Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4753
Revised: January 2, 2014
Accepted: February 17, 2014
Published online: April 28, 2014
Processing time: 209 Days and 20.8 Hours
AIM: To investigate the hepatoprotective effect of a Cichorium intybus L. extract (CIE) on CCl4-induced hepatic fibrosis in rats.
METHODS: Seventy-two male Wistar albino rats were randomly divided into six groups of twelve rats each. The normal control group was allowed free access to food and water. Liver injury was performed in the remaining five groups with an i.p. injection of a 1.0 mL/kg CCl4 and olive oil (2:3 v/v) mixture, twice weekly for 8 weeks. All rats, with the exception of the injury model group, were intragastrically (i.g.,) administered quantum satis (q.s.) dosages [CIE group: 6, 18, and 54 mg/kg, respectively; Fu Fang Bie Jia Ruan Gan Pian (FFBJRGP) group: 780 mg/kg]. The oral administration of different drugs was performed on the day before CCl4 administration and subsequently once per day for 8 wk. The serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), hexadecenoic acid (HA), laminin (LN), hydroxyproline (Hyp), and glutathione (GSH), malondialdehyde (MDA) and superoxide dismutase (SOD) in the rat livers were measured. Histopathological changes in the liver were assessed for each group using HE staining and a Masson Trichrome examination. The expression of transforming growth factor-β1 (TGF-β1) and α-smooth muscle actin (α-SMA) was examined by immunohistochemical analysis.
RESULTS: CIE at oral doses of 6, 18, and 54 g/kg per day showed a significant hepatoprotective effect, especially at a dose of 54 g/kg per day. CIE doses reduced the levels of AST (149.04 ± 34.44, P < 0.01), ALT (100.72 ± 27.19, P < 0.01), HA (548.50 ± 65.09, P < 0.01), LN (28.69 ± 3.32, P < 0.01) and Hyp (263.33 ± 75.82, P < 0.01). With regards to hepatoprotective activity, the CIE dose of 54 g/kg per day produced the largest significant effect by increasing GSH (3.11 ± 0.81), SOD (269.98 ± 33.77, P < 0.01) and reducing MDA (2.76 ± 0.51, P < 0.01) levels in the liver. The expressions of TGF-β1 and α-SMA were measured by immunohistology and found to be significantly reduced by CIE in a dose-dependent manner.
CONCLUSION: CIE may effectively protect against CCl4-induced hepatic fibrosis in rats; thus, it is a promising anti-fibrotic therapeutic agent.
Core tip:Cichorium intybus L. extract (CIE) is a traditional Uighur medicine that is commonly used in China and other Asian countries to nourish and improve the liver. The present study demonstrated that CIE has a hepatoprotective effect against CCl4-induced hepatotoxicity in rats. We propose that the increased levels of antioxidant enzymes and reduced levels of malondialdehyde are the major mechanism of CIE for preventing the development of liver fibrosis induced by CCl4.
-
Citation: Li GY, Gao HY, Huang J, Lu J, Gu JK, Wang JH. Hepatoprotective effect of
Cichorium intybus L., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol 2014; 20(16): 4753-4760 - URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4753
Cichorium intybus L. (Compositae), a traditional Uighur medicine, is a perennial herb from the asteraceae family with many commercial uses. Historically, Cichorium intybus L. (also known as chicory) was grown by ancient Egyptians as a medicinal plant, vegetable crop and for animal forage[1]. In addition to its alimentary use, chicory also has a history of medicinal use. Chicory roots have anti-microbial[2-4], anti-bacterial[5-8], anti-diabetic[9], immunoenhancement[10], anti-hepatotoxic[11-13], anti-hyperuricemia and anti-hypertriglyceridemia activities[14,15]. Chicory has been used as a digestive aid, diuretic, laxative, and mild sedative[16,17]. The water extract of Cichorium intybus L. showed a remarkable antioxidative effect on low density lipoprotein (LDL), and inhibitory effects on the production of thiobarbituric acid reactive substances and degradation of the fatty acids of LDL[18]. The potential anti-inflammatory activities of chicory have been investigated. An ethyl acetate chicory root extract produced a marked inhibition of prostaglandin E2 (PGE2) production in human colon carcinoma HT29 cells treated with the proinflammatory agent TNF-α[19]. Additionally, hepatoprotective agents have been discovered in the seeds of the plant[20].
Hepatic fibrosis is characterized by the excessive deposition of extracellular matrix (ECM) proteins, such as hyaluronic acid (HA), laminin (LN) and hydroxyproline (Hyp), which leads to severe pathophysiological disturbances, including remodeling of the liver architecture, development of intrahepatic shunts, liver insufficiency, portal hypertension, esophageal varices, ascites and encephalopathic coma. Despite the high incidence of hepatic fibrosis worldwide, no generally accepted anti-fibrogenic therapy is available[21-23]. Presently, many studies are assessing potential anti-fibrogenic drugs that have been used in traditional Chinese medicine for thousands of years[24].
The aim of the present study was to investigate the effects of a Cichorium intybus L. extract (CIE) on markers of liver function in the serum, antioxidant enzyme levels and hepatic histopathology of the liver in rats with CCl4-induced hepatotoxicity.
The herbs of Cichorium intybus L. were purchased from Shenyang and authenticated by pharmacognosist Dr. Jimin Xu. Ten kilograms of Cichorium intybus L. was powdered, decocted, refluxed three times with 100 L of ethanol, and then filtered. The filtrates were concentrated by rotary vacuum evaporation. The ethanolic extract was suspended in distilled water and extracted three times with the same volume of ethyl acetate. The extracts were concentrated once again by rotary vacuum evaporation and then lyophilized using a freeze dryer. The lyophilized powder was dissolved in sterilized distilled water before oral administration to experimental animals.
Seventy-two male Wistar albino rats (180-250 g) were obtained from Shenyang Pharmaceutical University. The institutional ethics committee of Shenyang Pharmaceutical University approved all the experiments. Rats were housed in specific standard laboratory conditions for one week, including a temperature-controlled environment (25 °C ± 2 °C), a relative humidity of 50% ± 5%, and with a regular 12 h light/12 h dark cycle. All animals were fed with a standard rodent chow diet and water ad libitum. Rats weighing 180-250 g were used for the analysis of CCl4-induced hepatotoxicity. Rats were randomly divided into six groups of twelve rats each. The normal control group was allowed free access to food and water. Liver injury was created in the remaining five groups with the i.p. injection of a 1.0 mL/kg CCl4 and olive oil (2:3 v/v) mixture, twice weekly for a total of 8 weeks. All rats, with the exception of the model group, were given intragastric (i.g.) administration of quantum satis (q.s.) dosages [CIE groups: 6, 18, and 54 mg/kg; Fu Fang Bie Jia Ruan Gan Pian (FFBJRGP) group: 780 mg/kg]. The oral administration of drugs was performed the day before CCl4 administration and then once per day for 8 wk.
Rats were euthanized by cervical dislocation the day after the last intragastric administration. Blood and liver tissue samples were harvested for further examination. The serum was obtained by centrifugation at 835 g for 10 min at 4 °C and then stored at -20 °C until subsequent use. Liver tissue was collected for the measurement of GSH, SOD and MDA levels, and determination of histopathological changes.
The blood was centrifuged at 835 g at 4 °C for 10 min to separate the serum. Markers of liver function, including aspartate transaminase (AST) and alanine transaminase (ALT), were measured with a UV spectrophotometer (Shimadzu UV-2401).
Serum HA, LN and Hyp levels were measured using an enzyme-linked immunosorbent assay kit obtained from Sigma-Aldrich Chemicals Co., United States.
The detection of measurement of lipid peroxidation by malondialdehyde (MDA) using a kit estimated liver peroxidation. The liver tissue was prepared as a 10% homogenate (w/v). The tubes were kept at 95 °C for 40 min. After cooling, tubes were centrifuged at 1484 g for 10 min, and the supernatant was subsequently measured with a UV spectrophotometer (Shimadzu UV-2401) at 532 nm. The protein content was determined using a Coomassie brilliant blue protein kit, and the data are expressed as nmol MDA per milligram of protein of liver tissue (nmol/mg protein).
A commercially available kit from the Jiancheng Biological Engineering Institute (Nanjing, China) determined the SOD activity following the protocol provided by the manufacturer. Data were expressed as SOD U/mg protein. A GSH kit from Jiancheng Biological Engineering Institute (Nanjing, China) measured the GSH activity using a modified protocol. The reaction was measured at 420 nm, and the enzyme activity was calculated as mg/g protein.
Rat liver tissue was fixed with 10% formalin for 24 h, dehydrated with a sequence of ethanol solutions, and embedded in paraffin. Serial sections were cut at a 5-μm thickness and stained with hematoxylin-eosin (HE) and masson trichrome. Stained tissue sections were assessed for the detection of changes in the magnitude of liver injury using a photomicroscope.
The activation of HSCs was identified by the immunohistochemical analysis using monoclonal α-SMA and TGF-β1 antibodies (Abcam, United Kingdom) in deparaffinized tissue sections. The primary antibody was diluted to 1:50 and the biotinylated goat anti-rabbit secondary antibody was diluted to 1:100. In this experiment, we used sections that were not incubated with the primary antibody as the negative control.
All data were expressed as the mean ± SD. Data were analyzed with a one-way analysis of variance. Fisher’s LSD test was used to calculate statistical significance, using SPSS software. Values of P < 0.05 and P < 0.01 were considered statistically significant.
Organ coefficients of the liver, spleen and kidney were evaluated in rats. Similar to previous studies[25,26], liver and spleen coefficients were significantly increased in rats that were exposed to CCl4 (P < 0.01); however, there was no difference in the kidney coefficient among all treated groups. As shown in Table 1, the CCl4-induced increase in the liver coefficient was reduced by 18 and 54 mg/kg CIE (P < 0.01, P < 0.05 respectively). A non-statistically significant protective effect against an increase in the spleen coefficient was observed in rats treated with CIE (6, 18 and 54 mg/kg) and FFBJRGP treatment (780 mg/kg). Groups treated with CIE showed a dose-dependent attenuation of CCl4-induced changes in the liver and spleen coefficients.
| Treatment | Liver coefficient % | Spleen coefficient % | Kidney coefficient % |
| Control | 2.18 ± 0.17 | 0.16 ± 0.01 | 0.65 ± 0.02 |
| CCl4 | 3.59 ± 0.62b | 0.26 ± 0.05b | 0.68 ± 0.08 |
| 6 g/kg CIE + CCl4 | 3.45 ± 0.27 | 0.29 ± 0.07 | 0.67 ± 0.09 |
| 18 g/kg CIE + CCl4 | 3.20 ± 0.21d | 0.27 ± 0.11 | 0.66 ± 0.07 |
| 54 g/kg CIE + CCl4 | 3.10 ± 0.13d | 0.22 ± 0.04 | 0.65 ± 0.04 |
| FFBJRGP (780 mg/kg) | 3.38 ± 0.37 | 0.24 ± 0.06 | 0.64 ± 0.08 |
The leakage of AST and ALT in the blood indirectly reflects liver failure caused by CCl4-induced hepatotoxicity. Table 2 shows that AST and ALT levels were significantly increased after the administration of CCl4 compared with the control group (P < 0.01). Pretreatment with CIE (6, 18, and 54 g/kg) significantly reduced the elevation of AST and ALT (P < 0.01) compared with the CCl4 group. Similarly, pretreatment with 780 mg/kg FFBJRGP significantly reduced the elevation of AST and ALT (P < 0.01).
The serum levels of HA, LN and Hyp were significantly increased after CCl4 administration compared with the control group (P < 0.01, Table 3). Treatment with FFBJRGP (780 mg/kg) significantly decreased the serum levels of HA, LN and Hyp compared with the CCl4 group (P < 0.05, P < 0.01). In addition, the serum levels of HA, LN and Hyp showed a dose-dependent decrease in the CIE groups (6, 18, and 54 mg/kg) compared with the CCl4 group (P < 0.05, P < 0.01).
| Treatment | HA (pg/mL) | LN (ng/mL) | HyP (μg/g) |
| Control | 315.46 ± 49.54 | 18.27 ± 3.50 | 165.57 ± 21.34 |
| CCl4 | 756.85 ± 57.82b | 40.47 ± 2.14b | 395.17 ± 94.31b |
| 6 g/kg CIE + CCl4 | 595.62 ± 76.19d | 27.92 ± 3.37d | 320.42 ± 63.35d |
| 18 g/kg CIE + CCl4 | 573.13 ± 65.42d | 28.17 ± 2.87d | 289.00 ± 89.99d |
| 54 g/kg CIE + CCl4 | 548.50 ± 65.09d | 28.69 ± 3.32d | 263.33 ± 75.82d |
| FFBJRGP (780 mg/kg) | 442.72 ± 18.84d | 30.70 ± 3.30d | 306.90 ± 51.85d |
GSH and SOD are antioxidants that can scavenge lipid peroxide radicals. MDA is an end product of the breakdown of polyunsaturated fatty acids and related esters, and its formation is an index of lipid peroxidation in many organ homogenates. Administration of CCl4 caused a significant decrease in the level of GSH and SOD and an increase in the MDA concentration compared with the normal control group (P < 0.01, Table 4). Pretreatment with 6, 18, and 54 g/kg of CIE significantly raised the level of SOD compared with the CCl4 group (P < 0.01, P < 0.05). The concentration of GSH showed an increase after CIE (6, 18, 54 g/kg) treatment, but the effect did not reach statistical significance. In addition, rats treated with 18 and 54 g/kg of CIE showed a significant reduction in MDA levels in the liver homogenate compared with the CCl4-exposed group (P < 0.01).
| Treatment | GSH | MDA | SOD |
| (mg/gprot) | (nmol/mgprot) | (U/mgprot) | |
| Control | 6.58 ± 0.62 | 1.93 ± 0.18 | 334.12 ± 13.75 |
| CCl4 | 2.52 ± 0.69b | 4.01 ± 1.00b | 193.58 ± 20.76b |
| 6 g/kg CIE + CCl4 | 2.93 ± 0.99 | 3.73 ± 0.61 | 223.23 ± 38.20d |
| 18 g/kg CIE + CCl4 | 3.03 ± 0.76 | 3.25 ± 0.73d | 241.29 ± 40.21d |
| 54 g/kg CIE + CCl4 | 3.11 ± 0.81 | 2.76 ± 0.51d | 269.98 ± 33.77d |
| FFBJRGP (780 mg/kg) | 4.44 ± 1.28d | 2.04 ± 0.70d | 274.43 ± 32.62d |
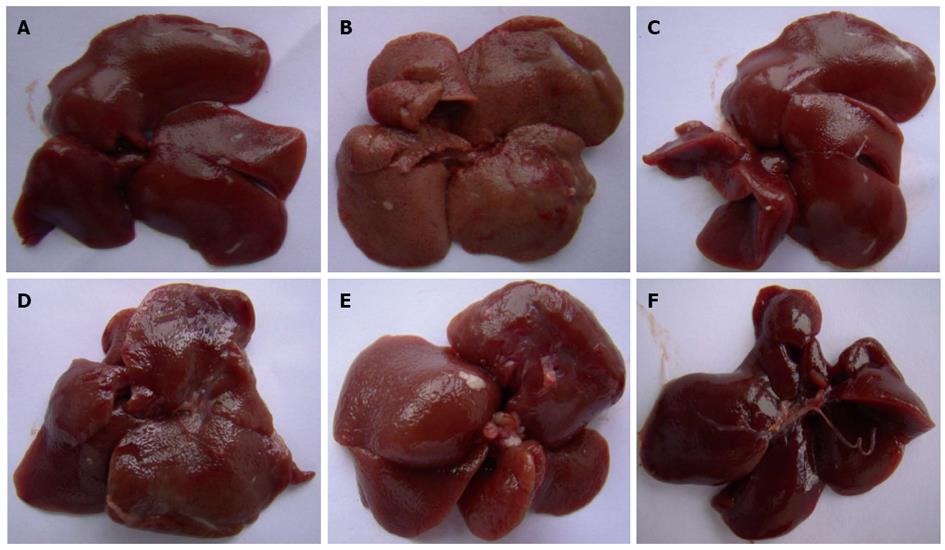
Without magnification, the liver tissue from the control group was observed to be deep red, moist and glossy. In the CCl4 group, the livers appeared swollen and had lost the luster on their surface. Pretreatment with CIE showed a dramatic and dose-dependent attenuation of the markers for liver injury (Figure 1).
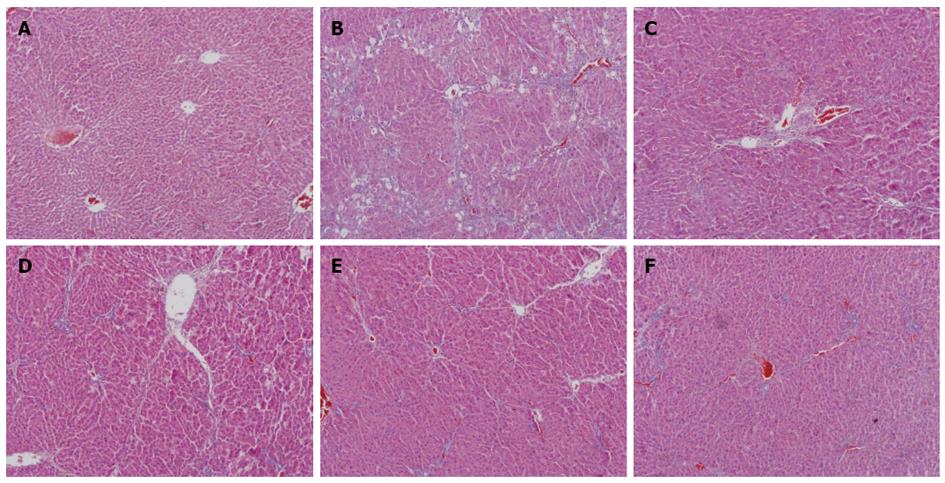
Figure 2 shows a magnified view of the changes in liver histopathology from the control, CCl4, CIE (6, 18, 54 g/kg) and FFBJRGP groups, as determined by an HE stain. Microscopic analysis showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces and a central vein in the control group (Figure 2A). However, CCl4-exposed tissue exhibited severe histopathological changes, such as centrilobular hepatic necrosis, Kupffer cells, ballooning degeneration and infiltrating lymphocytes (Figure 2B). Pretreatment with 780 mg/kg of FFBJRGP or CIE (6, 18, 54 g/kg) prevented the histopathological changes associated with CCl4-induced hepatotoxicity (Figure 2C-F) to varying degrees.
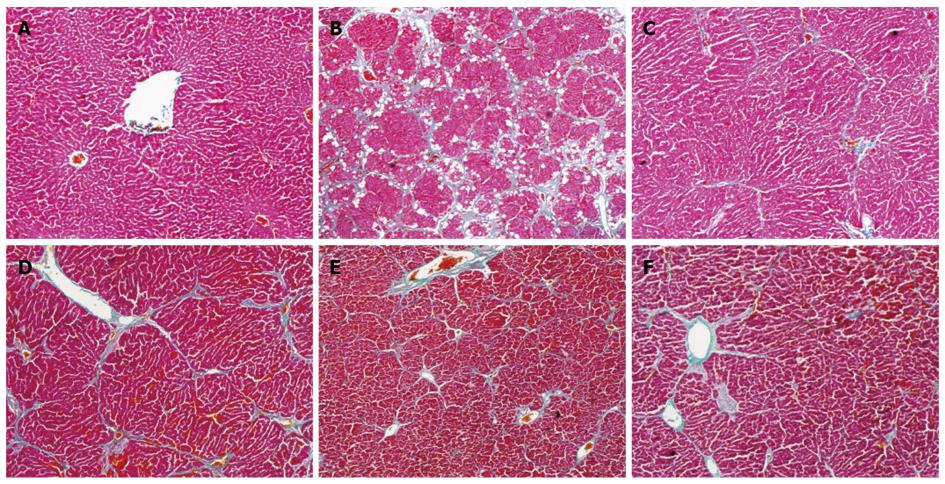
The histopathological changes in fibrosis that occurred in the CCl4 and CIE groups are shown in Figure 3. The livers of rats exposed to CCl4 showed an extensive accumulation of connective tissue that resulted in the formation of continuous fibrotic septa, nodules of regeneration and noticeable alterations in the central vein compared with the normal control (Figure 3A and B). The groups treated with CIE and FFBJRGP showed a less pronounced destruction of the liver architecture, without fibrosis (Figure 3C-F). Based on a microscopic examination, the severe hepatic fibrosis induced by CCl4 was markedly reduced by treatment with CIE. These data correlate with the results of the serum aminotransferase and hepatic antioxidant enzyme levels.
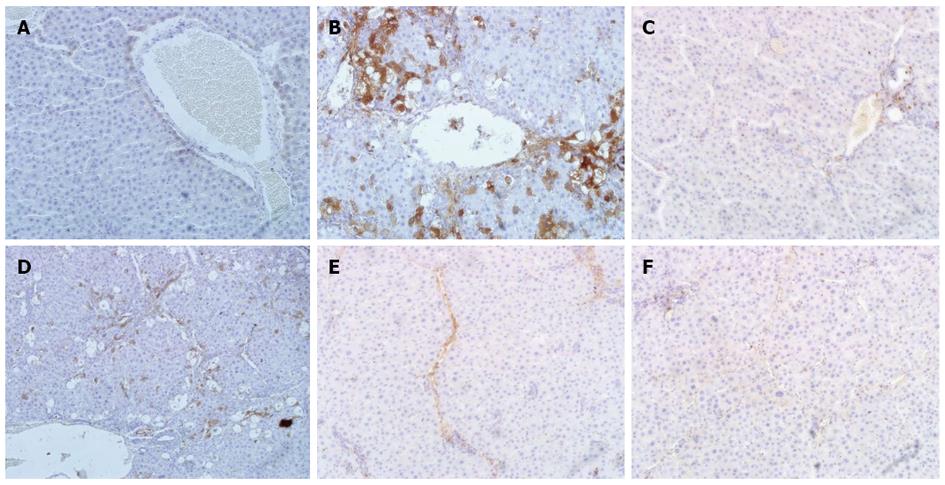
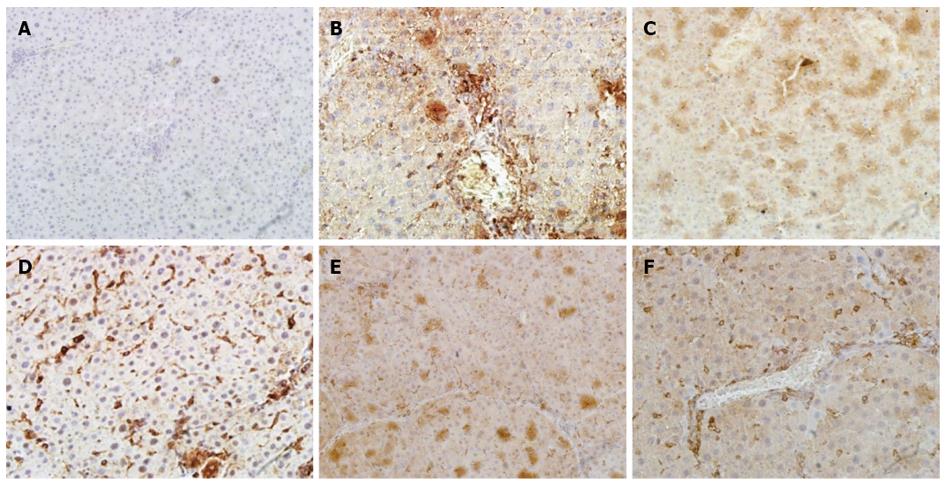
A histological examination of the liver of rats exposed to CCl4 revealed an increase and expansion of fibrous septa compared with normal control rats. α-SMA and TGF-β1 expressions increased in the liver of CCl4 rats compared with normal control rats (Figure 4A, B and Figure 5A, B). Treatment with CIE decreased α-SMA and TGF-β1 staining (Figure 4D-F and Figure 5D-F). Similarly, α-SMA and TGF-β1 expressions were significantly attenuated in the FFBJRGP group (Figure 4C and Figure 5C).
In the present study, CIE exhibited a hepatoprotective effect, as demonstrated by a significant decrease in AST and ALT concentrations and the prevention of histopathological changes in the liver of rats with CCl4-induced hepatotoxicity. Moreover, CIE attenuated the reduction of GSH and SOD, and decreased MDA levels in rats with CCl4-induced hepatotoxicity.
Transaminases are typically located in the cytoplasm; therefore, a decrease in the structural integrity of the liver can be reflected by an increase in the serum levels of these enzymes. It is generally accepted that the toxicity of carbon tetrachloride depends on the cleavage of the carbon-chlorine bond to generate a trichloromethyl free radical. This free radical reacts rapidly with oxygen to form a trichloromethyl peroxy radical that may contribute to hepatotoxicity and the subsequent increase in hepatic enzymes[27]. In the present study, the serum levels of the hepatic enzymes AST and ALT were increased, reflecting the hepatocellular damage in the CCl4-induced injury model. However, treatment with CIE lowered the AST and ALT levels of CCl4-exposed animals. Moreover, the liver index and serum levels of HA, LN, Hyp were increased in rats exposed to CCl4. CIE markedly decreased the serum levels of HA, LN, Hyp and the liver index, suggesting that the extract has hepatoprotective effects. In addition, these effects on liver function correlate with the histopathological changes observed from the microscopic examination of CIE-treated animals. Centrilobular hepatic necrosis, fatty changes, Kupffer cells, ballooning degeneration and infiltrating lymphocytes were found in rats exposed to CCl4. Treatment with CIE prevented these histopathological changes. Thus, our results suggest that attenuating the elevation of certain markers of liver failure may be the mechanism of protective action of CIE against CCl4-induced hepatotoxicity.
In conclusion, we demonstrated that CIE has hepatoprotective effects against CCl4-induced hepatotoxicity in rats. We propose that the observed enhancement of antioxidant enzymes and reduction in malondialdehyde are the major mechanisms of action of CIE in the prevention of CCl4-induced liver fibrosis. However, additional work is required to establish the efficacy of CIE as a potent anti-hepatic fibrosis drug.
We are grateful to Mr. Jimin Xu for identification of the plant materials.
Cichorium intybus L. (Compositae) is a traditional Uighur medicine with many commercial uses. Historically, Cichorium intybus L. was grown by the ancient Egyptians as a medicinal plant, vegetable crop and for animal forage. Previous studies have reported that Cichorium intybus L. exhibits various pharmacological effects, including anti-microbial, anti-hyperuricemia and anti-hypertriglyceridemia activities. Here, the authors investigated the effects of Cichorium intybus L. on markers of liver function, antioxidant enzyme levels and hepatic histopathology in the livers of rats with CCl4-induced hepatotoxicity.
Although Cichorium intybus L. has a long history of medicinal and alimentary use, there is no experimental research into its potential anti-hepatic fibrosis effects. This study demonstrates that this line of research is useful for the discovery of new anti-hepatic fibrosis drugs.
The present study is the first to report the anti-hepatic fibrosis effects of a Cichorium intybus L. extract on CCl4-induced hepatotoxicity.
This study establishes the efficacy of the Cichorium intybus L. extract as a potent anti-hepatic fibrosis drug.
Hepatic fibrosis is a dynamic process characterized by excessive deposition of extracellular matrix components, which can ultimately cause liver cirrhosis. In spite of the high incidence of hepatic fibrosis worldwide, no generally accepted anti-fibrogenic therapy is available. Current research is focused on the study of the potential anti-hepatic fibrosis effects of traditional Chinese medicines that have been used in China for thousands of years.
This is an interesting study in which the authors use a CCl4-induced model of hepatotoxicity to evaluate the protective effects of a Cichorium intybus L. extract. Interestingly, the results suggest that a Cichorium intybus L. extract enhances the levels of antioxidant enzymes and reduces malondialdehyde levels. These effects may be the major mechanism of action by which the Cichorium intybus L. extract prevents CCl4-induced liver fibrosis.
P- Reviewers: de Almeida Artifon EL, Vinken M S- Editor: Qi Y L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Alloush GA, Belesky DP, Clapham WM, Forage Chicory: A Plant Resource for Nutrient-Rich Sites. J Agron Crop Sci. 2003;189:96-104. [DOI] [Full Text] |
| 2. | Xu YM, Hu TM, Zhang CL, Wu HX, Yang YL. Anti-microbial Activities of the Extracts from Cichorium intybus L. Roots. Acta Bot Borea1 Occident Sin. 2006;26:615-619. |
| 3. | Mares D, Romagnoli C, Tosi B, Andreotti E, Chillemi G, Poli F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia. 2005;160:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Liu H, Wang Q, Liu Y, Chen G, Cui J. Antimicrobial and antioxidant activities of Cichorium intybus root extract using orthogonal matrix design. J Food Sci. 2013;78:M258-M263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Bischoff TA, Kelley CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Arefi AG. Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J Ethnopharmacol. 2004;95:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Heimler D, Isolani L, Vignolini P, Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem. 2009;114:765-770. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Rasmussen MK, Klausen CL, Ekstrand B. Regulation of cytochrome P450 mRNA expression in primary porcine hepatocytes by selected secondary plant metabolites from chicory (Cichorium intybus L.). Food Chem. 2014;146:255-263. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Petrovic J, Stanojkovic A, Comic Lj, Curcic S. Antibacterial activity of Cichorium intybus. Fitoterapia. 2004;75:737-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Pushparaj PN, Low HK, Manikandan J, Tan BK, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Kim JH, Mun YJ, Woo WH, Jeon KS, An NH, Park JS. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int Immunopharmacol. 2002;2:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Gilani AH, Janbaz KH. Evaluation of the liver protective potential of Cichorium intybus seed extract on Acetaminophen and CCl(4)-induced damage. Phytomedicine. 1994;1:193-197. [PubMed] [DOI] [Full Text] |
| 12. | Hassan HA, Yousef MI. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2010;48:2163-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Ahmed B, Al-Howiriny TA, Siddiqui AB. Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol. 2003;87:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Kong Y, Liu XQ, Zhang B. Effects of the Extract of Chicory on Serum Uric Acid and Lipid in the Quail with Hyperuricemia /Hypertriglyceridemia. Beijing Zhongyiyao Daxue Xuebao. 2004;27:29-31. |
| 15. | Azay-Milhau J, Ferrare K, Leroy J, Aubaterre J, Tournier M, Lajoix AD, Tousch D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): a comparative in vitro study with the effects of caffeic and ferulic acids. J Ethnopharmacol. 2013;150:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Bais HP, Ravishankar GA. Cichorium intybus L.-cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J Sci Food Agric. 2001;81:467-484. [DOI] [Full Text] |
| 17. | Figueira GM, Park KJ, Brod FPR, Honorio SL. Evaluation of desorption isotherms, drying rates and inulin concentration of chicory roots (Cichorium intybus L.) with and without enzymatic inactivation. J Food Eng. 2004;63:273–280. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kim TW, Yang KS. Antioxidative effects of cichorium intybus root extract on LDL (low density lipoprotein) oxidation. Arch Pharm Res. 2001;24:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Cavin C, Delannoy M, Malnoe A, Debefve E, Touché A, Courtois D, Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem Biophys Res Commun. 2005;327:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Gadgoli C, Mishra SH. Antihepatotoxic activity of Cichorium intybus. J Ethnopharmacol. 1997;58:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681-8685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 651] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Ahmad A, Ahmad R. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J Gastroenterol. 2012;18:155-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Tsai CF, Hsu YW, Chen WK, Chang WH, Yen CC, Ho YC, Lu FJ. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2009;47:2031-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Gao HY, Li GY, Lou MM, Li XY, Wei XY, Wang JH. Hepatoprotective effect of Matrine salvianolic acid B salt on Carbon Tetrachloride-Induced Hepatic Fibrosis. J Inflamm (Lond). 2012;9:16. [PubMed] |
| 27. | Zafar R, Mujahid Ali S. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J Ethnopharmacol. 1998;63:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |