Published online Feb 28, 2012. doi: 10.3748/wjg.v18.i8.794
Revised: June 15, 2011
Accepted: June 22, 2011
Published online: February 28, 2012
AIM: To study the long-term effects of endoscopic sphincterotomy on biliary epithelium.
METHODS: This is a prospective case-control study. A total of 25 patients with a median age of 71 years (range 49-89 years) and prior endoscopic sphincterotomy (ES) for benign disease formed the first group. The median time from ES was 42 mo (range 8-144 mo). Another 25 patients with a median age of 76 years (range 44-94 mo) and similar characteristics who underwent current endoscopic retrograde cholangiopancreatography (ERCP) and ES for benign disease formed the second group (control group). Brush cytology of the biliary tree with p53 immunocytology was performed in all patients of both groups. ERCPs and recruitment were conducted at the Endoscopic Unit of Aretaieion University Hospital and Tzaneio Hospital, Athens, from October 2006 to June 2010.
RESULTS: No cases were positive or suspicious for malignancy. Epithelial atypia was higher in the first group (32% vs 8% in the second group, P = 0.034). Acute cholangitis and previous biliary operation rates were also higher in the first group (acute cholangitis, 60% vs 24% in the second group, P = 0.01; previous biliary operation, 76% vs 24% in the second group, P = 0.001). Subgroup analysis showed that previous ES was the main causal factor for atypia, which was not related to the time interval from the ES (P = 0.407). Two patients (8%) with atypia in the first group were p53-positive.
CONCLUSION: ES causes biliary epithelial atypia that represents mostly reactive/proliferative rather than premalignant changes. The role of p53 immunoreactivity in biliary atypia needs to be further studied.
- Citation: Kalaitzis J, Vezakis A, Fragulidis G, Anagnostopoulou I, Rizos S, Papalambros E, Polydorou A. Effects of endoscopic sphincterotomy on biliary epithelium: A case-control study. World J Gastroenterol 2012; 18(8): 794-799
- URL: https://www.wjgnet.com/1007-9327/full/v18/i8/794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i8.794
The introduction of endoscopic retrograde cholangiopancreatography (ERCP) with endoscopic sphincterotomy (ES) in 1974 changed the treatment of choledocholithiasis[1]. Today, ES is a widely available endoscopic technique that is used not only for the removal of common bile duct stones but also in the treatment of a wide variety of biliary and pancreatic diseases[2]. An increasing number of young patients with a long life expectancy will undergo ES, and this raises concern for the long-term complications of the procedure and especially the late development of cholangiocarcinoma[3].
Carcinogenesis after surgical sphincteroplasty and biliary-enteric anastomosis has been well documented. Numerous case reports and large series have described a causal association between biliary-enteric drainage procedures for benign disease and the late development of cholangiocarcinoma[4-6]. The hypothesis is that the disruption of the sphincter of Oddi causes prolonged pancreatobiliary and duodenobiliary reflux, bacterial overgrowth and chronic inflammation of the bile ducts. Chronic inflammatory irritation induces hyperplasia, dysplasia and atypia of the epithelium that ultimately can lead to carcinogenesis[5].
ES also causes ablation of sphincter function, which raises concern about the late development of cholangiocarcinoma by the same mechanism as previously mentioned. In a study of long-term consequences of ES, 410 patients were followed for an average of 10 years after ES, and late development of cholangiocarcinoma was documented in 3 patients, which represents an elevated risk of malignancy[7]. Furthermore, the long-term effects of ES on sphincter function are not clear. In a small trial, the function of the biliary sphincter was permanently lost, and this was associated with bacterial colonisation and chronic inflammation of the biliary system[8]. In another study, pancreatobiliary reflux was present for the first year after ES and was minimised after wards[9]. Recently, two large population-based studies demonstrated an increase in the cholangiocarcinoma rate after ERCP mostly in the first five years, but there was no causal association between sphincterotomy and cholangiocarcinoma[10,11].
In the present study, we performed cytological evaluation with additional p53 immunocytology of the biliary epithelium one to twelve years after ES for benign disease.
The trial was approved by the Science Board of Aretaieion University Hospital and Tzaneio General Hospital and was registered at ClinicalTrials.org (protocol number NCT01135732). All patients gave informed consent for the ERCP procedure and for participation in the trial.
This was a prospective case-control study. ERCPs and recruitment of the patients were conducted at the Endoscopic Unit of Aretaieion University Hospital and Tzaneio General Hospital, Athens, from October 2006 to June 2010. Cytological evaluation and p53 immunocytology were performed at the Cytology Department of Tzaneio General Hospital. Analysis and interpretation of the data were completed in January 2011.
The patients were enrolled in two groups. A total cohort of twenty-five patients who underwent ERCP for benign disease and had a prior ES formed the first group (Table 1). Cytology brushing was performed at a median time of 42 mo from ES within a range of 8 to 144 mo. Exclusion criteria were biliary stricture, liver cirrhosis, viral hepatitis infection, sclerosing cholangitis, choledochal cysts, malignancy of the liver, bile ducts and pancreas, and any other known or suspected malignancy. To provide comparable data, a second group (control group) was formed of twenty-five patients of similar age and gender as the first group who underwent current ES for benign disease. The difference between the two groups was that the patients of the first group had a prior ES, while those of the control group had a current ES. The endoscopic procedures were performed by two specialised endoscopists (Vezakis A and Polydorou A).
| First group | Second group (control) | P value | |
| Patients | 25 | 25 | |
| Sex, male/female | 11/14 | 11/14 | 13 |
| Median age (yr) | 71 (range 49-89) | 76 (range 44-94) | 0.494 |
| ERCP indication | |||
| Stent removal | 4 (16) | - | |
| Biliary colic | 5 (20) | 17 (68) | 0.013 |
| Biliary pancreatitis | 1 (4) | 2 (8) | |
| Acute cholangitis | 15 (60) | 6 (24) | |
| Previous biliary operation1 | 19 (76) | 6 (24) | 0.0013 |
| Dilated bile ducts (ultrasound) | 14 (56) | 18 (72) | 0.233 |
| Median time from ES (mo)2 | 42 (range 8-144) | - |
Brush cytology was performed in all patients of the first group to evaluate the biliary epithelium. After cannulation of the common bile duct through the previous sphincterotomy, a 3.0-mm standard endoscopic cytology brush with a catheter sheath (Hobbs Medical, United States) was passed through the endoscope into the biliary tree under fluoroscopy. The brush was then pushed out of the catheter, and cellular material was obtained by moving it back and forth across the common and left or right hepatic ducts approximately ten times. The brush was then withdrawn into the catheter, and the catheter and brush were withdrawn from the endoscope as a unit. Similarly, brush cytology was performed in all patients of the control group immediately after performing the ES.
The cellular material obtained from each patient was immediately smeared onto five glass slides. Four slides were fixed with a 95% ethanol solution and one was air-dried. The brush was then fixed in cytospin (a solution composed of 50% ethanol, carbowax, and isopropanol) to obtain additional material if needed. Papanicolaou stain was applied to three out of four ethanol-fixed slides, and May-Grunwald-Giemsa stain was applied to the air-dried slide.
p53 immunostaining was performed on the fourth ethanol-fixed slide for all patients in both groups. The immunocytochemical method involved application of an anti-p53 primary antibody (monoclonal mouse anti-human p53, clone DO-7, Dako Athens, Greece, 1:100 dilution).
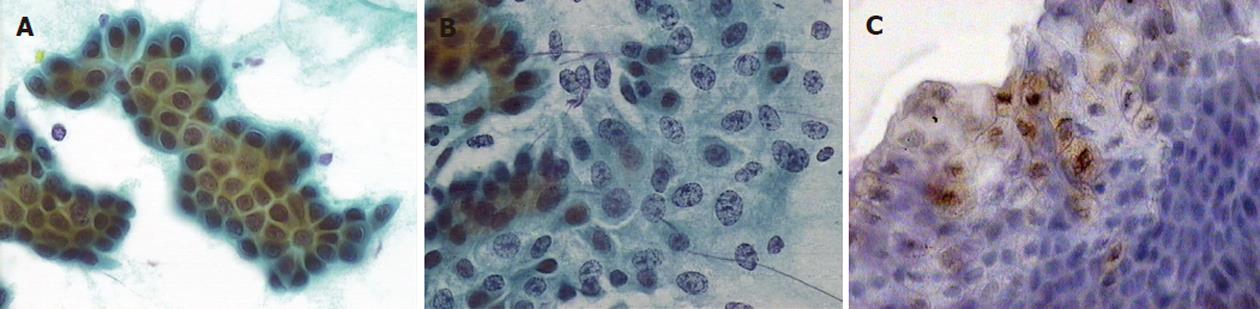
Samples were classified based on their morphological characteristics into one of five categories (Table 2)[12]. Positive immunocytochemical staining for p53 mutation was defined as the detection of more than five cells with strong intranuclear staining of p53 at high magnification (× 400) (Figure 1). Two specialised cytologists separately examined and evaluated the specimens to achieve more objective results.
| Cytological grade | Definition |
| Unsatisfactory | No diagnostic specimen: acellular or hypocellular; |
| Smears too thick, containing blood, obscured by inflammation or air-drying artefacts | |
| Negative for malignancy | Adequate cellular specimens containing benign cells consistent with normal elements of the target organ |
| Atypical/indeterminate | Minimally abnormal cellular findings that most likely represent reactive/reparative and inflammatory type changes; Malignancy is unlikely but cannot be completely ruled out |
| Suspicious for malignancy | Cytologic specimens exhibit abnormal cellular findings that display some, but not all, of the features of malignancy; Specimens containing highly abnormal cells in very limited numbers |
| Positive for malignancy | Unquestionable cellular features of malignancy |
Results were analysed using the χ2 test, Mann-Whitney test and regression analysis. Differences were considered significant at P < 0.05. Statistical analysis was performed with Minitab 16 statistical software.
All of the samples had adequate cellularity, and no case was judged too poor to be analysed. In one case, cytological evaluation by the two specialists was different, and an additional evaluation was then performed. No samples were suspect or positive for malignancy (Table 3). However, patients in the first group had a significantly higher proportion of atypia (32% vs 8% in the second group, P = 0.034). Acute cholangitis and previous biliary operation rates were also higher in the first group (acute cholangitis, 60% vs 24% in the second group, P = 0.01; previous biliary operation, 76% vs 24% in the second group, P = 0.001). On the contrary, the presence of dilated bile ducts detected by ultrasound, the extraction of common bile duct stones and the presence of dilated bile ducts without choledocholithiasis by ERCP did not differ between the two groups. A subgroup analysis of the patients with atypia demonstrated that previous ES was the only predisposing factor (Table 4).
| Atypical/indeterminate | Negative for malignancy | P value | |
| Patients | 10 | 40 | |
| Median age (yr) | 69.5 (SD = 11.3) | 74.5 (SD = 12.1) | 0.262 |
| Sex, male/female | 3/7 | 20/20 | 0.251 |
| Previous ES | 8 (80) | 17 (42) | 0.0341 |
| Acute cholangitis | 5 (50) | 16 (40) | 0.561 |
| Previous biliary operation | 6 (60) | 19 (47) | 0.481 |
| Stone extraction | 5 (50) | 22 (55) | 0.771 |
| Dilated bile ducts without lithiasis on ERCP | 4 (40) | 18 (45) | 0.781 |
| p53 immunocytology | |||
| Positive | 2 | 0 | |
| Negative | 8 | 40 | |
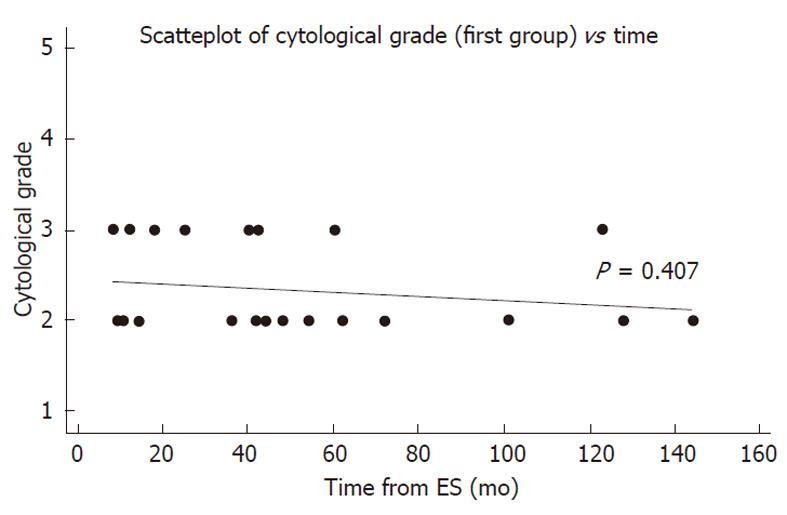
There was no correlation between atypia and the time interval from the previous ES (P = 0.407). Furthermore, 7 out of 8 cases of atypia in the first group occurred in the first five years after ES (Figure 2).
There were two samples with positive immunocytological staining for p53 in the first group and none in the second group; both positive samples were from patients with epithelial atypia (Table 5).
| Sex | Age (yr) | Indication ERCP | Previous biliary operation | Stone extraction | Cytological evaluation | Time from ES (mo) | Negative1 follow-up | |
| Patient 1 | Female | 49 | Acute cholangitis | Yes (open cholecystectomy) | No | Atypia | 18 | 3 yr 5 mo |
| Patient 2 | Female | 54 | Stent removal | Yes (open cholecystectomy) | Yes | Atypia | 25 | 3 yr 6 mo |
The main outcome of this trial was that no case was suspect or positive for malignancy for a median period of 42 mo after a previous ES. This finding is in agreement with several retrospective studies that examined the long-term complications of ES and did not report late development of cholangiocarcinoma[13-15].
However, there was a significant difference in the reported atypia of the biliary epithelium between the two groups in the present study. Subgroup analysis showed that only previous ES, and not acute cholangitis as one might expect, was directly connected to these changes. This result is not as controversial as it seems. Acute cholangitis as an indication for ERCP is a factor that represents a clinical condition at a specific moment that cannot express chronic subclinical inflammatory irritation of the epithelium. Furthermore, possible previous episodes of acute cholangitis were not included in the present study, as it is extremely difficult to have an accurate estimation. On the contrary, previous ES is a factor that includes and expresses these possible conditions, and the fact that it is associated with epithelial atypia demonstrates that these epithelial changes are possibly the result of a chronic process.
The reported difference in previous biliary operations between the two groups can be easily explained by the fact that a cholecystectomy was performed after ES for gallbladder lithiasis in most of the patients in the first group.
Cellular atypia may also represent premalignant chan-ges of the biliary epithelium[16]. It is known that carcinogenesis of the bile ducts is a very slow process that involves several cellular pathways such as growth autonomy, escape from senescence, unlimited replication, blockade of growth inhibitory signals, altered microenvironment and evasion of cell death[17]. One of the most commonly affected genes is the p53 tumour suppressor gene, which is inactivated in 21.7% to 76% of all cholangiocarcinomas[18]. Alterations in p53 have also been demonstrated in premalignant biliary lesions[19]. For these reasons and because of the availability of its use, p53 immunocytology was performed in all patients in both groups to assess possible premalignant lesions.
Immunocytological staining with p53 was positive in two young women who both had cellular atypia and also a clinical history that was negative for malignancy. False-positive p53 immunocytology results in biliary strictures are not known, as it is a relatively new method with only a few series reported, but it seems that p53 detection increases the sensitivity of brush cytology in cholangiocarcinoma detection[20,21]. Stewart et al[22] reported only one case that was p53-positive with cytological features of atypia, in whom clinical follow-up revealed no evidence of malignancy. Villanacci et al[21] reported two patients with mild dysplasia who were p53-positive and submitted to clinical follow-up. The two p53-positive cases in our study had a negative clinical follow-up at approximately 3.5 years. Nevertheless, these changes might represent premalignant conditions, and follow-up should continue.
Another important finding in this study is that the atypia of the biliary epithelium was not related to the time interval from ES as one could expect. In fact, seven out of eight cases of atypia were noticed in the first five years after ES. It seems that the causal factor for these epithelial changes ceases after a certain period of time. These data are in accordance with the results from the study by Sugiyama et al[9], in which the sphincterotomy length, pancreatobiliary reflux and pneumobilia gradually decreased at one and five years after ES. Interestingly, Mortensen et al[10], in their population-based study found elevated rates of cholangiocarcinoma within the first five years after ES, with percentage declination afterwards.
A major strength of this trial is the presence of a control group with almost matched sex and age. Brush cytology is an easy and safe technique with minimal complications[23]. A major limitation of this method is the relatively low sensitivity in detecting cholangiocarcinoma (30% to 57% in most published series)[23]. However, in this study, the intention was to collect cells from many sites of the biliary tree and not from a focal stenosis. In addition, p53 immunocytology is a method that seems to increase the sensitivity of brush cytology[21,22]. Finally, one could argue that the patients of the first group had recurrent biliary complications, and so the results cannot be fully extrapolated in asymptomatic patients with prior ES. The result is that many of these pathologic conditions are long-term complications of the ES. Furthermore, a human clinical trial with asymptomatic patients undergoing ERCP is not ethical.
In conclusion, no cases were positive or suspect for malignancy after a median period of 42 mo (range 8-144 mo) after ES for benign disease. Biliary epithelial atypia is likely to occur as a result of previous ES, primarily during the first five years after the procedure. Cytological findings represent reactive/proliferative rather than premalignant changes, but this must be confirmed by additional future studies. In addition, the role of p53-positive immunocytology in epithelial atypia needs to be further studied.
Carcinogenesis after surgical sphincteroplasty and biliary-enteric anastomosis has been well documented. Endoscopic sphincterotomy (ES) also causes ablation of sphincter function, and this raises concerns about the late development of cholangiocarcinoma.
Long-term effects of ES on biliary epithelium are not very clear. It seems that the biliary sphincter function is permanently lost, which results in duodenobiliary reflux. This study examines the effects of possible reflux on biliary epithelium and demonstrates that biliary atypia is most likely to occur after ES.
Several retrospective studies have examined the late complications of ES. This is the first prospective, case-control study that examines possible premalignant changes of biliary epithelium after ES using brush cytology and p53 immunocytology.
By understanding whether and how ES causes changes in biliary epithelium, tha authors contribute to a better knowledge of this commonly used procedure with emphasis on possible future adverse effects.
Brush cytology is an easy and safe technique of cytological examination. Mutation in the p53 oncogene is one of the most common mutations in the malignancy of biliary epithelium, thus p53 immunocytology indicates possible mutations in cytological samples that could represent premalignant changes.
The authors examined the long-term effects of ES on biliary epithelium. It is an interesting paper on a well-known subject. The results showed that reactive or proliferative change in biliary epithelium primarily occurred by ES.
Peer reviewer: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma - Sapienza, Viale del Policlinico 155, Roma 00161, Italy
S- Editor Yang XC L- Editor Logan S E- Editor Li JY
| 1. | Kawai K, Akasaka Y, Murakami K, Tada M, Koli Y. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 438] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ, Fanelli R. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Tham TC, Carr-Locke DL, Collins JS. Endoscopic sphincterotomy in the young patient: is there cause for concern? Gut. 1997;40:697-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Bettschart V, Clayton RA, Parks RW, Garden OJ, Bellamy CO. Cholangiocarcinoma arising after biliary-enteric drainage procedures for benign disease. Gut. 2002;51:128-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg. 2001;234:210-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Hakamada K, Sasaki M, Endoh M, Itoh T, Morita T, Konn M. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121:488-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Takahata S, Konomi H, Matsunaga H, Yokohata K, Takeda T, Utsunomiya N, Ikeda S. Long-term consequence of endoscopic sphincterotomy for bile duct stones. Gastrointest Endosc. 1998;48:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Bergman JJ, van Berkel AM, Groen AK, Schoeman MN, Offerhaus J, Tytgat GN, Huibregtse K. Biliary manometry, bacterial characteristics, bile composition, and histologic changes fifteen to seventeen years after endoscopic sphincterotomy. Gastrointest Endosc. 1997;45:400-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Sugiyama M, Atomi Y. Does endoscopic sphincterotomy cause prolonged pancreatobiliary reflux? Am J Gastroenterol. 1999;94:795-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Mortensen FV, Jepsen P, Tarone RE, Funch-Jensen P, Jensen LS, Sørensen HT. Endoscopic sphincterotomy and long-term risk of cholangiocarcinoma: a population-based follow-up study. J Natl Cancer Inst. 2008;100:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Strömberg C, Luo J, Enochsson L, Arnelo U, Nilsson M. Endoscopic sphincterotomy and risk of malignancy in the bile ducts, liver, and pancreas. Clin Gastroenterol Hepatol. 2008;6:1049-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc. 2001;54:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Bergman JJ, van der Mey S, Rauws EA, Tijssen JG, Gouma DJ, Tytgat GN, Huibregtse K. Long-term follow-up after endoscopic sphincterotomy for bile duct stones in patients younger than 60 years of age. Gastrointest Endosc. 1996;44:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 150] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Karlson BM, Ekbom A, Arvidsson D, Yuen J, Krusemo UB. Population-based study of cancer risk and relative survival following sphincterotomy for stones in the common bile duct. Br J Surg. 1997;84:1235-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccalà G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy. 2002;34:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Moss SF, Blaser MJ. Mechanisms of disease: Inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2:90-97; quiz 1 p following 113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 520] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 19. | Nakanishi Y, Zen Y, Kondo S, Itoh T, Itatsu K, Nakanuma Y. Expression of cell cycle-related molecules in biliary premalignant lesions: biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum Pathol. 2008;39:1153-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kim YS, Kim HG, Han J, Hur CJ, Kim BS, Jung JT, Kwon JG, Kim EY, Cho CH, Sohn YK. The Significance of p53 and K-ras Immunocytochemical Staining in the Diagnosis of Malignant Biliary Obstruction by Brush Cytology during ERCP. Gut Liver. 2010;4:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Villanacci V, Cestari R, Giulini S, Cengia P, Missale G, Berenzi A, Rossi E, Bonardi M, Baiocchi L, Bassotti G. Immunocytochemical assessment of p53 protein to detect malignancy in increased cell-yield brush cytology from the biliopancreatic tree. Dig Dis Sci. 2009;54:789-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Stewart CJ, Burke GM. Value of p53 immunostaining in pancreatico-biliary brush cytology specimens. Diagn Cytopathol. 2000;23:308-313. [PubMed] [Cited in This Article: ] |
| 23. | de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L, Watkins JL, Lehman GA. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002;56:720-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |