherapy sensitivity[14,15].
This is the first report examining the relation between IGF-1R expression and tumor radiosensitivity in rectal cancer. Results showed that IGF-1R hyperexpression is associated with a poor response to radiotherapy. In contrast, all 10 subjects with negative IGF-1R expression showed a good response to radiotherapy (8 cases of RCRG1 and 2 cases of RCRG2). The potential underlying mechanism may involve a previous report in which Héron-Milhavet et al[16] reported that the binding of IGF to IGF-1R could ameliorate radiation-induced cell damage in an IGF-1R positive mouse fibroblast NIH-3T3 cell line. This mechanism at least partially involves activation of MDM2/p53/p21 mediated DNA damage repair signaling machinery. As a matter of fact, IGF-IGF-1R binding activates DNA damage repair signaling cascades which include PI3K, MAPK and 14-3-3 protein etc.[17]. Part of these signaling molecules activates DNA damage repair genes, including GADD45 and APEN, which facilitates DNA repair in those scenarios and is considered to be involved in the therapeutic effect of radiation[18]. Recently, IGF-1R has been also associated with new partners, such as major vault proteins, BCL-2, BAX, or Ku70/80, related to regulation of apoptosis, and nonhomologous end-joining DNA repair[19-22]. Thus we propose that the higher protein and mRNA expression of IGF-1R may indicate the stronger DNA damage repair and anti-apoptosis capability, which means more surviving tumor cells and radiotherapy resistance.
In addition, there was a significant up-regulation of IGF-1R protein and mRNA in remaining rectal adenocarcinoma cells after radiotherapy compared to that before radiotherapy. One potential explanation for this observation indicates that IGF-1R hyperexpressed part of tumor may be the part with better tolerance to radiation, which culminates in better survival after radiotherapy. An alternative explanation suggests that this phenomenon may represent a self-protection reaction to radiotherapy. By reactive up-regulation of IGF-1R expression, there is an increase of DNA damage repair in tumor cells which warrants stronger protection to radiation. Therefore, tumor cells lack of reactive feedback will be eliminated by radiation. This is probably why remaining rectal adenocarcinoma cells, though very few in number, showed strong expression of IGF-1R after radiotherapy. Given that this reactive IGF-1R upregulation does exist in pathophysiological condition, blockage of IGF/IGR-1R signal transduction may enhance rectal cancer sensitivity to radiation. As a hot target for specific tumor treatment, monoclonal antibodies to IGF-1R and small molecule kinase inhibitors have entered the clinical trial stage of development[23] Thus a discussion of regulation of this pathway combined with radiotherapy stands as an important question for clinical practice.
In general, our results show that IGF-1R may serve as a novel radiotherapy sensitivity indicator for rectal cancer patients. IGF-1R hyperexpression in rectal cancer may indicate a better tolerance to preoperative radiotherapy. Further investigations may be directed to address the following questions: (1) the protective effect of IGF-1 in radiotherapy should be confirmed in colorectal cancer cells in vitro; and (2) how IGF-1/IGF-1R signal transduction regulates feedback to radiotherapy mediated cell stress.
Conditions with increased insulin-like growth factor-1 (IGF-1) levels, such as acromegaly, are associated with an increased risk of malignancy, especially in colorectal cancer. This implies that IGF-1/insulin-like growth factor receptor-1 (IGF-1R) may play an important role in the pathogenesis of colorectal carcinoma. Studies on IGF/IGF-1R signaling revealed that the binding of IGF-1 to its receptor activates a series of signal transduction events related to DNA damage repair, which is considered part of the radiation protection mechanism. Based on these preliminary data, the current study sought to examine the relative expression of IGF-1R in biopsies collected before and after radiotherapy, which may provide exploratory evidence on the relationship between IGF-1R expression and sensitivity to radiotherapy.
The outcome of rectal cancer radiotherapy varies dramatically in different patients. The sensitivity to radiotherapy remains as a major concern for clinicians. Even though there is a rapid increase in related literature, the significance of those molecular biomarkers in clinical contexts remains controversial, which warrants further investigations on by-path biomarkers for radiotherapy sensitivity.
This is the first report examining the relation between IGF-1R expression and tumor radiosensitivity in rectal cancer. Results showed that IGF-1R hyperexpression is associated with poor response to radiotherapy. In addition, there was a significant up-regulation of IGF-1R protein and mRNA in remaining rectal adenocarcinoma cells after radiotherapy compared to that before radiotherapy. Based on the DNA repair theory, authors could partially interpret our data: the higher protein and mRNA expression of IGF-1R may indicate the stronger DNA damage repair and anti-apoptosis capability, which means more surviving tumor cells and radiotherapy resistance. But blockage of IGF/IGR-1R signal transduction may enhance rectal cancer sensitivity to radiation.
As a hot target for specific tumor treatment, monoclonal antibodies to IGF-1R and small molecule kinase inhibitors have entered the clinical trial stage of development. Thus a discussion of regulation of this pathway combined with radiotherapy stands as an important question for clinical practice. This article can stand as part of the theoretical basis.
Authors investigated the relationship between insulin-like growth factor-1 expression and preoperative response of radiotherapy in rectal cancers. They showed IGF-1R overexpression was significantly correlated with a poor response to radiotherapy, from the results of immunohistochemistry and reverse transcription-polymerase chain reaction. The results look informative to the gastroenterologists.
P- Reviewer: George V, Muguruma N, Wittmann T S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Martijn H, Voogd AC, van de Poll-Franse LV, Repelaer van Driel OJ, Rutten HJ, Coebergh JW. Improved survival of patients with rectal cancer since 1980: a population-based study. Eur J Cancer. 2003;39:2073-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644-5650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 540] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 3. | Rule W, Meyer J. Current status of radiation therapy for the management of rectal cancer. Crit Rev Oncog. 2012;17:331-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Rivera S, Villa J, Quero L, Hennequin C. Adjuvant radiotherapy for rectal cancer: recent results, new questions. Clin Res Hepatol Gastroenterol. 2011;35:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Renehan AG, O’Connell J, O’Halloran D, Shanahan F, Potten CS, O’Dwyer ST, Shalet SM. Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm Metab Res. 2003;35:712-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab. 2001;86:2935-2941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Shiratsuchi I, Akagi Y, Kawahara A, Kinugasa T, Romeo K, Yoshida T, Ryu Y, Gotanda Y, Kage M, Shirouzu K. Expression of IGF-1 and IGF-1R and their relation to clinicopathological factors in colorectal cancer. Anticancer Res. 2011;31:2541-2545. [PubMed] [Cited in This Article: ] |
| 8. | Trojanek J, Ho T, Del Valle L, Nowicki M, Wang JY, Lassak A, Peruzzi F, Khalili K, Skorski T, Reiss K. Role of the insulin-like growth factor I/insulin receptor substrate 1 axis in Rad51 trafficking and DNA repair by homologous recombination. Mol Cell Biol. 2003;23:7510-7524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Wheeler JM, Warren BF, Mortensen NJ, Ekanyaka N, Kulacoglu H, Jones AC, George BD, Kettlewell MG. Quantification of histologic regression of rectal cancer after irradiation: a proposal for a modified staging system. Dis Colon Rectum. 2002;45:1051-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999;6:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Zaider M, Hanin L. Tumor control probability in radiation treatment. Med Phys. 2011;38:574-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Komuro Y, Watanabe T, Hosoi Y, Matsumoto Y, Nakagawa K, Saito S, Ishihara S, Kazama S, Tsuno N, Kitayama J. Prediction of tumor radiosensitivity in rectal carcinoma based on p53 and Ku70 expression. J Exp Clin Cancer Res. 2003;22:223-228. [PubMed] [Cited in This Article: ] |
| 13. | Komuro Y, Watanabe T, Hosoi Y, Matsumoto Y, Nakagawa K, Tsuno N, Kazama S, Kitayama J, Suzuki N, Nagawa H. The expression pattern of Ku correlates with tumor radiosensitivity and disease free survival in patients with rectal carcinoma. Cancer. 2002;95:1199-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Smith FM, Reynolds JV, Miller N, Stephens RB, Kennedy MJ. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. Eur J Surg Oncol. 2006;32:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Héron-Milhavet L, LeRoith D. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem. 2002;277:15600-15606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Peruzzi F, Prisco M, Morrione A, Valentinis B, Baserga R. Anti-apoptotic signaling of the insulin-like growth factor-I receptor through mitochondrial translocation of c-Raf and Nedd4. J Biol Chem. 2001;276:25990-25996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Dittmann KH, Gueven N, Mayer C, Rodemann HP. The radioprotective effect of BBI is associated with the activation of DNA repair-relevant genes. Int J Radiat Biol. 1998;74:225-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lloret M, Lara PC, Bordón E, Rey A, Falcón O, Apolinario RM, Clavo B, Ruiz A. MVP expression is related to IGF1-R in cervical carcinoma patients treated by radiochemotherapy. Gynecol Oncol. 2008;110:304-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lloret M, Lara PC, Bordón E, Fontes F, Rey A, Pinar B, Falcón O. Major vault protein may affect nonhomologous end-joining repair and apoptosis through Ku70/80 and bax downregulation in cervical carcinoma tumors. Int J Radiat Oncol Biol Phys. 2009;73:976-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Amsel AD, Rathaus M, Kronman N, Cohen HY. Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc Natl Acad Sci USA. 2008;105:5117-5122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Wang Q, Gao F, May WS, Zhang Y, Flagg T, Deng X. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell. 2008;29:488-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Hofmann F, García-Echeverría C. Blocking the insulin-like growth factor-I receptor as a strategy for targeting cancer. Drug Discov Today. 2005;10:1041-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |