Published online Jan 28, 2013. doi: 10.3748/wjg.v19.i4.482
Revised: November 20, 2012
Accepted: December 15, 2012
Published online: January 28, 2013
AIM: To investigate retrograde tracer transport by gastric enteric neurons in insulin resistant mice with low or high glycosylated hemoglobin (Hb).
METHODS: Under anesthesia, the retrograde tracer fluorogold was superficially injected into the fundus or antrum using a microsyringe in KK Cg-Ay/J mice prior to onset of type 2 diabetes mellitus (T2DM; 4 wk of age), at onset of T2DM (8 wk of age), and after 8, 16, or 24 wk of untreated T2DM and in age-matched KK/HIJ mice. Six days later, mice were sacrificed by CO2 narcosis followed by pneumothorax. Stomachs were removed and fixed. Sections from fundus, corpus and antrum were excised and mounted on a glass slide. Tracer-labeled neurons were viewed using a microscope and manually counted. Data were expressed as the number of neurons in short and long descending and ascending pathways and in local fundus and antrum pathways, and the number of neurons in all regions labeled after injection of tracer into either the fundus or the antrum.
RESULTS: By 8 wk of age, body weights of KKAy mice (n = 12, 34 ± 1 g) were heavier than KK mice (n = 17, 29 ± 1 g; F (4, 120) = 4.414, P = 0.002] and glycosylated Hb was higher [KK: (n = 7), 4.97% ± 0.04%; KKAy: (n = 6), 6.57% ± 0.47%; F (1, 26) = 24.748, P < 0.001]. The number of tracer labeled enteric neurons was similar in KK and KKAy mice of all ages in the short descending pathway [F (1, 57) = 2.374, P = 0.129], long descending pathway [F (1, 57) = 0.922, P = 0.341], local fundus pathway [F (1, 53) = 2.464, P = 0.122], local antrum pathway [F (1, 57) = 0.728, P = 0.397], and short ascending pathway [F (1, 53) = 2.940, P = 0.092]. In the long ascending pathway, fewer tracer-labeled neurons were present in KKAy as compared to KK mice [KK: (n = 34), 302 ± 17; KKAy: (n = 29), 230 ± 15; F (1, 53) = 8.136, P = 0.006]. The number of tracer-labeled neurons was decreased in all mice by 16 wk as compared to 8 wk of age in the short descending pathway [8 wk: (n = 15), 305 ± 26; 16 wk: (n = 13), 210 ± 30; F (4, 57) = 9.336, P < 0.001], local antrum pathway [8 wk: (n = 15), 349 ± 20; 16 wk: (n = 13), 220 ± 33; F (4, 57) = 8.920, P < 0.001], short ascending pathway [8 wk: (n = 14), 392 ± 15; 16 wk: (n = 14), 257 ± 33; F (4, 53) = 17.188, P < 0.001], and long ascending pathway [8 wk: (n = 14), 379 ± 39; 16 wk: (n = 14), 235 ± 26; F (4, 53) = 24.936, P < 0.001]. The number of tracer-labeled neurons decreased at 24 wk of age in the local fundus pathway [8 wk: (n = 14), 33 ± 11; 24 wk: (n = 12), 3 ± 2; F (4, 53) = 5.195, P = 0.001] and 32 wk of age in the long descending pathway [8 wk: (n = 15), 16 ± 3; 32 wk: (n = 12), 3 ± 2; F (4, 57) = 2.944, P = 0.028]. The number of tracer-labeled enteric neurons was correlated to final body weight for local fundus and ascending pathways [KK: (n = 34), r = -0.746, P < 0.001; KKAy: (n = 29), r = -0.842, P < 0.001] as well as local antrum and descending pathways [KK (n = 36), r = -0.660, P < 0.001; KKAy (n = 31), r = -0.622, P < 0.001]. In contrast, glycosylated Hb was not significantly correlated to number of tracer-labeled neurons [KK (n = 17), r = -0.164, P = 0.528; KKAy (n = 16), r = -0.078, P = 0.774].
CONCLUSION: Since uncontrolled T2DM did not uniformly impair tracer transport in gastric neurons, long ascending neurons may be more susceptible to persistent hyperglycemia and low effective insulin.
- Citation: LePard KJ, Cellini J. Age-dependent slowing of enteric axonal transport in insulin-resistant mice. World J Gastroenterol 2013; 19(4): 482-491
- URL: https://www.wjgnet.com/1007-9327/full/v19/i4/482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i4.482
Diabetes mellitus (DM), characterized by persistent hyperglycemia and a deficiency of effective plasma insulin, can result in neuropathy of autonomic neurons. In rodent models of type 1 DM (T1DM) and type 2 DM (T2DM), neuropathies of the parasympathetic vagus nerve[1-3] and enteric neurons located within the wall of the gut[4,5] have been described. Many factors likely contribute to the development of autonomic neuropathy[6,7] including accumulation of sorbitol in nerves[8,9], oxidative stress[10,11], and somal deficiency of neurotrophins[7,12-14].
Neurotrophins, proteins that support the proper functioning of neurons, are retrogradely transported from the innervated organ to the soma by an axonal active transport mechanism. Active axonal transport of proteins in the vagus nerve was impaired in animal models of DM. Early studies using nerve ligation demonstrated reduced axonal transport of opiate receptors[15] and choline acetyltransferase[8] in the vagus nerve of streptozotocin (STZ) rats with partial reversal by immediate treatment with insulin[15] or an aldose reductase inhibitor[8]. Retrograde transport by the vagus nerve of endogenous neurotrophins[8] or experimental neuronal tracers injected into the stomach[16,17] was impaired after 16 or 24 wk of uncontrolled hypoinsulinemia and hyperglycemia in STZ rats. In a rodent model of T2DM, the db/db mouse, axonal transport of acetylcholinesterase[18,19] and choline acetyltransferase[20] in peripheral nerves was impaired, but only after at least 25 wk[18,19], approximately double the time observed in STZ models of T1DM[16,17,21]. To date, active axonal transport of proteins by enteric neurons located within the wall of the gut has not been evaluated in rodent models of DM. We hypothesized that retrograde transport of the neural tracer fluorogold (FG) would be impaired in enteric neurons, but only after prolonged insulin resistance and hyperglycemia.
Coordinated contraction and relaxation of fundus, corpus and antrum is required for normal storage and emptying of gastric contents[22,23]. Enteric neural control of contraction and relaxation of gastric smooth muscle varied by region in rodent models of DM[24,25]. Therefore, tracer transport in ascending and descending enteric neurons innervating the fundus and antrum, respectively, were evaluated.
These studies used an obese mouse model of T2DM, the KKAy mouse. As the result of an agouti mutation on a diabetes susceptible background (KK strain), the KKAy mouse overeats normal chow, accumulates adipose tissue, and develops overt hyperglycemia. Since both mouse groups are insulin resistant[26-28], but only the KKAy mouse group develops persistent hyperglycemia[26], the contribution of overt hyperglycemia to enteric retrograde axonal transport could be determined. We hypothesized that the combination of insulin resistance and overt hyperglycemia in KKAy mice would accelerate the onset of impaired FG transport by enteric neurons as compared to KK mice.
Female KK Cg-Ay/J mice (KKAy, strain 2468, n = 60), an obese model of T2DM, and age-matched female KK/HIJ mice (KK, stock number 2106, n = 70), were obtained at 3-6 wk of age (Jackson Laboratories, Bar Harbor, ME, United States). Initially, KKAy mice were screened for onset of T2DM by urine glucose. Since most KKAy mice became diabetic at 8 wk of age, onset of T2DM was defined as 8 wk of age and T2DM was untreated for 8, 16, or 24 wk. Body weight was recorded weekly. At sacrifice, drops of whole blood were used to determine glycosylated hemoglobin (Hb) (A1C Now+® Multi Test A1C System; Bayer, Tarrytown, NY, United States) and stress-induced glucose (BD Logic blood glucose monitor; Becton, Dickinson, and Co., Franklin Lakes, NJ, United States). Urine was drained from the bladder using a syringe and tested for glucose using uristix (Bayer). Urine was considered positive for glucose if glucose was ≥ 500 mg/dL.
Surgeries were performed prior to onset of T2DM (4 wk of age), at onset of T2DM (8 wk of age), and after 8, 16, or 24 wk of untreated T2DM (i.e., 16, 24, and 32 wk of age, respectively). The number of mice at each age is shown in Table 1. Both KKAy and age-matched KK mice surgeries were performed on the same day using the same tracer solution. Surgeries were performed under ketamine (100-150 mg/kg, ip; Fort Dodge Animal Health, Fort Dodge, IA, United States) and xylaxine (8 mg/kg, ip; Vedco, St. Joseph, MO, United States) anesthesia. The neural tracer FG was used to assess active axonal retrograde transport in enteric neural pathways.
| Age (wk) | Fundus | Antrum |
| KK mice | ||
| 4 | 6 | 6 |
| 8 | 8 | 9 |
| 16 | 8 | 7 |
| 24 | 6 | 8 |
| 32 | 6 | 6 |
| KKAy mice | ||
| 4 | 6 | 6 |
| 8 | 6 | 6 |
| 16 | 6 | 6 |
| 24 | 6 | 7 |
| 32 | 5 | 6 |
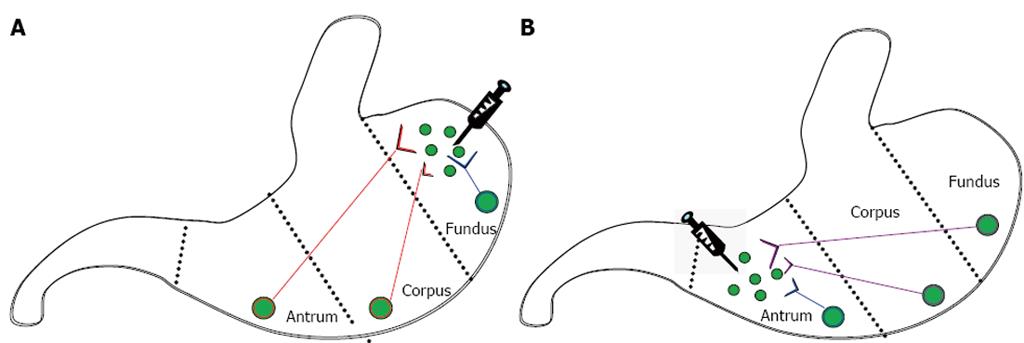
The tracer solution containing 4% FG (hydroxystilbamidine methanesulfonate; Invitrogen Molecular Probes) in dimethyl sulfoxide was superficially injected into the ventral fundus or ventral antrum at 5 sites, 2 μL each, using a microsyringe (702RN, 25 μL; Hamilton, Reno, NV, United States) with replaceable needle (3/8, point style 2, 33 G, Hamilton). An analgesic (buprenorphine, 0.05 mg/kg, sc; Hospira, Inc., Lake Forest, IL, United States) was administered immediately after surgery. Ketoprofen (5 mg/kg, ip; Fort Dodge Animal Health) was administered 6 h after surgery and the following day.
To promote selective labeling, care was taken to inject the tracer into the gastric wall and avoid leakage of the tracer into the abdominal cavity. Other experiments performed in the lab using the same injection technique have shown that ventral injection of tracer into the stomach wall resulted in unilateral FG labeling of efferent vagal neurons in the brainstem dorsal motor nucleus[29]. If tracer were not confined to the stomach wall, brainstem labeling would be bilateral.
Six days after tracer injection, mice were sacrificed by CO2 narcosis followed by pneumothorax. Stomachs were removed, placed in oxygenated Krebs solution, trimmed, opened along the lesser curvature, and emptied of contents. The fundus was cut away from the corpus/antrum. Tissues were stretched and pinned, mucosa side up, in a sylgard-lined Petri dish then fixed overnight at 37 °C in 4% paraformaldehyde in 0.1 mol/L PBS. The mucosa was removed. Using a razor blade, the center of each fundus, corpus, and antrum region along the greater curvature was cut out and mounted on a glass slide then coverslipped. The approximate distances of the excised sections from fundus injection sites were 0.6, 2.2, and 3.3 cm for fundus, corpus, and antrum sections, respectively. The approximate distances from antrum injection sites were 3.2, 1.1, and 0.4 cm for fundus, corpus sections, and antrum sections, respectively.
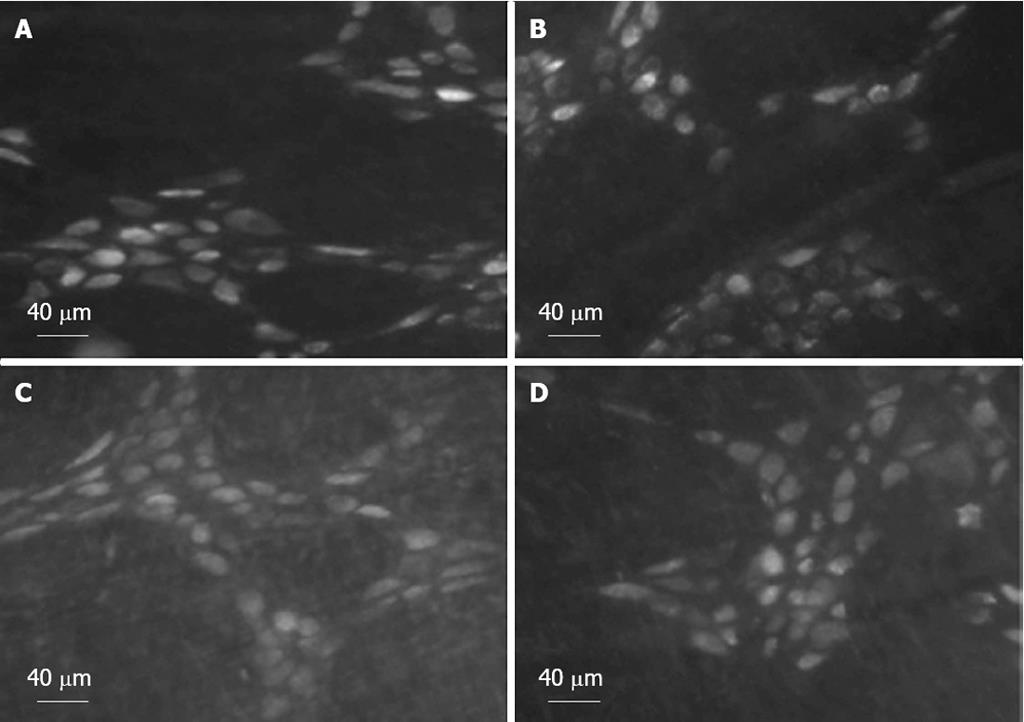
Tracer-labeled neurons were viewed at 20× using a microscope (Leica DMIL with UV light source; Leica Microsystems Inc., Buffalo Grove, IL, United States) with attached digital camera (MagnaFire; Optronics, Goleta, CA, United States) using the Chroma filter for FG (11006V3 Gold: Ex. 300-390, Em. ≥ 515). Digital images (1280 by 1024 pixels) were 0.327 μm/pixel. Digital images were in 32 bit RGB color at an image resolution of 100 dpi and a computer screen resolution of 92 dpi. Labeled neurons were manually counted with the assistance of ImagePro Plus (version 4.5.0.29, Media Cybernetics, Silver Spring, MD, United States). The numbers of labeled neurons in 20 adjacent images (418.6 μm by 334.8 μm) of the fundus and in 12 adjacent images of the corpus and antrum (total area: 2.8, 1.7, and 1.7 mm2, respectively) were counted and data were expressed as (1) total number of tracer-labeled neurons in that pathway; and (2) total number of neurons in all three regions labeled after tracer injection into the fundus or antrum.
Six pathways in the stomach were assessed in this study (Figure 1). Enteric neurons in ascending pathways had cell bodies in the corpus (short ascending pathways) or antrum (long ascending pathways) and nerve terminals in the ventral fundus, while those in descending pathways had cell bodies in the fundus (long descending pathways) or corpus (short descending pathways) and nerve terminals in the ventral antrum. Enteric neurons in fundic local pathways had both cell bodies and nerve terminals in the ventral fundus, while those in antral local pathways had both cell bodies and nerve terminals in the ventral antrum.
All data were expressed as mean ± SE. Data were statistically analyzed using SPSS (version 18.0, Chicago, IL). Independent factors for analysis of variance included KK or KKAy, age (4, 8, 16, 24, or 32 wk) or pathway (ascending or descending). Both main effects of one independent factor and interactions of two independent factors were evaluated and Sidak correction was used for post-hoc analysis. The ratios of KK and KKAy mice with positive urine glucose were compared using χ2. Correlations were performed using Pearson correlation (r), linear regression (r2) was performed using SigmaPlot (version 12.0, Systat Software, Inc., San Jose, CA). The number inside the bar in the graphs indicates the number of animals in the group. A P value < 0.05 was considered statistically significant with aP < 0.05, bP < 0.01.
Body weights of KK and KKAy mice were similar at 4 wk of age (Table 2), then KKAy mice became heavier [interaction: KK/KKAy and age F (4, 120) = 4.414, P = 0.002]. Body weight plateaued in KK and KKAy mice at 24 and 16 wk of age, respectively.
| Body weight (g) | HbA1c (%) | Urine glucose1 | |
| 4 wk of age | |||
| KK | 19.2 ± 0.4 | < 4.0 | 0/6 |
| (n = 12) | (n = 3) | ||
| KKAy | 19.9 ± 0.5 | < 4.0 | 2/6 |
| (n = 12) | (n = 3) | ||
| 8 wk of age | |||
| KK | 29 ± 1d | 5.0 ± 0.0 | 0/6 |
| (n = 17) | (n = 7) | ||
| KKAy | 34 ± 1bd | 6.6 ± 0.5b | 1/31 |
| (n = 12) | (n = 6) | ||
| 16 wk of age | |||
| KK | 35 ± 1df | 5.1 ± 0.1 | 0/13 |
| (n = 15) | (n = 3) | ||
| KKAy | 43 ± 1bdf | 7.5 ± 0.7b | 8/111 |
| (n = 12) | (n = 3) | ||
| 24 wk of age | |||
| KK | 40 ± 1dfh | 5.3 | 0/12 |
| (n = 14) | (n = 2) | ||
| KKAy | 45 ± 1bdf | 7.0 ± 0.9b | 7/111 |
| (n = 13) | (n = 3) | ||
| 32 wk of age | |||
| KK | 39 ± 2df | 5.0 ± 0.1 | 0/9 |
| (n = 12) | (n = 5) | ||
| KKAy | 47 ± 1bdf | 5.6 ± 0.5b | 3/11 |
| (n = 11) | (n = 5) |
Glucose control was impaired in KKAy mice (Table 2). Glycosylated Hb, an index of average plasma glucose over the previous 40 d for a mouse[30], was elevated in KKAy as compared to KK mice [main effect F (1, 26) = 24.748, P < 0.001] but did not change with age [main effect: F (3, 26) = 1.916, P = 0.152]. For all mice 8 wk of age and older, glycosylated Hb was higher in KKAy mice (6.53 ± 0.31, n = 17) than in KK mice (5.02 ± 0.04, n = 17). The ability to raise blood glucose in response to stress was greater in KKAy as compared to KK mice. Stress-induced hyperglycemia was highest at 16 wk in KKAy mice (535 ± 47, n = 7), but remained steady in KK mice (297 ± 20, n = 9) [interaction: KK/KKAy and age F (4, 67) = 2.609, P = 0.043]. Glucose was detected in the urine of some KKAy mice prior to onset of T2DM, but was not detected in any KK mice.
Six days after injection of tracer into the fundus or antrum, FG accumulated in the cell bodies of enteric neurons of the fundus, corpus, and antrum. Tracer-labeled enteric neurons of the corpus are shown in Figure 2.
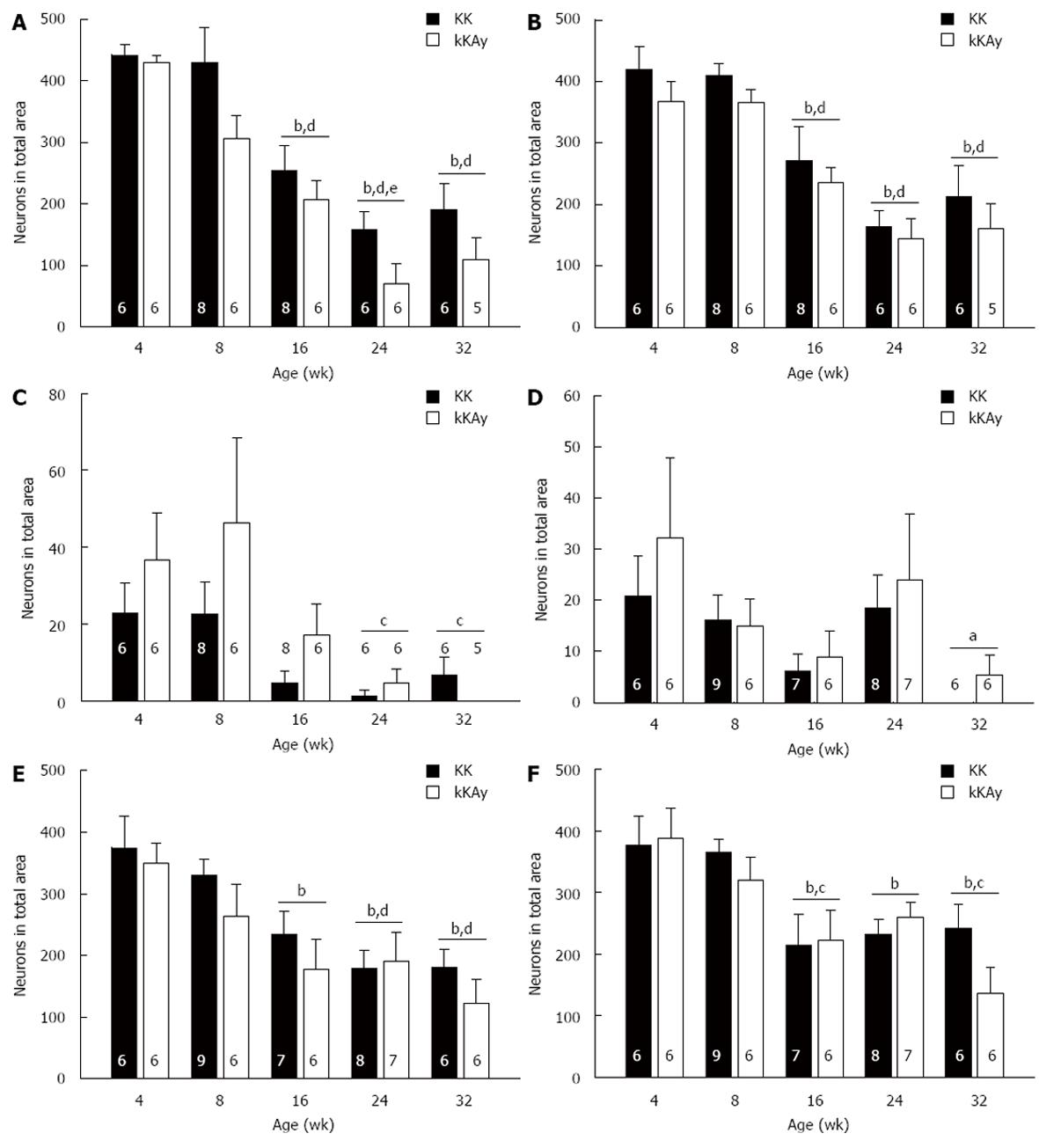
In the long ascending pathway (Figure 3A), significantly fewer FG-labeled enteric neurons were detected in KKAy as compared to KK mice. For both KK and KKAy mice, the number of FG-labeled neurons was reduced by 16 wk of age and reached its lowest value at 24 wk of age.
In the short ascending pathway (Figure 3B), there was a trend for fewer FG-labeled enteric neurons in KKAy as compared to KK mice. By 16 wk of age, the numbers of FG-labeled neurons were reduced as compared to 4 and 8 wk of age. The numbers of FG-labeled neurons remained low at 24 and 32 wk of age.
In the long (Figure 3D) and short (Figure 3E) descending pathways, similar numbers of FG-labeled enteric neurons were detected in KK as compared to KKAy mice. The total number of FG-labeled enteric neurons reached its lowest value at 24 wk of age for the short descending pathway and 32 wk of age for the long descending pathway.
A similar dominance of ascending projections as compared to descending projections reported in guinea pig stomach[31,32] was observed in this mouse study. At 4 wk of age, more FG-labeled neurons were in the ascending pathways as compared to the descending pathways for KK and KKAy mice [main effect: F (1, 20) = 98.981, P < 0.001; KK: ascending (n = 6), 858 ± 45; descending (n = 6), 397 ± 55; KKAy: ascending (n = 6), 800 ± 37; descending (n = 6), 384 ± 37].
Similar numbers of FG-labeled fundic (Figure 3C) and antral (Figure 3F) enteric neurons were detected in KK as compared to KKAy mice. In the local fundic pathway, the total number of FG-labeled enteric neurons was variable, reaching its highest value at 8 wk and its lowest value at 24 wk of age. In contrast, in the antral pathway, the total number of FG-labeled enteric neurons was decreased by 16 wk in KK and KKAy mice.
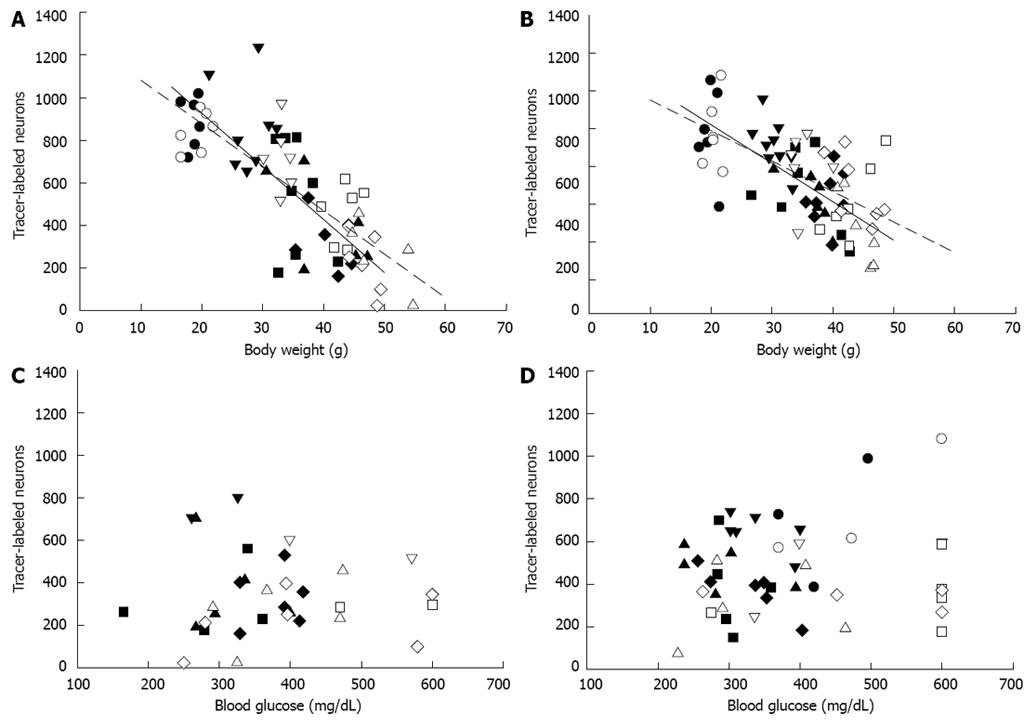
The total number of FG-labeled neurons in all sections after injection into either the fundus or antrum was significantly correlated to mouse final body weight, but not stress-elevated blood glucose (Figure 4). As KK mice aged and body weight increased, the total number of enteric neurons labeled by fundus injection (Figure 4A) decreased (n = 34, r = -0.746, P < 0.001). In the heavier KKAy mice, the correlation was stronger (n = 29, r = -0.842, P < 0.001). As a result of injection into the antrum (Figure 4B), the total number of FG-labeled neurons similarly decreased with body weight in both groups of mice [KK (n = 36), r = -0.660, P < 0.001; KKAy (n = 31), r = -0.622, P < 0.001]. In contrast, stress-elevated blood glucose was not correlated with the number of FG-labeled neurons [fundus injection (Figure 4C): KK (n = 17), r = -0.097, P = 0.712; KKAy (n = 15), r = 0.328, P = 0.232; antrum injection (Figure 4D): KK (n = 25), r = 0.174, P = 0.407; KKAy (n = 20), r = 0.308, P = 0.187]. In addition, HbA1c was not correlated to number of FG-labeled neurons for antrum injection [KK (n = 17), r = -0.164, P = 0.528; KKAy (n = 16), r = -0.078, P = 0.774]. Data was not available for fundus injection.
The principal findings of these studies were that (1) insulin resistance, in the absence of persistent hyperglycemia, slowed retrograde FG transport in KK mice at 16 wk of age, the approximate age of onset of insulin resistance[26-28]; and (2) persistent hyperglycemia, as indicated by elevated HbA1c, significantly slowed tracer transport in long ascending pathways in KKAy as compared to KK mice, but the age of onset was unchanged. Persistent hyperglycemia did not significantly accelerate the age of onset of neuropathy in KKAy as compared to KK mice.
Reduced numbers of FG-labeled neurons may be due to many factors related to enteric neuropathy. Enteric neurons undergoing neuropathy may take up less tracer because of limited endocytosis[33]. Retrograde transport of tracer may be impaired due to glycosylation of axonal cytoskeletal proteins[34-36]. The density of nerve terminals in the tracer injection field may be reduced due to degeneration of nerve terminal processes[3,37,38] or to low rates of neuronal apoptosis[1,39] as a consequence of metabolic disturbances[40]. In addition, impaired active axonal transport of growth factors to the neuronal soma, including insulin and neurotrophins[11,14,41-44], may both promote and exacerbate neuropathy[6,7].
Specific mechanism(s) in distinct populations of neurons may contribute more significantly to neuropathy. Many factors play a role in the development of diabetic neuropathy[6,7,45], but treatments targeting a single mechanism do not prevent neuropathy of all autonomic neurons[46-48]. In this study, long ascending enteric neurons, but not long descending enteric neurons, displayed significantly slower tracer transport in KKAy as compared to KK mice despite similar axonal lengths. Ascending enteric neurons are predominantly cholinergic[31,49-51]. Impaired axonal transport has been reported for acetylcholinesterase[18,19] and muscarinic receptors[15] and debated for choline acetyltransferase[8,20,43,52] in rodent models of DM. Hence, active tracer transport in this subpopulation of autonomic neurons with a cholinergic phenotype may be more susceptible to combined persistent hyperglycemia and low effective insulin.
Age-related degeneration of axonal branches[37,40] may contribute to the reduction in the number of FG-labeled neurons observed in older as compared to younger mice. In young NMRI mice, the number of myenteric neurons per ganglion section did not change between 1 and 3 mo of age[53]. Similarly, in this study, neuron density was unchanged from 4 to 8 wk of age. In rats, gastric myenteric neurons have been reported to be less susceptible to age-related degeneration than intestinal myenteric neurons[54,55]. The density of gastric myenteric neurons was unchanged until 27 mo of age, when density was reduced in the forestomach, but not corpus or antrum[55]. In this study, the initial reduction in FG labeled neurons was observed at 16 wk of age in KK and KKAy mice. Hence, in mice with insulin resistance, the reduced delivery of target organ trophic factors back to the cell soma may be associated with increased susceptibility of enteric neurons, especially those with cholinergic phenotype[54], to age-related degeneration[37,56].
A heavier body weight, reflecting a higher degree of adiposity and therefore insulin resistance, contributed more strongly to enteric neuropathy than severity of hyperglycemia. The correlation of total FG-labeled neurons with body weight was stronger for heavier KKAy as compared to lighter KK mice. In contrast, the severity of hyperglycemia was not correlated to total FG-labeled neurons. Other studies have also reported that insulin deficiency, but not hyperglycemia, was associated with severity of peripheral neuropathy[14,38,57-59].
An increase in stomach size may result in a lower density of nerve terminals in the injection field for FG endocytosis and subsequent reduction in FG labeling. The weights of stomachs from 16 wk old KK and KKAy mice were similar [personal observation, KK (n = 4), 277 ± 40 mg; KKAy (n = 4), 263 ± 33 mg, P = 0.80] suggesting that stomachs from KKAy mice were not hypertrophied.
Insulin resistance itself in the absence of persistent hyperglycemia slowed tracer transport in 16-wk-old KK mice. The addition of persistent hyperglycemia in KKAy mice further slowed tracer transport in only long ascending enteric neurons without accelerating the age of onset. Slowed active tracer transport in enteric neurons implies that retrograde transport of endogenous neurotrophins required for maintenance of normal nerve function may also be impaired[6,21,33,60]. Given the codependence of neurons and the target tissue on neurotrophins to preserve organ function[6], neuropathy resulting from impaired retrograde transport of neurotrophins may result in imprecise coordination of gastric regions by ascending and descending inhibitory and excitatory enteric reflexes[61,62], thereby contributing to asynchronous gastric motor function[63,64].
Diabetes mellitus (DM), characterized by persistent hyperglycemia and a deficiency of effective plasma insulin, can result in neuropathy of autonomic neurons. In rodent models of type 1 DM (T1DM) and type 2 DM (T2DM), neuropathies of the parasympathetic vagus nerve and enteric neurons located within the wall of the gut have been described. Many factors likely contribute to the development of autonomic neuropathy including accumulation of sorbitol in nerves, oxidative stress, and somal deficiency of neurotrophins.
Under anesthesia, the retrograde tracer fluorogold was superficially injected into the fundus or antrum using a microsyringe in KK Cg-Ay/J mice prior to onset of T2DM (4 wk of age), at onset of T2DM (8 wk of age), and after 8, 16, or 24 wk of untreated T2DM and in age-matched KK/HIJ mice. Six days later, mice were sacrificed by CO2 narcosis followed by pneumothorax. Data were expressed as the number of neurons in short and long descending and ascending pathways and in local fundus and antrum pathways, and the number of neurons in all regions labeled after injection of tracer into either the fundus or the antrum.
The principal findings of these studies were that (1) insulin resistance, in the absence of persistent hyperglycemia, slowed retrograde fluorogold transport in KK mice at 16 wk of age, the approximate age of onset of insulin resistance; and (2) persistent hyperglycemia, as indicated by elevated HbA1c, significantly slowed tracer transport in long ascending pathways in KKAy as compared to KK mice, but the age of onset was unchanged. Persistent hyperglycemia did not significantly accelerate the age of onset of neuropathy in KKAy as compared to KK mice.
Insulin resistance itself in the absence of persistent hyperglycemia slowed tracer transport in 16-wk-old KK mice. The addition of persistent hyperglycemia in KKAy mice further slowed tracer transport in only long ascending enteric neurons without accelerating the age of onset. Slowed active tracer transport in enteric neurons implies that retrograde transport of endogenous neurotrophins required for maintenance of normal nerve function may also be impaired.
The manuscript is formally well written and figures are clear and understandable. Experimental design and methodology are suitable for publication.
P- Reviewers Binder HJ, Wex T S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Tay SS, Wong WC. Short- and long-term effects of streptozotocin-induced diabetes on the dorsal motor nucleus of the vagus nerve in the rat. Acta Anat (Basel). 1994;150:274-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kniel PC, Junker U, Perrin IV, Bestetti GE, Rossi GL. Varied effects of experimental diabetes on the autonomic nervous system of the rat. Lab Invest. 1986;54:523-530. [PubMed] [Cited in This Article: ] |
| 3. | Robertson DM, Sima AA. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: a morphometric study. Diabetes. 1980;29:60-67. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4:271-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell Mol Life Sci. 2003;60:2445-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Tomlinson DR, Townsend J, Fretten P. Prevention of defective axonal transport in streptozocin-diabetic rats by treatment with “Statil” (ICI 128436), an aldose reductase inhibitor. Diabetes. 1985;34:970-972. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Belai A, Calcutt NA, Carrington AL, Diemel LT, Tomlinson DR, Burnstock G. Enteric neuropeptides in streptozotocin-diabetic rats; effects of insulin and aldose reductase inhibition. J Auton Nerv Syst. 1996;58:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Hounsom L, Corder R, Patel J, Tomlinson DR. Oxidative stress participates in the breakdown of neuronal phenotype in experimental diabetic neuropathy. Diabetologia. 2001;44:424-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann NY Acad Sci. 2002;959:368-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Tomlinson DR, Mayer JH. Defects of axonal transport in diabetes mellitus--a possible contribution to the aetiology of diabetic neuropathy. J Auton Pharmacol. 1984;4:59-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Liu W, Yue W, Wu R. Effects of diabetes on expression of glial fibrillary acidic protein and neurotrophins in rat colon. Auton Neurosci. 2010;154:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Pierson CR, Zhang W, Murakawa Y, Sima AA. Insulin deficiency rather than hyperglycemia accounts for impaired neurotrophic responses and nerve fiber regeneration in type 1 diabetic neuropathy. J Neuropathol Exp Neurol. 2003;62:260-271. [PubMed] [Cited in This Article: ] |
| 15. | Laduron PM, Janssen PF. Impaired axonal transport of opiate and muscarinic receptors in streptozocin-diabetic rats. Brain Res. 1986;380:359-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lee PG, Cai F, Helke CJ. Streptozotocin-induced diabetes reduces retrograde axonal transport in the afferent and efferent vagus nerve. Brain Res. 2002;941:127-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Odekunle A. Impairment of transneuronal traffic in Streptozotocin-induced diabetes, a WGA-HRP neurohistochemical study in the rat. J Biomed Sci. 2006;13:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Vitadello M, Couraud JY, Hässig R, Gorio A, Di Giamberardino L. Axonal transport of acetylcholinesterase in the diabetic mutant mouse. Exp Neurol. 1983;82:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Norido F, Canella R, Zanoni R, Gorio A. Development of diabetic neuropathy in the C57BL/Ks (db/db) mouse and its treatment with gangliosides. Exp Neurol. 1984;83:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Calcutt NA, Willars GB, Tomlinson DR. Axonal transport of choline acetyltransferase and 6-phosphofructokinase activities in genetically diabetic mice. Muscle Nerve. 1988;11:1206-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Lee PG, Hohman TC, Cai F, Regalia J, Helke CJ. Streptozotocin-induced diabetes causes metabolic changes and alterations in neurotrophin content and retrograde transport in the cervical vagus nerve. Exp Neurol. 2001;170:149-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Cannon WB, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267-273. [Cited in This Article: ] |
| 23. | Cannon WB. The nature of gastric peristalsis. Am J Physiol. 1911;29:250-266. [Cited in This Article: ] |
| 24. | Cellini J, DiNovo K, Harlow J, LePard KJ. Regional differences in neostigmine-induced contraction and relaxation of stomach from diabetic guinea pig. Auton Neurosci. 2011;160:69-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612-G619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970;17:23-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 207] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3:212-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Reddi AS, Camerini-Davalos RA. Hereditary diabetes in the KK mouse: an overview. Adv Exp Med Biol. 1988;246:7-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Fullmer R, LePard KJ. Gastric vagal fluorogold labeled neurons are reduced in obese diabetic agouti mice. Downers Grove, IL: Midwestern University 2009; . [Cited in This Article: ] |
| 30. | Van Putten LM. The life span of red cells in the rat and the mouse as determined by labeling with DFP32 in vivo. Blood. 1958;13:789-794. [PubMed] [Cited in This Article: ] |
| 31. | Schemann M, Reiche D, Michel K. Enteric pathways in the stomach. Anat Rec. 2001;262:47-57. [PubMed] [Cited in This Article: ] |
| 32. | Michel K, Reiche D, Schemann M. Projections and neurochemical coding of motor neurones to the circular and longitudinal muscle of the guinea pig gastric corpus. Pflugers Arch. 2000;440:393-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Tomlinson DR, Fernyhough P, Diemel LT, Maeda K. Deficient neurotrophic support in the aetiology of diabetic neuropathy. Diabet Med. 1996;13:679-681. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Cullum NA, Mahon J, Stringer K, McLean WG. Glycation of rat sciatic nerve tubulin in experimental diabetes mellitus. Diabetologia. 1991;34:387-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Jeyabal PV, Kumar R, Gangula PR, Micci MA, Pasricha PJ. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil. 2008;20:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | McLean WG. The role of axonal cytoskeleton in diabetic neuropathy. Neurochem Res. 1997;22:951-956. [PubMed] [Cited in This Article: ] |
| 37. | Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 524] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 38. | Sima AA, Zhang W, Xu G, Sugimoto K, Guberski D, Yorek MA. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43:786-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Guo C, Quobatari A, Shangguan Y, Hong S, Wiley JW. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil. 2004;16:335-345. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Wiley JW. Aging and neural control of the GI tract: III. Senescent enteric nervous system: lessons from extraintestinal sites and nonmammalian species. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1020-G1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Burnstock G, Mirsky R, Belai A. Reversal of nerve damage in streptozotocin-diabetic rats by acute application of insulin in vitro. Clin Sci (Lond). 1988;75:629-635. [PubMed] [Cited in This Article: ] |
| 42. | Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Jakobsen J, Brimijoin S, Skau K, Sidenius P, Wells D. Retrograde axonal transport of transmitter enzymes, fucose-labeled protein, and nerve growth factor in streptozotocin-diabetic rats. Diabetes. 1981;30:797-803. [PubMed] [Cited in This Article: ] |
| 44. | Schmidt RE, Modert CW, Yip HK, Johnson EM. Retrograde axonal transport of intravenously administered 125I-nerve growth factor in rats with streptozotocin-induced diabetes. Diabetes. 1983;32:654-663. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Sima AA. Pathological mechanisms involved in diabetic neuropathy: can we slow the process? Curr Opin Investig Drugs. 2006;7:324-337. [PubMed] [Cited in This Article: ] |
| 46. | Shotton HR, Clarke S, Lincoln J. The effectiveness of treatments of diabetic autonomic neuropathy is not the same in autonomic nerves supplying different organs. Diabetes. 2003;52:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Shotton HR, Adams A, Lincoln J. Effect of aminoguanidine treatment on diabetes-induced changes in the myenteric plexus of rat ileum. Auton Neurosci. 2007;132:16-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Shotton HR, Broadbent S, Lincoln J. Prevention and partial reversal of diabetes-induced changes in enteric nerves of the rat ileum by combined treatment with alpha-lipoic acid and evening primrose oil. Auton Neurosci. 2004;111:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Pfannkuche H, Reiche D, Firzlaff U, Sann H, Schemann M. Enkephalin-immunoreactive subpopulations in the myenteric plexus of the guinea-pig fundus project primarily to the muscle and not to the mucosa. Cell Tissue Res. 1998;294:45-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Reiche D, Schemann M. Mucosa of the guinea pig gastric corpus is innervated by myenteric neurones with specific neurochemical coding and projection preferences. J Comp Neurol. 1999;410:489-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 51. | Schemann M, Schaaf C. Differential projection of cholinergic and nitroxidergic neurons in the myenteric plexus of guinea pig stomach. Am J Physiol. 1995;269:G186-G195. [PubMed] [Cited in This Article: ] |
| 52. | Mayer JH, Herberg L, Tomlinson DR. Axonal transport and nerve conduction and their relation to nerve polyol and myo-inositol levels in spontaneously diabetic BB/D rats. Neurochem Pathol 1984-. 1985;2:285-293. [PubMed] [Cited in This Article: ] |
| 53. | El-Salhy M, Sandström O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107:93-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Cowen T. Selective vulnerability in adult and ageing mammalian neurons. Auton Neurosci. 2002;96:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Romanovsky D, Cruz NF, Dienel GA, Dobretsov M. Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin-treated rats. Neurobiol Dis. 2006;24:384-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Hoybergs YM, Meert TF. The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett. 2007;417:149-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Zhang W, Sima AA. Experimental rat models of types 1 and 2 diabetes differ in sympathetic neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:450-460. [PubMed] [Cited in This Article: ] |
| 60. | Li Y, Owyang C. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G461-G469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Yuan SY, Brookes SJ, Costa M. Distension-evoked ascending and descending reflexes in the isolated guinea-pig stomach. J Auton Nerv Syst. 1997;62:94-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Hennig GW, Brookes SJ, Costa M. Excitatory and inhibitory motor reflexes in the isolated guinea-pig stomach. J Physiol. 1997;501:197-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol. 2007;103:1560-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |