Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.14004
Revised: May 12, 2014
Accepted: June 14, 2014
Published online: October 14, 2014
AIM: To highlight magnetic resonance enterography (MRE) for diagnosis of patients with refractory iron deficiency anemia and normal endoscopy results.
METHODS: Fifty-three patients diagnosed with iron deficiency anemia refractory to treatment and normal gastroscopy and colonoscopy results were admitted to this prospective study between June 2013 and December 2013. All patients underwent a standardized MRE examination with a 1.5 Tesla magnetic resonance imaging system using two six-channel phased-array abdominal coils. Adequate bowel distention and fast imaging sequences were utilized to achieve diagnostic accuracy. All segments of the small bowel, duodenum, jejunum, and ileum were examined in detail. All cases were examined independently by two radiologists with > 5 years of experience in abdominal magnetic resonance imaging. A consensus reading was performed for each patient following image examination. Both radiologists were blinded to patient history, laboratory findings, and endoscopy results.
RESULTS: Twenty (37.7%) male and 33 (62.3%) female patients were included in the study. The mean age of the patients was 52.2 ± 13.6 years (range: 19-81 years, median 51.0). The age difference between the male and female patient groups was not statistically significant (54.8 ± 16.3 years vs 50.7 ± 11.7 years). MRE results were normal for 49 patients (92.5%). Four patients had abnormal MRE results. One patient with antral thickening was diagnosed with antral gastritis in the second-look gastroscopy. One patient had focal wall thickening in the 3rd and 4th portions of the duodenum. The affected areas were biopsied in a subsequent duodenoscopy, and adenocarcinoma was diagnosed. One patient had a fistula and focal contrast enhancement in the distal ileal segments, consistent with Crohn’s disease. One patient had focal wall thickening with luminal narrowing in the mid-jejunum that was later biopsied during a double-balloon enteroscopy, and lymphoma was diagnosed.
CONCLUSION: MRE is a non-invasive and effective alternative for evaluating possible malignancies of the small intestines and can serve as a guide for a second-look endoscopy.
Core tip: This study stresses the importance of magnetic resonance enterography (MRE) for small bowel pathologies in patients with iron deficiency anemia refractory to treatment and normal gastroscopy and colonoscopy findings. The prospect of occult bleeding must be considered in such patients. This study of 53 patients demonstrates that in cases of negative upper endoscopy and colonoscopy, MRE is a non-invasive and effective examination method for the evaluation of potential neoplastic processes of the small intestines. Furthermore, MRE can serve as a guide for a second-look endoscopy and double-balloon enteroscopy.
- Citation: Cengic I, Tureli D, Aydin H, Bugdayci O, Imeryuz N, Tuney D. Magnetic resonance enterography in refractory iron deficiency anemia: A pictorial overview. World J Gastroenterol 2014; 20(38): 14004-14009
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/14004.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.14004
Iron deficiency due to nutritional causes is the most frequent cause of microcytic hypochromic anemia. Occult gastrointestinal losses, intestinal malabsorption, chronic infection, and inflammatory conditions of the gastrointestinal tract can all lead to iron deficiency anemia refractory to treatment. Occult gastrointestinal bleeding is challenging for clinicians to evaluate, especially when considering small bowel segments as the potential culprit. The stomach and colon are easily visualized using endoscopic techniques. However, traditional endoscopy has limited success rates in evaluation of the jejunum and ileum due to the organs’ length and location[1,2].
Magnetic resonance enterography (MRE), owing its success to the development of rapid imaging sequences, is a viable alternative to more conventional methods studying the small bowel. The aim of the present study was to determine the role of MRE in evaluating small bowel pathologies in patients with iron deficiency anemia refractory to treatment who have normal gastroscopy and colonoscopy findings.
Fifty-three patients diagnosed with iron deficiency anemia refractory to treatment and normal gastroscopy and colonoscopy results were admitted to this prospective study between June 2013 and December 2013. An institutional review board approval was obtained prior to the study. Each patient provided written informed consent. All patients underwent a standardized MRE examination with a 1.5 Tesla magnetic resonance imaging (MRI) system (Magnetom Symphony; Siemens, Munich, Bavaria, Germany) using two six-channel phased-array abdominal coils.
Adequate bowel distention and fast imaging sequences were utilized to achieve diagnostic accuracy. MRE was performed with patients in the supine position. Forty minutes prior to the examination, an oral solution containing 120 g of polyethylene glycol 3350 and electrolytes (Golytely; Braintree Laboratories Inc., Braintree, MA, United States) diluted in 1.5 L of water was used as the biphasic oral contrast agent. To decrease bowel motility and prevent peristaltic artifacts, 0.5 mg of glucagon (Glucagen; Novo-Nordisk, Bagsvaerd, Denmark) was administered intravenously 5 min before the examination. An additional dose of 0.5 mg glucagon was injected after the non-contrast sequences and just before the intravenous contrast administration.
After the localizer scout images were obtained, the following were applied sequentially: (1) coronal T2-weighted fast imaging with steady-state precession (FISP) [TR/TE 3.85/1.93 ms, slice thickness 5 mm, slice gap 0 mm, field of view (FOV) 450 mm]; (2) axial T2-weighted FISP (TR/TE 4.11/2.06 ms, slice thickness 5 mm, slice gap 0 mm, FOV 350 mm); (3) coronal T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) (TR/TE 800/81, slice thickness 6 mm, slice gap 0 mm, FOV 400 mm); (4) axial T2-weighted HASTE (TR/TE 1200/81, slice thickness 6 mm, slice gap 0 mm, FOV 360 mm); and (5) axial fat-saturated 3D volumetric interpolated breath-hold examination (VIBE) (TR/TE 5.13/2.33, slice thickness 5 mm, slice gap 0.2 mm, FOV 340 mm); and (6) coronal fat-saturated 3D VIBE (TR/TE 5.12/2.50, slice thickness 5 mm, slice gap 0.2 mm, FOV 366 mm). Gadolinium-based contrast agent [1 mmol/mL gadobutrol (Gadovist), Bayer Schering, Berlin-Wedding, Germany] was given intravenously as a 0.65 mg/kg bolus dose, followed by axial fat-saturated 3D VIBE (TR/TE 5.13/2.33, slice thickness 5 mm, slice gap 0.2 mm, FOV 340 mm) and coronal fat-saturated 3D VIBE (TR/TE 5.12/2.50, slice thickness 5 mm, slice gap 0.2 mm, FOV 366 mm).
Two radiologists with > 5 years of experience in abdominal MRI examined all cases independently, followed by a consensus reading for each patient. Both radiologists were blinded to patient history, laboratory findings, and endoscopy results. All of the small intestine segments were examined in Digital Imaging and Communications in Medicine image formats on a Picture Archiving Communication System workstation (Leonardo; Siemens).
Demographic variables were analyzed using the Student’s t-test (SPSS version 17, SPSS Inc., Chicago, IL, United States). A P value < 0.05 was considered statistically significant.
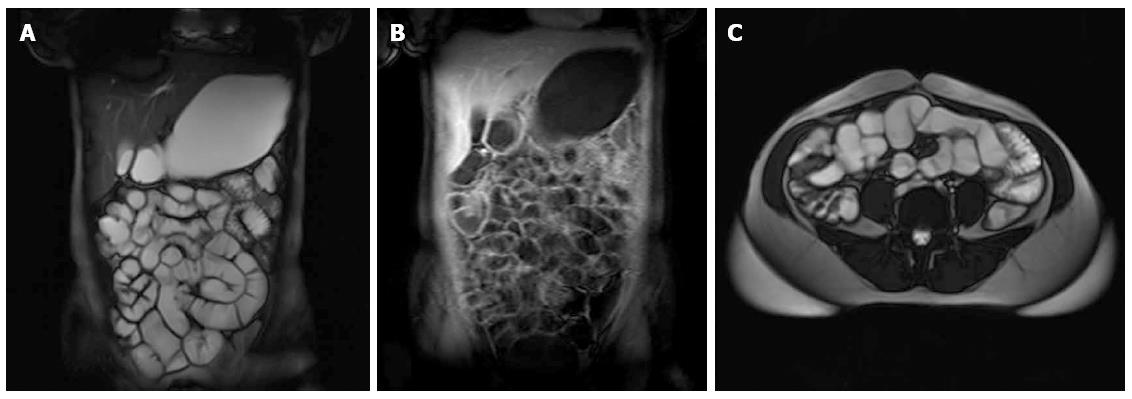
Twenty (37.7%) male and 33 (62.3%) female patients were included in the study. The mean patient age was 52.2 ± 13.6 years (range: 19-81 years, median 51.0). There was no significant age difference between the male and female patient groups (54.8 ± 16.3 vs 50.7 ± 11.7 years). Forty-nine of the 53 patients (92.5%) had normal MRE findings (Figure 1). The mean age of patients with normal MRE was 51.2 ± 13.4 years, and the mean age of patients with abnormal findings was 64.5 ± 10.9 years. This difference was not statistically significant.
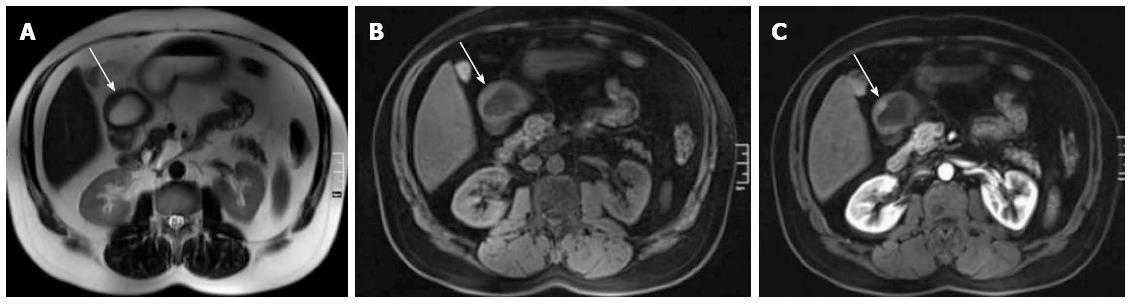
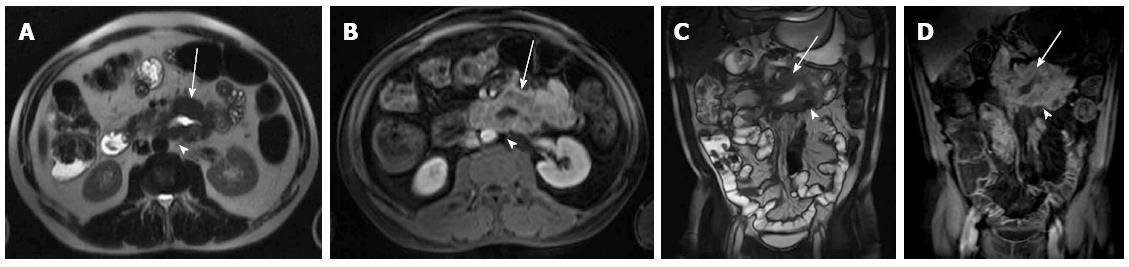
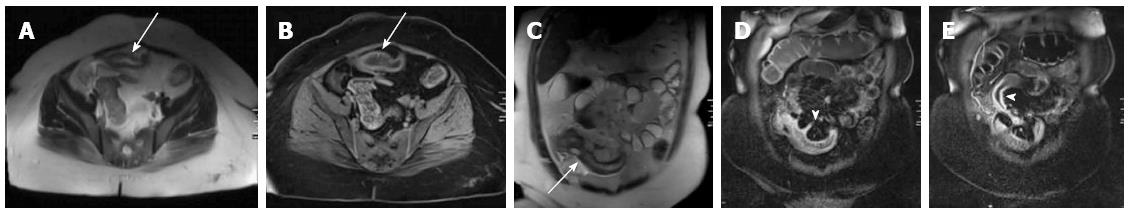
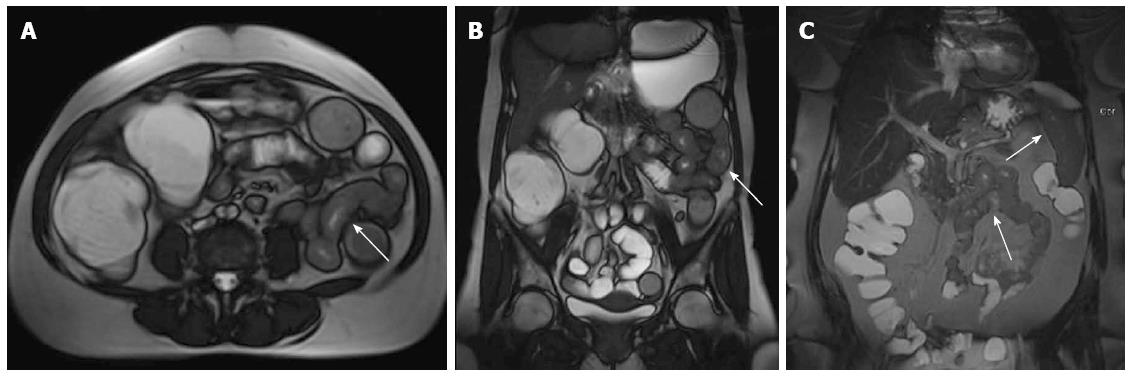
Four patients had the following abnormalities in MRE images: (1) antral thickening (Figure 2); (2) asymmetric focal wall thickening and contrast enhancement in the 3rd and 4th portions of the duodenum (Figure 3); (3) a fistula and focal contrast enhancement in distal ileal segments (Figure 4); and (4) segmental wall thickening with luminal narrowing in the mid-jejunum (Figure 5). The antral thickening was shown to be antral gastritis in the second-look gastroscopy. Double-balloon enteroscopy revealed a narrow segment in the distal duodenum, which made the impression of duodenal wall infiltration with the mucosa eroded and fragile. The biopsy result was adenocarcinoma of the duodenum. The images of the abnormality in the ileal segment were almost pathognomonic for Crohn’s disease. A biopsy from the distal ileum confirmed the diagnosis. The mid-jejunum segment was biopsied during double-balloon enteroscopy and diagnosed as lymphoma.
Occult gastrointestinal bleeding is an important cause of iron deficiency anemia refractory to treatment. The condition causes recurrent or persistent blood loss despite negative initial endoscopic and radiologic evaluations, regardless of the fecal occult blood test result. Approximately 5% of all gastrointestinal bleeding is occult and is frequently caused by lesions of the small bowel[3-7].
The stomach and colon can be evaluated very efficiently with traditional endoscopic techniques. On the other hand, jejunal and proximal ileal segment evaluations are limited owing to their length and location[8-11]. New methods have been devised for better evaluation of the small bowel including capsule endoscopy and double-balloon enteroscopy[12-16]. Barium studies of the small bowel are limited due to the fact that only abnormalities of the lumen can be visualized. In order to visualize the extraluminal structures, cross-sectional imaging techniques may be used, such as computed tomography (CT), ultrasonography, and MRI[17]. The main disadvantage of CT enterography is its use of ionizing radiation. On the other hand, radiation exposure is not a concern in ultrasonography, making this method more suitable, especially for younger patients. However, ultrasonography is quite limited due to obscuring of the field of view by bowel gas.
Due to long acquisition times, which lead to respiratory and peristalsis artifacts, MRI of the small intestines was not technically possible until rather recently. The development of rapid imaging techniques and the possibility to obtain images in a single breath-hold has made this feasible. The lack of ionizing radiation and increased patient comfort during the procedure, compared to conventional or magnetic resonance enteroclysis, made MRE a more favorable method for examining the small bowel[18-20]. In addition, when compared to CT, magnetic resonance has four-times more contrast resolution[21].
A weakness of MRE, compared to magnetic resonance enteroclysis, is the suboptimal distension of the jejunum. Although this problem is less pronounced with the use of hyperosmolar oral contrast agents such as polyethylene glycol, it may still be a cause for concern. All hyperosmolar luminal contrast agents have a probability of inducing diarrhea, causing patient discomfort. On the other hand, patient comfort and tolerability is much better with MRE[20,22].
One of the major MRE applications is the ability to monitor disease activity and extraluminal complications of Crohn’s disease, such as mesenteric inflammation and fistula formation[23-26]. MRE may also be used for evaluation of patients with symptoms related to jejunoileal segments, ruling out neoplastic processes of the small bowel. High patient acceptance and absence of ionizing radiation has made MRE a very feasible alternative to the more conventional methods, such as colonoscopy, especially when used for frequent follow-ups of Crohn’s disease[27-30].
Double-balloon enteroscopy allows for visualization and tissue sample harvesting from all segments of the small bowel. However, the method’s value as a diagnostic and therapeutic technique clearly increases when used in conjunction with MRE. In our study, MRE made it possible to identify those patients who needed further evaluation with double-balloon enteroscopy (3/53). MRE also guided the operator in choosing the place of entry-oral in two patients or anal in one case-by identifying the target intestinal segment prior to the procedure.
In summary, patients with iron deficiency anemia refractory to treatment must be examined for occult bleeding. In cases of negative upper endoscopy and colonoscopy, MRE presents a non-invasive and effective examination method for evaluating the possibility of neoplastic processes of the small intestine. Moreover, MRE can serve as a guide for a second-look endoscopy and double-balloon enteroscopy.
Microcytic hypochromic anemia is most frequently caused by iron deficiency due to nutritional causes. Occult gastrointestinal losses, intestinal malabsorption, chronic infection, and inflammatory conditions of the gastrointestinal tract can all lead to iron deficiency anemia refractory to treatment. Clinicians are presented with a challenge when evaluating occult gastrointestinal bleeding, especially when small bowel segments are considered as the potential culprit.
Magnetic resonance enterography (MRE) is a more favorable method for small bowel examination than either conventional or magnetic resonance enteroclysis due to the lack of ionizing radiation and increased patient comfort during the procedure.
As this 53-patient study demonstrates, MRE is a non-invasive and effective examination method for evaluating the possibility of neoplastic processes of the small intestine in cases of negative upper endoscopy and colonoscopy. This paper is the first to report a study of this magnitude.
Visualization and tissue sample collection of whole small bowel segments is possible with double-balloon enteroscopy. However, the method’s value as a diagnostic and therapeutic technique increases when used in conjunction with MRE. MRE can serve as a guide for a second-look endoscopy and double-balloon enteroscopy.
MRE, a rapid magnetic resonance imaging technique, allows for the acquisition of images in a single breath-hold. Small bowel distension is achieved by using hyperosmolar oral contrast agents such as polyethylene glycol.
This manuscript is of great interest. The authors propose MRE as a non-invasive and effective alternative for evaluating possible malignancies of the small intestine. Furthermore, the procedure may serve as a guide for a second-look endoscopy for those patients suffering from iron deficiency anemia refractory to treatment.
P- Reviewer: Li YZ S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Gralnek IM. Obscure-overt gastrointestinal bleeding. Gastroenterology. 2005;128:1424-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Rockey DC. Occult gastrointestinal bleeding. N Engl J Med. 1999;341:38-46. [PubMed] [Cited in This Article: ] |
| 4. | Singh V, Alexander JA. The evaluation and management of obscure and occult gastrointestinal bleeding. Abdom Imaging. 2009;34:311-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Leighton JA, Goldstein J, Hirota W, Jacobson BC, Johanson JF, Mallery JS, Peterson K, Waring JP, Fanelli RD, Wheeler-Harbaugh J. Obscure gastrointestinal bleeding. Gastrointest Endosc. 2003;58:650-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | American Gastroenterological Association medical position statement: evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Moawad FJ, Veerappan GR, Wong RK. Small bowel is the primary source of obscure gastrointestinal bleeding. Gastroenterology. 2008;135:1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Atar M, Kadayifci A. Transnasal endoscopy: Technical considerations, advantages and limitations. World J Gastrointest Endosc. 2014;6:41-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo SE. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75:373-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Appleyard M, Fireman Z, Glukhovsky A, Jacob H, Shreiver R, Kadirkamanathan S, Lavy A, Lewkowicz S, Scapa E, Shofti R. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 401] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Di Caro S, May A, Heine DG, Fini L, Landi B, Petruzziello L, Cellier C, Mulder CJ, Costamagna G, Ell C. The European experience with double-balloon enteroscopy: indications, methodology, safety, and clinical impact. Gastrointest Endosc. 2005;62:545-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | May A, Nachbar L, Pohl J, Ell C. Endoscopic interventions in the small bowel using double balloon enteroscopy: feasibility and limitations. Am J Gastroenterol. 2007;102:527-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Larkman DJ, Nunes RG. Parallel magnetic resonance imaging. Phys Med Biol. 2007;52:R15-R55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Maris T, Prassopoulos P. MR imaging of the small bowel with a true-FISP sequence after enteroclysis with water solution. Invest Radiol. 2000;35:707-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Negaard A, Sandvik L, Berstad AE, Paulsen V, Lygren I, Borthne A, Klow NE. MRI of the small bowel with oral contrast or nasojejunal intubation in Crohn’s disease: randomized comparison of patient acceptance. Scand J Gastroenterol. 2008;43:44-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Bushong SC. Magnetic resonance imaging: physical and biological principles. 3rd ed. St. Louis: Mosby 2003; 7-9. [Cited in This Article: ] |
| 22. | Laghi A, Carbone I, Catalano C, Iannaccone R, Paolantonio P, Baeli I, Trenna S, Passariello R. Polyethylene glycol solution as an oral contrast agent for MR imaging of the small bowel. AJR Am J Roentgenol. 2001;177:1333-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Sinha R, Verma R, Verma S, Rajesh A. MR enterography of Crohn disease: part 1, rationale, technique, and pitfalls. AJR Am J Roentgenol. 2011;197:76-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Messaris E, Chandolias N, Grand D, Pricolo V. Role of magnetic resonance enterography in the management of Crohn disease. Arch Surg. 2010;145:471-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Siddiki HA, Fidler JL, Fletcher JG, Burton SS, Huprich JE, Hough DM, Johnson CD, Bruining DH, Loftus EV, Sandborn WJ. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Ippolito D, Invernizzi F, Galimberti S, Panelli MR, Sironi S. MR enterography with polyethylene glycol as oral contrast medium in the follow-up of patients with Crohn disease: comparison with CT enterography. Abdom Imaging. 2010;35:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, Bruesewitz MR, McCollough CH, Sandborn WJ, Loftus EV. Crohn Disease: mural attenuation and thickness at contrast-enhanced CT Enterography--correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Masselli G, Casciani E, Polettini E, Gualdi G. Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn’s disease. Eur Radiol. 2008;18:438-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |