Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9497
- This article has been corrected.
- See: World J Gastroenterol. Jul 14, 2021; 27(26): 4246-4247
Revised: February 14, 2014
Accepted: April 8, 2014
Published online: July 28, 2014
AIM: To investigate the effect of knockdown of Forkhead box M1 (FoxM1) on the proliferation and invasion capacities of human gallbladder carcinoma (GBC)-SD cells.
METHODS: Four FoxM1 shRNAs were transfected into GBC-SD cells with Lipofectamine 2000 to select the appropriate shRNA for down-regulation of FoxM1. A recombinant lentivirus for shFoxM1 (Lv-shFoxM1), which expresses FoxM1-specific shRNA, and a negative control carrying green fluorescent protein, which expresses a scrambled RNA, were constructed. After transfection with the recombinant adenovirus and screened with puromycin, RT-PCR and Western blot were utilized to evaluate the inhibition efficiency. Cell viability was evaluated by MTT assay, and cell migration and invasion were assessed using the Transwell system. Cells were suspended in serum-free medium and seeded into Transwell inserts either uncoated (for migration assay) or coated (for invasion assay) with growth factor-reduced Matrigel. To verify the involvement of FoxM1 in the senescence of tumor cells, staining of senescence β-galactosidase (SA β-gal), the widely used biomarker of cellular senescence, was also performed.
RESULTS: After successful transfection of four FoxM1 small interfering RNAs (shRNAs) with Lipofectamine 2000, the shF1822 was selected as the most appropriate shRNA according to its obvious inhibitory effect. The recombinant adenovirus was then constructed with the shF1822 and successfully transfected into the GBC-SD cells, resulting in the significant inhibition of FoxM1 expression at both the mRNA and protein levels, compared with the negative control (P < 0.05). After transfection, down-regulation of FoxM1 significantly inhibited cell viability according to the MTT assay (P < 0.05). In addition, Transwell migration and invasion assays also suggested the suppression of invasion ability of the transfected cells. SA β-gal staining showed that down-regulation of FoxM1 could induce more senescent GBC cells (P < 0.05), suggesting the possible involvement of the senescence process of the FoxM1-deficient cells in GBC.
CONCLUSION: FoxM1 is functionally involved in viability of GBC cells, partially dependent on the inducement of cellular senescence, and is a potential target for GBC therapy.
Core tip: Gallbladder cancer is characterized by early metastases, thus it is in urgent need to identify novel therapies to enhance the therapeutic effect. We have previously reported that Forkhead box M1 (FoxM1) expression was closely correlated with gallbladder carcinoma differentiation, Nevin stage and metastasis, indicating the potential roles of FoxM1 in gallbladder carcinoma. In this study, by regulating the expression of FoxM1 with small interfering RNAs, we demonstrated the impact of FoxM1 on cellular viability in a human gallbladder carcinoma cell line, which was probably via the regulation of cellular senescence, revealing FoxM1 as a potential target for gallbladder carcinoma therapy.
- Citation: Tao J, Xu XS, Song YZ, Qu K, Wu QF, Wang RT, Meng FD, Wei JC, Dong SB, Zhang YL, Tai MH, Dong YF, Wang L, Liu C. Down-regulation of FoxM1 inhibits viability and invasion of gallbladder carcinoma cells, partially dependent on inducement of cellular senescence. World J Gastroenterol 2014; 20(28): 9497-9505
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9497
Gallbladder carcinoma (GBC) is the fifth most common malignant tumor of the digestive system, and the most common malignant neoplasm of the biliary tract, with an incidence of 1-2 cases/100000 worldwide. However, in Eastern countries such as China and South America, this neoplasm is more common, with an incidence up to 96 cases/100000[1,2]. GBC is characterized by early lymph node and distant metastases, thus only 10% of patients present with early-stage disease and are candidates for surgical resection[3]. Currently, the prognosis of advanced GBC is very poor, as the overall survival is less than 1 year following diagnosis[4]. As no specific chemotherapy or radiotherapy for the disease has emerged with satisfying effects, many researchers have been trying to explore new approaches to benefit GBC patients, but only achieved limited progress[5-8]. It is then in urgent need to identify novel therapies to enhance the therapeutic effect and improve the survival of GBC patients.
Forkhead box M1 (FoxM1), which is characterized by the forkhead box domain, is a proliferation-associated transcription factor that has important roles in cellular proliferation, cell cycle progression, tissue repair and carcinogenesis[9,10]. We have previously reported the involvement of FoxM1 in cellular senescence in hepatocellular carcinoma (HCC) through p53-FoxM1 pathway, indicating a new promising target for treating digestive cancers[11,12]. In addition, our previous study also showed that FoxM1 expression was closely correlated with GBC differentiation, Nevin stage and metastasis, and that GBC patients with highly expressed FoxM1 would have a poorer overall survival by multivariate analysis, indicating the potential roles of FoxM1 in GBC[13]. Thus in this study, by regulating the expression of FoxM1 with small interfering RNAs (siRNAs), we explored the impact of FoxM1 on cellular viability in a human GBC cell line, which was probably via the regulation of cellular senescence.
The human GBC cell line GBC-SD was purchased from the Cell Bank of the Chinese Academic of Sciences (Shanghai branch), and were cultured in DMEM medium containing 10% fetal bovine serum (Gibco, Grand Island, NY, United States) at 37 °C with 5% CO2. At 24 h after cell seeding in the culture dish, the recombinant adenovirus vector containing specific shRNA was transfected into the GBC-SD cells with Lipofectamine 2000, at different multiplicities of infection.
RNA interference mediated by duplexes of 21-nucleotide RNA was performed in GBC-SD cells. The following four shRNAs of FoxM1 were synthesized by Shanghai GenePharma Co (Shanghai, China): FoxM1-homo-461 (5’-GCT GGG ATC AAG ATT ATT AAC-3’), FoxM1-homo-579 (5’-GCA GTA GTG GGC CCA ACA AAT-3’), FoxM1-homo-1044 (5’-GGA AGC GCA TGA CTT TGA AAG-3’) and FoxM1-homo-1822 (5’-GGA AAT GCT TGT GAT TCA ACA-3’). A negative control shRNA duplex (shRNA-NC, FoxM1-homo-NC 5’-ACT ACC GTT GTT ATA GGT G-3’), labeled with the fluorophore FAM, was used to detect the transfection efficiency. The shRNA-NC did not target any known mammalian gene and was synthesized by Shanghai GenePharma Co (Shanghai, China). shRNA transfection was carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) according to the procedure recommended by the manufacturer.
A recombinant lentivirus for shFoxM1, which expresses FoxM1-specific shRNA, was purchased from GenePharma (Shanghai, China). A negative control carrying green fluorescent protein, which expresses a scrambled RNA, was constructed as a control. The virus containing the construct was isolated using plaque screening, purification and amplification. Protocol of lentivirus infection was according to the GenePharma Recombinant Lentivirus Operation Manual (http://www.genepharma.com).
Total RNA was isolated from cell groups using the RNAfast200 Total RNA Extract Kit (Fastgene, Shanghai, China). Primers used in PCR were designed according to the reported FoxM1 cDNA sequence. The primer sequences were 5’-CAC CCC AGT GCC AAC CGC TAC TTG-3’ (forward), and 5’-AAA GAG GAG CTA TCC CCT CCT CAG-3’ (reverse). The primer sequences of β-actin were 5’-CGC GAG AAG ATG ACC CAG AT-3’ (forward) and 5’-GCA CTG TGT TGG CGT ACA GG-3’ (reverse). PCR cycling parameters included an initial denaturation step at 95 °C for 5 min; 35 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min; and a final elongation phase at 72 °C for 7 min. Each sample was assayed in triplicate.
Cells were lysed in ice-cold RIPA lysis buffer (Beyotime Inc., NanTong, China). The total protein concentration was determined with the Bradford reagent (Beyotime Inc.). Equivalent amounts of proteins were then separated by 12% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, United States). The membranes were subsequently immunoblotted with the appropriate primary antibody at 4 °C for 12 h, and then incubated with HRP conjugated anti-goat or anti-rabbit antibody (Santa Cruz, CA, United States). Signals were detected on X-ray films using the ECL detection system (Pierce, Rockford, IL, United States). Equal protein loading was assessed by the expression of β-actin.
GBC-SD cells were seeded at 5 × 103 cells per well in 96-well flat-bottom plates before transfection. For the assay, 20 μL of MTT solution (5 g/L) was added to each well, and the cells were incubated for 4 h. Then supernatants were removed and formazan crystals were dissolved in 200 mL dimethylsulfoxide. After the insoluble crystals were completely dissolved, the absorbance values at 490 nm were measured using a microplate reader (Bio-Rad, Hercules, CA, United States).
Senescence β-galactosidase (SA β-gal) staining is widely used to assess cellular senescence in vivo and in vitro, with the positive green or blue-colored staining of β-galactosidase at pH 6.0 being remarkably increased in senescent cells. Senescent cells in the GBC-SD cell line were analyzed using a SA β-gal staining kit (Beyotime Inc., Nantong, China) according to the manufacturer’s instructions. The percentage of SA-β-gal positive cells was calculated by counting the cells in five random fields (at least 100 cells) using bright-field microscopy. The staining results were recorded as ‘‘positive’’ or ‘‘negative’’, according to the method reported by te Poele et al[14].
Cell invasion and migration assays were performed using Transwell permeable supports with 8 μm pore size (Costar, Cambridge, MA, United States). Cells were suspended in serum-free medium and seeded into Transwell inserts either uncoated (for migration assay) or coated (for invasion assay) with growth factor-reduced Matrigel (BD Biosciences, Bedford, MA). Bottom wells were filled with complete medium, and after 24 h, the invaded cells were fixed with methanol and stained with a crystal violet solution. The number of cells that penetrated the membrane was determined by counting the mean cell number in five randomly selected high-power fields.
All data are expressed as mean ± SD. To compare the means of normally distributed variables, analysis of variance or Student’s t test was applied. A P value less than 0.05 was considered significant in all tests. All analyses were performed using the SPSS software version 19.0 (SPSS Inc, Chicago, IL, United States).
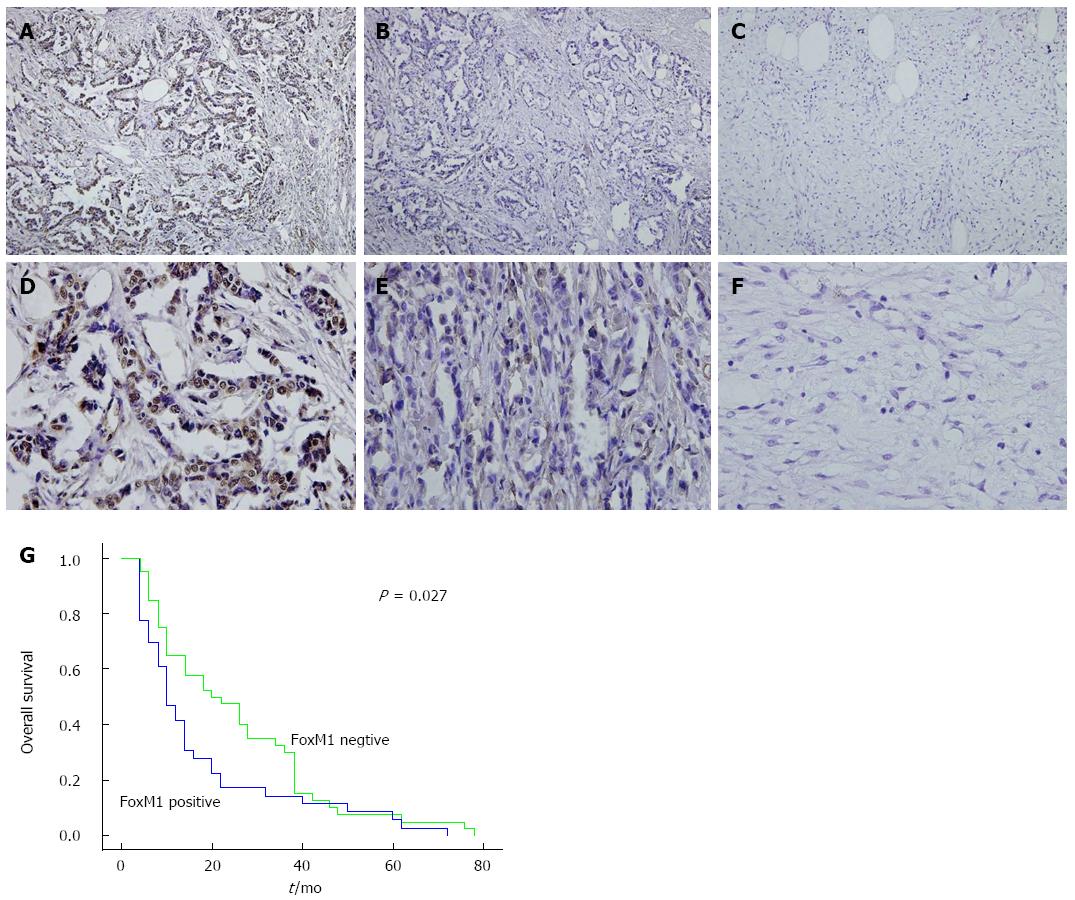
The expression of FoxM1 has been reported to be associated with the prognosis of cancer patients in a series of tumors[15-17]. Likewise, our previous study has also demonstrated the potential impact of FoxM1 on the poor prognosis of GBC patients[13]. As shown in Figure 1A-F, the expression of FoxM1 in GBC tissues was significantly higher than that in the pericarcinoma tissues and adjacent non-GBC gallbladder samples (P < 0.05). Furthermore, the Kaplan-Meier analysis also demonstrated that GBC patients with positive FoxM1 expression had a poorer overall survival than those with negative FoxM1 expression (P < 0.05) (Figure 1G)[13].
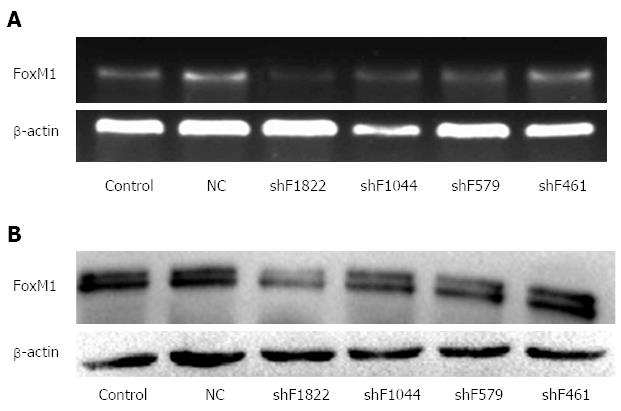
To investigate the inhibitory efficiency of the shRNAs in GBC-SD cells, PCR and Western blot were performed 48 h after the shRNA transfection. We found that the FoxM1-homo-1822 was more efficient and could significantly inhibit the expression of FoxM1 (Figure 2). Thus, the FoxM1-homo-1822 was selected as the optimal shRNA of FoxM1 in the following experiments.
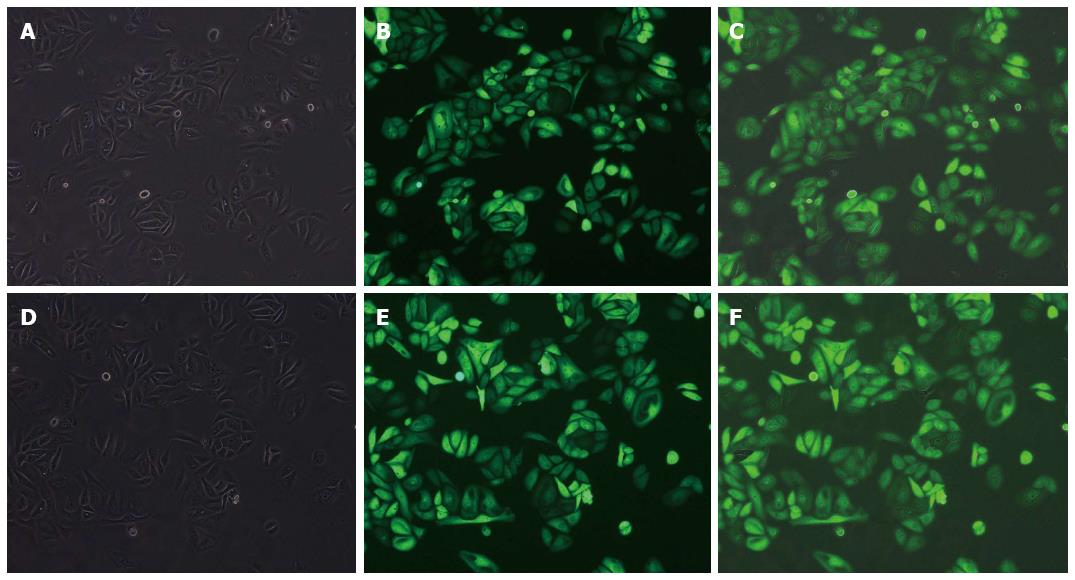
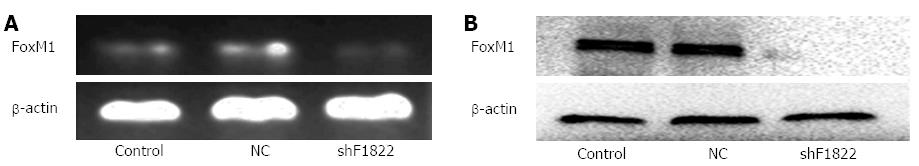
GBC-SD cells, which have a high level of FoxM1 expression, were stably transfected with the FoxM1 shRNA or negative control (NC-shRNA) (Figure 3). After 3-wk screening with puromycin, the total RNA and protein of the transfected cells were isolated and analyzed by RT-PCR and Western blot, respectively. Compared with the blank (no siRNA) and NC-shRNA transfected cells, the expression of FoxM1 was significantly suppressed in cells transfected with FoxM1 shRNA, at both the mRNA and protein levels (P < 0.05) (Figure 4).
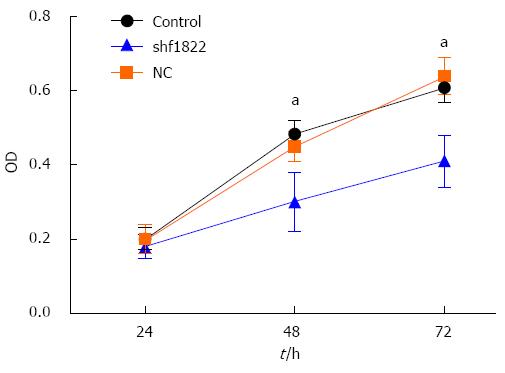
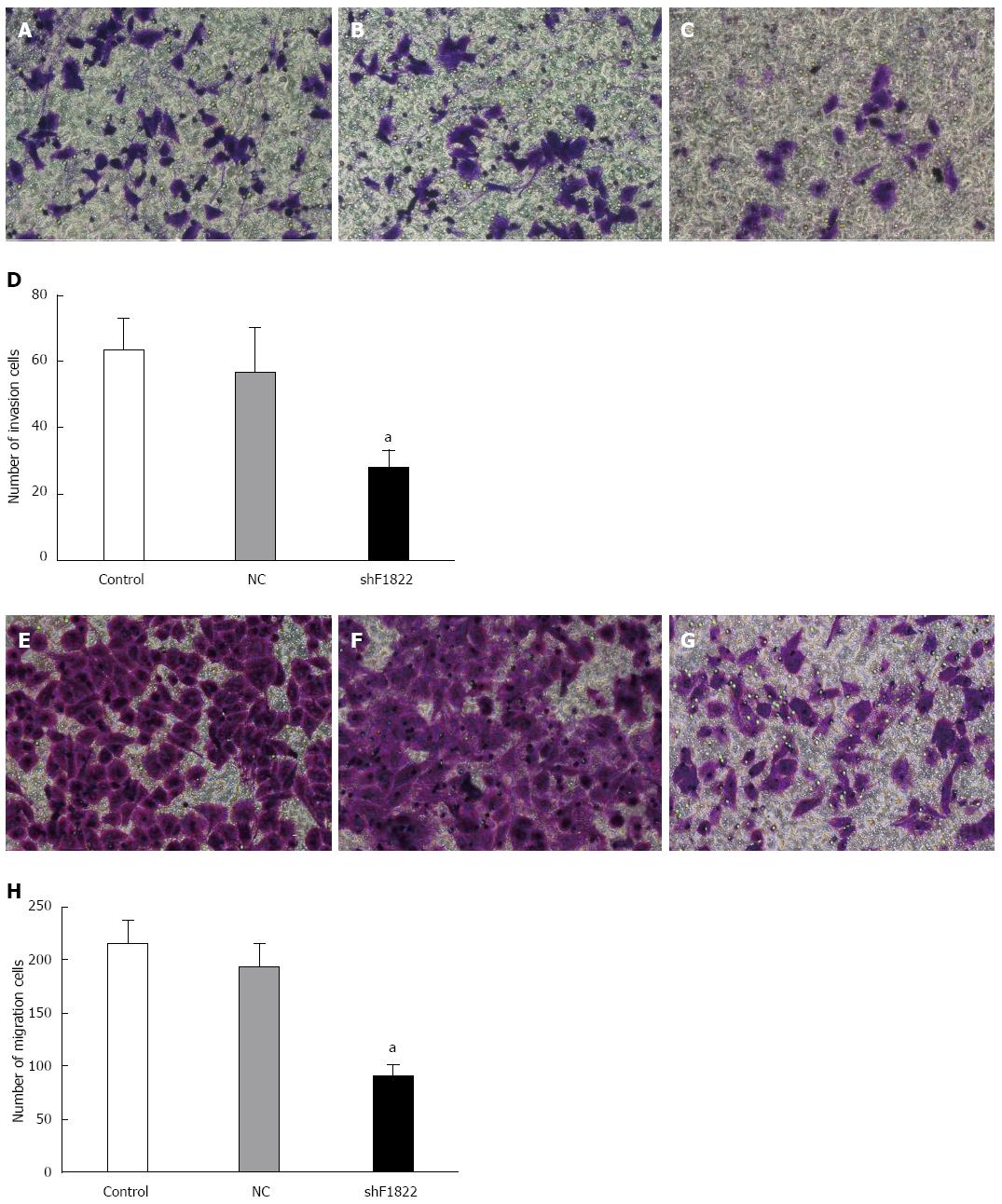
As shown in Figure 5, down-regulation of FoxM1 expression in GBC-SD cells caused significant inhibition of cell viability compared with the control and NC-shRNA cells (P < 0.05). Furthermore, the Transwell system was utilized to determine whether down-regulation of FoxM1 affected the invasive and migratory ability of GBC-SD cells. Compared with the control and NC-shRNA cells, suppression of FoxM1 significantly inhibited the invasion and migration of GBC-SD cells, as indicated by a marked decrease in the number of cells that invaded the bottom well (P < 0.05, Figure 6).
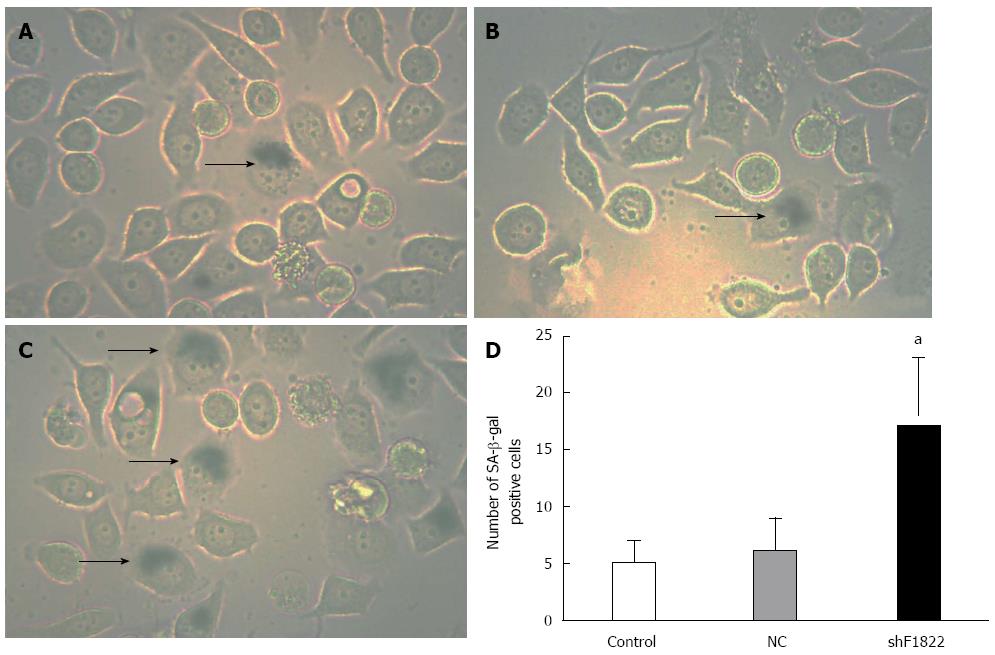
We further examined the mechanism behind FoxM1 depletion-mediated impaired viability in GBC-SD cells. As a significant molecule for regulating cellular senescence in a series of malignancies, FoxM1 depletion might also trigger a cellular senescence program in GBC. SA-β-gal staining was thus performed[18]. Positive SA-β-gal staining was observed in approximately 20% of GBC-SD cells treated with FoxM1 siRNA, which was significantly higher than those in the control group and NC-shRNA group (P < 0.05, Figure 7).
In the previous and present study, we demonstrated the overexpression of FoxM1 in both GBC-SD cells and primary GBC tissues. FoxM1, a transcription factor ubiquitously expressed in most cancer cells, is essential for sustaining proliferation of GBC-SD cells. We have previously demonstrated the correlation between high FoxM1 expression and the poor prognosis of GBC patients, and in the present study, the impaired viability and invasion ability of GBC-SD cells were also induced by down-regulation of FoxM1. Furthermore, SA-β-gal staining indicated that FoxM1 down-regulation could induce the senescence of GBC-SC cells, suggesting that the impaired viability of GBC-SC cells was probably partially dependent on the inducement of cellular senescence.
FoxM1, a critical regulator of cell cycle progression, has important roles in cell proliferation, organogenesis, senescence and carcinogenesis[9,10]. Genome-wide gene expression profiling of cancers has independently identified FoxM1 as one of the genes whose expression is most commonly up-regulated in human solid tumors, such as liver, prostate, brain, breast, lung, colon, and pancreatic tumors[19]. In addition, subsequent studies also demonstrated that high expression of FoxM1 predicted the poor prognosis of several malignancies, such as gastric cancer, lung cancer, and liver cancer[15,16,20]. Likewise, we discovered that the FoxM1 was also up-regulated in GBC. Furthermore, according to our previous results, we demonstrated that high expression of FoxM1 was closely correlated with differentiation, Nevin stage and metastasis of GBC, and thus the prognosis of GBC patients[13].
Consistent with the observed decreased cell proliferation, FoxM1 deficiency has been reported to be associated with reduced expression of many cell cycle-regulatory genes that are known to potently promote progression through the S-, G2- and M-phases of the cell cycle. Thus, knockdown of FoxM1 was supposed to be associated with impaired proliferation of tumor cells. Previous studies have also verified the inhibition of viability in FoxM1-deficient cells in a series of tumor cells, indicating the possible similar effect of FoxM1 on GBC cells[12,21,22]. In our study, consistent with the supposition, we found that down-regulation of FoxM1 by shRNA transfection could significantly inhibit the viability of GBC-SD cells. In addition, Transwell assays also demonstrated suppressed migration and invasion ability of the GBC-SC cells transfected with the FoxM1 shRNA, further verifying the impaired proliferation of FoxM1-deficient cells.
Cellular senescence is an irreversible growth arrest, characterized by decreased cell proliferation, combined with accumulation of mitotic defects and chromosomal instability[23]. This permanent exit from the cell cycle represents an important tumor suppression mechanism. In accordance with its proliferation stimulating function, FoxM1 prevents both oncogene- and oxidative stress-induced premature senescence[24]. Previous studies demonstrated that, early-passage FoxM1-/- mouse embryonic fibroblasts (MEFs), immortalized FoxM1-/- MEFs, and pancreata from mice with a pancreas-specific knockout of FoxM1 displayed premature senescence, indicating the prevention of premature senescence by FoxM1[24-26]. Accordingly, silencing of FoxM1 by RNA interference in U2OS cells resulted in cellular senescence[24]. We have also discovered that in HepG2 and SMMC-7721 HCC cells, knockdown of FoxM1 led to cellular senescence, whereas the senescent cells were decreased by FoxM1 overexpression[11]. Likewise, in this study, to elucidate the functional relevance of FoxM1 in GBC, SA-β-gal staining was performed to determine the senescent cells. Our data suggested that lower expression of FoxM1 in GBC-SD cells was associated with more senescent cells, indicating the involvement of FoxM1 in the prevention of senescence in GBC.
In conclusion, as FoxM1 was highly expressed in GBC, our results for the first time demonstrated that down-regulation of FoxM1 expression in GBC-SC cells by RNA interference could inhibit cell proliferation, invasion and induce cellular senescence, providing clear evidence that FoxM1 is a potential therapeutic target for the treatment of GBC.
The incidence of gallbladder carcinoma is currently rising faster in Oriental countries than Western world, though the etiology is largely unknown. Progression of this disease is associated with a proliferation-associated transcription factor Forkhead box M1 (FoxM1), a crucial molecular in cell cycle progression.
FoxM1 overexpression is common in most malignant tumors of the digestive system. However, whether FoxM1 is also up-regulated in gallbladder carcinoma, and the specific roles of FoxM1 in gallbladder carcinoma have not been unequivocally addressed. In this study, the authors demonstrate that overexpression of FoxM1 could be a potential mechanism for mediating cellular senescence and viability in gallbladder carcinoma.
Recent reports have highlighted the importance of proliferation-associated transcription factors, including FoxM1, in gastrointestinal carcinogenesis. Our previous study reported that FoxM1 was also overexpressed in gallbladder carcinoma, and that FoxM1 expression was closely correlated with GBC differentiation, Nevin stage and metastasis. Furthermore, this in vitro studies suggest that this protein may be the cause of the repression of senescence observed in this malignancy, and that silencing this protein significantly impaired the cellular viability in gallbladder carcinoma, suggesting that it is a potential therapy target.
By understanding how FoxM1 is functionally involved in cellular viability in gallbladder carcinoma and by blocking its expression, this study proposes a future strategy for therapeutic intervention in the treatment of patients with gallbladder carcinoma.
Cellular senescence is an irreversible growth arrest, characterized by decreased cell proliferation, combined with accumulation of mitotic defects and chromosomal instability. This permanent exit from the cell cycle represents an important tumor suppression mechanism. Non-surprisingly, in accordance with its proliferation stimulating function, FoxM1 prevents both oncogene- and oxidative stress-induced premature senescence.
The authors examined the functional involvement of FoxM1 in gallbladder carcinoma. This research is well organized and of relevant interest and scientific innovation.
P- Reviewer: Ferrarese AG, Shi BM, Zhu YL S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167-176. [PubMed] [Cited in This Article: ] |
| 3. | Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Marino D, Leone F, Cavalloni G, Cagnazzo C, Aglietta M. Biliary tract carcinomas: from chemotherapy to targeted therapy. Crit Rev Oncol Hematol. 2013;85:136-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Jia J, Li S, Gong W, Ding J, Fang C, Quan Z. mda-7/IL-24 induces apoptosis in human GBC-SD gallbladder carcinoma cells via mitochondrial apoptotic pathway. Oncol Rep. 2011;25:195-201. [PubMed] [Cited in This Article: ] |
| 6. | Li JY, Quan ZW, Zhang Q, Liu JW. The synergistic inhibitory effect of somatostatin-doxorubicin co-treatment on gallbladder carcinoma. BMC Cancer. 2007;7:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol. 2005;11:2431-2437. [PubMed] [Cited in This Article: ] |
| 8. | Fan YZ, Fu JY, Zhao ZM, Chen CQ. Inhibitory effect of norcantharidin on the growth of human gallbladder carcinoma GBC-SD cells in vitro. Hepatobiliary Pancreat Dis Int. 2007;6:72-80. [PubMed] [Cited in This Article: ] |
| 9. | Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 833] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Qu K, Xu X, Liu C, Wu Q, Wei J, Meng F, Zhou L, Wang Z, Lei L, Liu P. Negative regulation of transcription factor FoxM1 by p53 enhances oxaliplatin-induced senescence in hepatocellular carcinoma. Cancer Lett. 2013;331:105-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Wu QF, Liu C, Tai MH, Liu D, Lei L, Wang RT, Tian M, Lü Y. Knockdown of FoxM1 by siRNA interference decreases cell proliferation, induces cell cycle arrest and inhibits cell invasion in MHCC-97H cells in vitro. Acta Pharmacol Sin. 2010;31:361-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Wang R, Song Y, Xu X, Wu Q, Liu C. The expression of Nek7, FoxM1, and Plk1 in gallbladder cancer and their relationships to clinicopathologic features and survival. Clin Transl Oncol. 2013;15:626-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876-1883. [PubMed] [Cited in This Article: ] |
| 15. | Li X, Qiu W, Liu B, Yao R, Liu S, Yao Y, Liang J. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J Transl Med. 2013;11:204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Xu N, Jia D, Chen W, Wang H, Liu F, Ge H, Zhu X, Song Y, Zhang X, Zhang D. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PLoS One. 2013;8:e59412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363-9367. [PubMed] [Cited in This Article: ] |
| 19. | Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Frau M, Feo F, Pascale RM. Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J Hepatol. 2013;59:830-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Wang Z, Zheng Y, Park HJ, Li J, Carr JR, Chen YJ, Kiefer MM, Kopanja D, Bagchi S, Tyner AL. Targeting FoxM1 effectively retards p53-null lymphoma and sarcoma. Mol Cancer Ther. 2013;12:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Yang C, Chen H, Yu L, Shan L, Xie L, Hu J, Chen T, Tan Y. Inhibition of FOXM1 transcription factor suppresses cell proliferation and tumor growth of breast cancer. Cancer Gene Ther. 2013;20:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486-2492. [PubMed] [Cited in This Article: ] |
| 24. | Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20:1853-1866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875-10894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 495] [Article Influence: 26.1] [Reference Citation Analysis (0)] |