Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4578
Revised: April 6, 2012
Accepted: April 12, 2012
Published online: September 7, 2012
AIM: To evaluate clinicopathologic parameters and the clinical significance related lymphovascular invasion (LVI) by immunohistochemical staining (IHCS) in endoscopic submucosal dissection (ESD).
METHODS: Between May 2005 and May 2010, a total of 348 lesions from 321 patients (mean age 63 ± 10 years, men 74.6%) with early gastric cancer (EGC) who met indication criteria after ESD were analyzed retrospectively. The 348 lesions were divided into the absolute (n = 100, differentiated mucosal cancer without ulcer ≤ 20 mm) and expanded (n = 248) indication groups after ESD. The 248 lesions were divided into four subgroups according to the expanded ESD indication. The presence of LVI was determined by factor VIII-related antigen and D2-40 assessment. We compared LVI IHCS-negative group with LVI IHCS-positive in each group.
RESULTS: LVI by hematoxylin-eosin staining (HES) and IHCS were all negative in the absolute group, while was observed in only the expanded groups. The positive rate of LVI by IHCS was higher than that of LVI by HES (n = 1, 0.4% vs n = 11, 4.4%, P = 0.044). LVI IHCS-positivity was observed when the cancer invaded to the mucosa 3 (M3) or submucosa 1 (SM1) levels, with a predominance of 63.6% in the subgroup that included only SM1 cancer (P < 0.01). In a univariate analysis, M3 or SM1 invasion by the tumor was significantly associated with a higher rate of LVI by IHCS, but no factor was significant in a multivariate analysis. There were no cases of tumor recurrence or metastasis during the median 26 mo follow-up.
CONCLUSION: EGCs of the absolute group are immunohistochemically stable. The presence of LVI may be carefully examined by IHCS in an ESD expanded indication group with an invasion depth of M3 or greater.
- Citation: Jeon SR, Cho JY, Bok GH, Lee TH, Kim HG, Cho WY, Jin SY, Kim YS. Does immunohistochemical staining have a clinical impact in early gastric cancer conducted endoscopic submucosal dissection? World J Gastroenterol 2012; 18(33): 4578-4584
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4578.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4578
The incidence of early gastric cancer (EGC) has increased in Asia, and particularly in South Korea and Japan[1]. Endoscopic submucosal dissection (ESD) can be used for en bloc and complete resection (CR), and is widely applied for the curative treatment of EGC[2-4]. Studies comparing the long-term results of EGC treatment have demonstrated no significant increase in the five-year survival rate of patients following ESD[2,5,6]. However, these techniques are necessary for proper diagnosis of tumor histology to check whether the tumor has been completely removed or has recurred and to predict the possibility of lymph node (LN) metastasis, thereby determining the need for additional surgery.
Assessment of lymphovascular invasion (LVI) is usually performed on the basis of conventional hematoxylin-eosin staining (HES), sometimes lacks objectivity possibly because of the inability to distinguish lymphatics from blood vessels[7]. Although the clinical significance of molecular-based methods using immunohistochemical staining (IHCS) of resected tissues is controversial, IHCS has been used to evaluate the presence of LVI, which a risk factor for LN metastasis[8-10]. Thus, we evaluated possible correlations between IHCS and other clinicopathologic parameters in ESD specimens and the clinical significance of IHCS.
Data were retrospectively collected from patients who underwent ESD at a single, tertiary-care, academic medical center. Between May 2005 and May 2010, a total of 348 lesions (321 patients) that met the absolute and expanded indication criteria after ESD were included. This study was approved by our hospital ethics committee and institutional review board. Written informed consent was obtained from all patients prior to the procedure.
The pre-ESD assessment of tumor size and depth of invasion was performed by conventional endoscopy and endoscopic ultrasonography. Contrast-enhanced computed tomography was also performed in all patients before treatment to exclude involvement of lymph nodes or distant metastasis.
ESD was performed as reported previously[11,12]. All ESD specimens were examined by an experienced pathologist (Jin SY). Resected specimens were fixed in 10% formalin and sectioned serially in 2-mm intervals, then subjected to histological analysis. All slices were embedded in a paraffin block. The section margin, depth of invasion, and LVI were observed carefully. A one-piece resection was defined as an en bloc resection. The definition of CR was: (1) en bloc resection or complete reconstruction in two piecemeal resection cases; (2) being free of cancer at both the vertical (VM) and lateral margins (LM); (3) no submucosal invasion deeper than 500 μm from the muscularis mucosa; and (4) no evidence of vascular or lymphatic invasion. Incomplete resection was defined as resections that did not meet the curative criteria. Incompletely resected lesions confined to the mucosa with positive LM were scheduled for an additional ESD procedure, argon plasma coagulation, or surgery.
In pathology specimens obtained by ESD, the absolute indication criteria for ESD was intramucosal differentiated adenocarcinoma without ulceration ≤ 20 mm in diameter. The expanded indication criteria for ESD were: (1) intramucosal differentiated adenocarcinoma without ulceration > 20 mm in diameter; (2) intramucosal differentiated adenocarcinoma with ulceration ≤ 30 mm in diameter; (3) submucosa 1 (SM1) differentiated adenocarcinoma ≤ 30 mm in diameter; and (4) intramucosal undifferentiated adenocarcinoma without ulceration ≤ 20 mm in diameter. In this order, the expanded indication group was divided into four subgroups (groups 1, 2, 3 and 4).
Serial sections (4 μm-thick) were deparaffinized in xylene. Samples were rehydrated in a graded alcohol series, and heated for 10 min in a microwave oven. This procedure was repeated twice after immersion in a citrate buffer solution to increase the reproducibility of endogenous antigens. To detect endogenous peroxidase, the samples were placed in 3% hydrogen peroxide solution for 10 min and rinsed with phosphate-buffered saline (PBS). Nonspecific binding sites were blocked by incubation in 3% normal non-immune serum for 10 min. Then the slides were washed with PBS and incubated with biotinylated polyvalent secondary antibody at room temperature for 15 min. After washing with PBS, the slides were incubated with streptavidine peroxidase for 15 min and washed a second time. Immunoreactive proteins were visualized by incubation with amine-ethyl carbazole for 3 to 5 min. The samples were counterstained with Mayer’s HE and mounted using crystal mount (Biomeda, Foster City, CA, United States).
Primary antibodies specific for the mucin and gastric phenotypic markers, MUC5AC (CLH2, Novocastra Lab. Ltd, Newcastle, United Kingdom) and MUC6 (CLH5, Novocastra Lab. Ltd, Newcastle, United Kingdom), the intestinal phenotypic marker MUC2 (CCP58, Biogenx, San Ramon, CA, United States), and CD10 (56C6, Signet Lab. Oakland, CA, United States) were used. Samples that were positive for MUC5AC or MUC6 were classified as having a gastric phenotype, and those positive for MUC2 or CD10 were classified as having an intestinal phenotype. Samples that were positive for at least one marker of both phenotypes were considered to have a mixed phenotype, and samples showing no marker immunoreactivity were considered to be an unclassified phenotype.
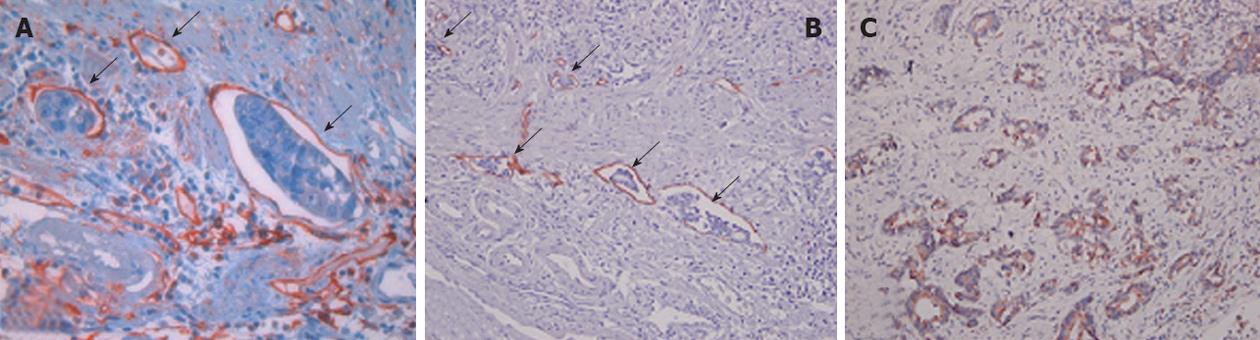
For the vascular endothelial marker, primary antibodies against factor VIII-related antigen (FVIIIRAg, 1:3000; DAKO, Carpenteria, CA, United States) were used. For the lymphatic endothelial marker, primary antibodies against D2-40 (1:200; LI-D, Covance Inc., Cumberland, VA, United States) were used. Primary antibodies against vascular endothelial growth factor (VEGF) (1:50; SC-152, Santa Cruz Biotechnology, Santa Cruz, CA, United States) were also applied. Vascular or LVI was indicated by clusters of tumor cell in vessels or lymphatic vasculature, the endothelia of which stained positive for FVIIIRAg or D2-40, respectively. VEGF was considered positive when anti-VEGF antibodies stained the tumor cytoplasm. High microvessel density (MVD) and lymphovascular density (LVD) were detected by increased intra-tumor staining of factor VIII and D2-40 (Figure 1).
Endoscopic follow-up examinations were performed routinely at 1, 3, 6 and 12 mo, and then annually after ESD to assess the completeness of resection and to detect metachronous lesions. During the follow-up period, biopsy samples were taken from the ESD ulcer scar or other suspicious mucosal abnormalities. If a residual lesion or metachronous lesion was noted, additional ESD or surgery was planned.
Statistical analyses were performed using the SPSS software (version 15.0; SPSS, Chicago, IL, United States). Categorical variables were analyzed by the Chi-square test or Fisher’s exact test and non-categorical variables were analyzed by the t test to verify the relationship between IHCS and other clinicopathological parameters. Non-categorical variables were compared among the four groups using an analysis of variance test. A multivariate analysis was conducted using a logistic regression method. P values ≤ 0.05 indicated statistical significance.
In the absolute group, the mean age of the 100 patients (100 lesions) was 62.2 years (range: 33-86 years) and included 70 men (70%). In the expanded group, the mean age of the 221 patients (248 lesions) was 62.9 years (range: 33-91 years) and included 189 men (76.2%). The dominant tumor location in both groups was the lower third of the stomach. Macroscopically, the flat/depressed type was most common (n = 131, 52.8%), and ulcers were observed in 64.9% (n = 161) of patients in the expanded group.
Of 248 lesions in the expanded group, 238 (96%) were differentiated and 10 (4%) were poorly differentiated adenocarcinoma. Twenty-eight lesions (11.3%) were SM1 cancer. The mean tumor size was 21.2 mm (range: 3-86 mm). Twenty-four (9.7%) were LM-positive two (0.8%) were VM-positive. The mixed mucin phenotype (n = 90, 36.3%) was predominant. A total of 72 (29%) were VEGF positive and 59 (23.8%) exhibited a high MVD/LVD. The CR rate by IHCS was significantly lower than that by HES (84.3% vs 88.3%, P < 0.01). The endoscopic and histopathologic characteristics of the lesions in the absolute and expanded groups are summarized in Table 1.
| Groups | Absolute (n = 100) | Expanded (n = 248) | P value |
| Age (yr), mean (range) | 62.2 (33-86) | 62.9 (33-91) | 0.589 |
| Male (%) | 70 (70) | 189 (76.2) | 0.277 |
| Location (%) | 0.183 | ||
| Upper | 3 (3) | 21 (8.5) | |
| Middle | 31 (31) | 69 (27.8) | |
| Lower | 66 (66) | 158 (63.7) | |
| Macroscopic type (%) | < 0.001 | ||
| Elevated | 59 (59) | 56 (22.6) | |
| Flat/depressed | 36 (36) | 131 (52.8) | |
| Mixed | 5 (5) | 61 (24.6) | |
| Endoscopic ulcer (%) | < 0.001 | ||
| Present | 0 (0) | 161 (64.9) | |
| Absent | 100 (100) | 87 (35.1) | |
| Resection (%) | 0.053 | ||
| En-bloc | 100 (100) | 234 (94.4) | |
| Complete reconstruction | 0 (0) | 8 (3.2) | |
| ≥ 3 piecemeal | 0 (0) | 6 (2.4) | |
| Histology (%) | 0.069 | ||
| Differentiated | 100 (100) | 238 (96) | |
| Undifferentiated | 0 (0) | 10 (4) | |
| Depth (%) | < 0.001 | ||
| M2 | 59 (59) | 89 (35.9) | |
| M3 | 41 (41) | 131 (52.8) | |
| SM1 | 0 (0) | 28 (11.3) | |
| Tumor size (mean, range) | 11.4 (2-20) | 21.2 (3-86) | < 0.001 |
| ≤ 20 mm (%) | 100 (100) | 131 (52.8) | |
| > 20 mm (%) | 0 (0) | 117 (47.2) | |
| Lateral margin (%) | < 0.001 | ||
| Positive | 0 (0) | 24 (9.7) | |
| Negative | 100 (100) | 224 (90.3) | |
| Vertical margin (%) | 0.500 | ||
| Positive | 0 (0) | 2 (0.8) | |
| Negative | 100 (100) | 246 (99.2) | |
| Phenotype (%) | 0.312 | ||
| Gastric | 24 (24) | 66 (26.6) | |
| Intestinal | 31 (31) | 92 (37) | |
| Mixed | 45 (45) | 90 (36.3) | |
| VEGF (%) | 0.096 | ||
| Positive | 79 (79) | 72 (29) | |
| Negative | 21 (21) | 176 (71) | |
| High MVD/LVD (%) | 0.116 | ||
| Positive | 16 (16) | 59 (23.8) | |
| Negative | 84 (84) | 189 (76.2) | |
| LVI HES | 0.713 | ||
| Positive | 0 (0) | 1 (0.4) | |
| Negative | 100 (100) | 247 (99.6) | |
| LVI IHCS | 0.038 | ||
| Positive | 0 (0) | 11 (4.4) | |
| Negative | 100 (100) | 237 (95.6) |
As mentioned above, the 248 lesions were divided into four groups according to the expanded ESD indication. Groups 1, 2, 3 and 4 contained 71 (28.6%), 140 (56.4%), 27 (10.8%) and 10 (4.7%) lesions, respectively. LVI HES-positivity was also defined as when LVI was positive by HE staining, and LVI IHCS-positivity was also defined as when LVI was positive by IHCS. Of a number of clinicopathological factors, only LVI IHCS-positivity showed significant differences between subgroups. LVI HES-positivity was observed only in group 4, which included undifferentiated carcinoma. While LVI IHCS-positivity was observed in all subgroups, there was a predominance of 63.6% (7/11) in group 3, which included only SM1 cancer (P < 0.001) (Table 2).
| Group 1 (n = 71) | Group 2 (n = 140) | Group 3 (n = 27) | Group 4 (n = 10) | P value | |
| Age > 60 (yr, %) | 44 (62) | 82 (58.6) | 21 (77.8) | 6 (60) | 0.502 |
| Male (%) | 56 (78.9) | 104 (74.3) | 24 (88.9) | 5 (50) | 0.455 |
| Location (%) | 0.730 | ||||
| Upper + corpus | 19 (26.8) | 15 (10.7) | 11 (40.7) | 1 (10) | |
| Lower + angle | 52 (73.2) | 125 (89.3) | 16 (59.3) | 9 (90) | |
| Macroscopic type (%) | < 0.001 | ||||
| Elevated | 45 (63.4) | 4 (2.9) | 4 (14.8) | 3 (30) | |
| Flat/depressed | 26 (36.6) | 136 (97.1) | 23 (85.2) | 7 (70) | |
| Endoscopic ulcer (%) | < 0.001 | ||||
| Present | 0 (0) | 140 (100) | 20 (74.1) | 1 (10) | |
| Absent | 71 (100) | 0 (0) | 7 (25.9) | 9 (90) | |
| Resection, piece (%) | 0.177 | ||||
| ≤ 2 | 68 (95.8) | 137 (97.9) | 27 (100) | 10 (100) | |
| ≥ 3 | 3 (4.2) | 3 (2.1) | 0 (0) | 0 (0) | |
| Depth (%) | 0.003 | ||||
| M2 | 33 (46.5) | 52 (37.1) | 0 (0) | 4 (40) | |
| M3 + SM1 | 38 (53.5) | 88 (62.9) | 27 (100) | 6 (60) | |
| Tumor size, mm (%) | < 0.001 | ||||
| ≤ 20 | 0 (0) | 105 (75) | 16 (59.3) | 10 (100) | |
| > 20 | 71(100) | 35 (25) | 11 (40.7) | 0 (0) | |
| Lateral margin (%) | 0.091 | ||||
| Positive | 10 (14.1) | 11 (7.9) | 1 (3.7) | 2 (20) | |
| Negative | 61 (85.9) | 129 (92.1) | 26 (96.3) | 8 (80) | |
| Vertical margin (%) | 0.170 | ||||
| Positive | 2 (2.8) | 0 (0) | 0 (0) | 0 (0) | |
| Negative | 69 (97.2) | 140 (100) | 27 (100) | 10 (100) | |
| Phenotype (%) | 0.107 | ||||
| Intestinal | 31 (43.7) | 50 (35.7) | 9 (33.3) | 2 (20) | |
| Non-intestinal | 40 (56.3) | 90 (64.3) | 18 (66.7) | 8 (80) | |
| VEGF (%) | 0.003 | ||||
| Positive | 17 (23.9) | 34 (24.3) | 16 (59.3) | 5 (50) | |
| Negative | 54 (76.1) | 106 (75.7) | 11 (40.7) | 5 (50) | |
| High LVD/MVD (%) | < 0.001 | ||||
| Positive | 11 (15.5) | 26 (18.6) | 16 (59.3) | 6 (60) | |
| Negative | 60 (84.5) | 114 (81.4) | 11 (40.7) | 4 (40) | |
| LVI HES | 0.005 | ||||
| Positive | 0 (0) | 0 (0) | 0 (0) | 1 (10) | |
| Negative | 71 (100) | 140 (100) | 27 (100) | 9 (90) | |
| LVI IHCS | < 0.001 | ||||
| Positive | 1 (1.4) | 1 (0.7) | 7 (25.9) | 2 (20) | |
| Negative | 70 (98.6) | 139 (99.3) | 20 (74.1) | 8 (80) | |
LVI HES-positivity was confirmed by LVI IHCS; however, of 11 lesions identified as LVI IHCS-positive, only one was also LVI HES-positive (100% vs 9.1%, P = 0.044) (Table 3).
| ESD indication | LVI IHCS | Total | P value | |||
| Negative | Positive | |||||
| Absolute | LVI HES | Negative | 100 | 0 | 100 | |
| Positive | 0 | 0 | 0 | |||
| Total | 100 | 0 | 100 | |||
| Expanded | LVI HES | Negative | 237 | 10 | 247 | 0.044 |
| Positive | 0 | 1 | 1 (0.4%) | |||
| Total | 237 | 11 (4.4%) | 248 | |||
The association between LVI IHCS and various clinicopathological factors and IHCS markers was analyzed (Table 4). In this statistical analysis, we combined the three tumor location and depth of invasion categories into two “upper + corpus” and “lower + angle”, and “mucosa 2 (M2)” and “M3 + SM1”, respectively. Phenotype was categorized into intestinal and non-intestinal type. LVI IHCS-positivity was detected in 4.4% (11/248). In a univariate analysis, M3 or SM1 invasion of the tumor, and the presence of VEGF and a high LVD/MVD were factors significantly associated with a higher rate of LVI by IHCS. However, there were no significant associations between LVI by IHCS and age, sex, location, macroscopic type, endoscopic ulcer, resection pieces, histology, tumor size, lateral margin, vertical margin, or phenotype. In a multivariate analysis, there were no significant associations between LVI in IHCS and these factors (Table 4).
| LVI IHCS | P value | ||
| Negative (n = 237) | Positive (n = 11) | ||
| Age, yr (%) | 62.6 ± 9.9 | 69.4 ± 10.2 | 0.054 |
| ≤ 60 | 92 (38.8) | 3 (27.3) | |
| > 60 | 145 (61.2) | 8 (72.7) | |
| Male (%) | 182 (76.8) | 7 (63.6) | 0.297 |
| Location (%) | 0.667 | ||
| Upper + corpus | 44 (18.6) | 2 (18.2) | |
| Lower + angle | 193 (81.4) | 9 (81.8) | |
| Macroscopic type (%) | 0.715 | ||
| Elevated | 53 (22.4) | 3 (27.3) | |
| Flat/depressed | 184 (77.6) | 8 (72.7) | |
| Endoscopic ulcer (%) | 0.524 | ||
| Present | 155 (65.4) | 6 (54.5) | |
| Absent | 82 (34.6) | 5 (45.5) | |
| Resection, piece (%) | 0.760 | ||
| ≤ 2 | 231 (97.5) | 11 (100) | |
| ≥ 3 | 6 (2.5) | 0 (0.0) | |
| Histology (%) | 0.066 | ||
| Differentiated | 229 (96.6) | 9 (81.8) | |
| Undifferentiated | 8 (3.4) | 2 (18.2) | |
| Depth (%) | 0.009 | ||
| M2 | 89 (37.6) | 0 (0) | |
| M3 + SM1 | 148 (62.4) | 11 (100) | |
| Tumor size, mm (%) | 21.2 ± 12.8 | 21.2 ± 16.7 | 0.986 |
| ≤ 20 | 125 (52.7) | 6 (54.5) | |
| > 20 | 112 (47.3) | 5 (45.5) | |
| Lateral margin (%) | 0.289 | ||
| Positive | 22 (9.3) | 2 (18.2) | |
| Negative | 215 (90.7) | 9 (81.8) | |
| Vertical margin (%) | 0.913 | ||
| Positive | 2 (0.8) | 0 (0) | |
| Negative | 235 (99.2) | 11 (100) | |
| Phenotype (%) | 0.751 | ||
| Intestinal | 89 (37.6) | 3 (27.3) | |
| Non-intestinal | 148 (62.4) | 8 (72.7) | |
| VEGF (%) | < 0.001 | ||
| Positive | 62 (26.2) | 10 (90.9) | |
| Negative | 175 (73.8) | 1 (9.1) | |
| High LVD/MVD (%) | < 0.001 | ||
| Positive | 48 (20.3) | 11 (100) | |
| Negative | 189 (79.7) | 0 (0.0) | |
Of the 321 patients who underwent ESD, 5 (1.6%) required additional surgery (Table 5). The reasons for additional surgery were an insufficient gap between the tumor and LM or VM in four patients and patient need in one. No lesions exhibited LN metastasis. Two of the five patients had a residual tumor in the gastric wall. There were no cases of tumor recurrence or metastasis after ESD, including those patients who underwent additional surgery, during the median 26 mo follow-up.
| Sex/age (yr) | Type | Site | Ulcer | Pathology | Size (mm) | LM/VM | Depth | LVI HES | LVI IHCS | Residual tumor | LN |
| Female/67 | IIc | Lower | - | MD | 30 | -/+ | M | - | - | + | - |
| Male/74 | III | Middle | + | WD | 14 | +/- | M | + | + | - | - |
| Female/64 | III | Middle | + | MD | 19 | +/- | M | - | - | - | - |
| Female/54 | I | Middle | - | MD | 68 | +/- | M | - | + | + | - |
| Male/35 | I | Middle | - | MD | 22 | -/- | M | - | - | - | - |
LVI and increased LVD detected by IHCS are associated with LN metastasis in gastric cancer[13-18], and are correlated with an unfavorable prognosis in other types of cancer[19-23]. However, detection of LVI by HES is difficult for several reasons. Primarily, the distinction between blood vessels and lymphatic vessels is subjective. Furthermore, artifacts or artificial spaces formed during sample preparation may mimic the tubular structure of vasculature[7]. Antibodies to FVIIIRAg help to distinguish true vascular endothelial cells therefore increasing the sensitivity of detection of vascular invasion[24,25] whereas the monoclonal antibody D2-40 specific for lymphatic endothelial cells enhances detection of lymphatic invasion[26-28]. In a previous study using post-operative specimens to diagnose lymphatic invasion, D2-40 was more sensitive for detection of lymphatic invasion (positive rate 44% vs 27% for D2-40 vs HE)[27]. Thus, we evaluated the clinical significance of IHCS, using for example FVIIIRAg and D2-40, in patients with the expanded indication criteria after ESD. To the best of our knowledge, no study has evaluated the possible correlations between IHCS and other clinicopathological parameters in the expanded indication of ESD.
In this study, 11 lesions in the only expanded indication group, but not the absolute indication group, displayed LVI-positivity. This is likely because lymphatics existed only in the deep mucosa and abundant microvessels were found in the muscularis mucosa[27]. Differentiated mucosal cancer, which belongs to the absolute indication group was LVI-negative. Because the EGCs of the absolute indication group were immunohistochemically stable, the clinical implication of IHCS in this group is insignificant.
In the expanded indication, comparing the LVI IHCS-negative and LVI IHCS-positive groups, M3 or SM1 invasion of the tumor and the presence of VEGF and a high LVD/MVD were factors significantly associated with a higher rate of LVI by IHCS. However, there were no significant associations between LVI by IHCS and these factors in a multivariate analysis. This may be because, first, the number of patients in the LVI IHCS-positive group was too small compared to the LVI IHCS-negative group. Second, although the specimens were examined by a highly experienced pathologist, the interpretation of the IHCS results was not always accurate. Nonetheless, we must not overlook the fact that LVI-positivity was observed when the cancer invaded the M3 or SM1 layer in the LVI IHCS-positive group. Resected specimens examined by HES showed an LVI false-negative rate of 4% and a false-positive rate of 0%. The positive rate of LVI by IHCS was significantly higher than that of LVI by HES (4% vs 0.4%, P = 0.044). In other words, 10 tumors, which had been diagnosed as no LVI by HES, were correctly diagnosed as having LVI after IHCS. Only 1 of 11 (9%) LVI, confirmed by IHCS, was correctly diagnosed by HES. These results suggest that LVI cannot be predicted by HES alone and also that IHCS is somewhat more helpful than HES for identifying LVI. Therefore, the presence of LVI should be carefully examined by IHCS when invasion with a depth of M3 or greater has been detected in an expanded indication.
The major limitations of this study are its retrospective design, the relatively short follow-up period and the limited number of patients with LVI by IHCS. Because the number of LVI by IHCS was small and additional surgical treatment was not provided to all patients who were LVI-positivity by IHCS, we were unable to explain the association between LVI IHCS-positivity and LN metastasis. According to the Japanese classification of gastric carcinoma[29], resected specimens were cut thinner (vs surgical specimens; 2 mm vs 4 mm). Therefore, the quality of pathologic interpretation of resected specimens was better than that of surgical specimens. We found no case of metastasis in the LVI IHCS-positive group during the median 26 mo follow-up. However, because a previous study reported that LN metastasis was confirmed in 89% of D2-40 positive cases, compared to 41% of suspected cases of metastasis based on HES[14], a future large and long-term follow-up study is needed to assess via IHCS the relationship of LVI with LN metastasis, tumor recurrence, and patient survival.
Examination by IHCS may be required because LVI can be undetected in an ESD expanded indication group with an invasion depth of M3 or greater if we rely only on HES.
Lymphovascular invasion (LVI) detected by immunohistochemical staining (IHCS) is associated with lymph node (LN) metastasis in gastric cancer. The clinical significance of IHCS in early gastric cancer (EGC) in association with endoscopic submucosal dissection (ESD) is controversial. No study has evaluated the possible correlations between IHCS and other clinicopathological parameters in ESD specimens.
Relationships between LN micrometastasis and clinicopathological findings based on D2-40 and factor VIII-related antigen (FVIIIRA) IHCS in gastric cancer have been reported. Several studies have reported that IHCS is more sensitive for detection of LVI.
LVI by hematoxylin-eosin stain (HES) and IHCS were all negative in an ESD absolute group; this group was immunohistochemically stable. However, in an ESD expanded group, the LVI IHCS-positive rate was significantly higher than that of LVI by HES. LVI IHCS-positivity was observed when the cancer invaded the mucosa 3 (M3) or submucosa 1 (SM1) levels. Therefore, the presence of LVI should be carefully examined by IHCS in an expanded group with an invasion depth of M3 or SM1.
In an ESD expanded indication group with an invasion depth of M3 or greater, IHCS should be used because LVI-positivity can remain undetected by HES. However, a large-scale prospective study is required.
ESD, a method that can be used for en bloc and complete resection of EGC, is widely applied for the curative treatment of EGC. IHCS is widely used in the diagnosis of abnormal cells using specific molecular markers, such as D2-40 and FVIIIRA.
Because the number of LVIs detected by IHCS was small and additional surgical treatment was not provided to all patients who were LVI-positive by IHCS, it was unable to explain the association between LVI IHCS-positivity and LN metastasis. However, this study implies the clinical significance of IHCS in an ESD expanded indication group, and reports a useful methodology.
Peer reviewers: Magdy El-Salhy, Professor, Department of Gastroenterology, School of Medicine, Stord Helse-Fonna Hospital, 5409 Stord, Norway; Dr. Cuneyt Kayaalp, Professor, Depatment of General Surgery, Inonu University, 44315 Malatya, Turkey
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 476] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 4. | Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, Lise M. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077-1085. [PubMed] [Cited in This Article: ] |
| 6. | Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596-602. [PubMed] [Cited in This Article: ] |
| 7. | Sako A, Kitayama J, Ishikawa M, Yamashita H, Nagawa H. Impact of immunohistochemically identified lymphatic invasion on nodal metastasis in early gastric cancer. Gastric Cancer. 2006;9:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [Cited in This Article: ] |
| 9. | Kim H, Kim JH, Park JC, Lee YC, Noh SH, Kim H. Lymphovascular invasion is an important predictor of lymph node metastasis in endoscopically resected early gastric cancers. Oncol Rep. 2011;25:1589-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775-784. [PubMed] [Cited in This Article: ] |
| 11. | Lee TH, Cho JY, Chang YW, Kim JO, Lee JS, Cho WY, Kim HG, Kim WJ, Park YS, Jin SY. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011;74:772-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Chung JI, Kim N, Um MS, Kang KP, Lee D, Na JC, Lee ES, Chung YM, Won JY, Lee KH. Learning curves for colonoscopy: a prospective evaluation of gastroenterology fellows at a single center. Gut Liver. 2010;4:31-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Kikuchi Y, Tsuchiya A, Ando Y, Yoshida T, Takenosita S. Immunohistochemical detection of lymph node microinvolvement in node-negative gastric cancer. Gastric Cancer. 1999;2:173-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Ishida K, Katsuyama T, Sugiyama A, Kawasaki S. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer. 1997;79:1069-1076. [PubMed] [Cited in This Article: ] |
| 16. | Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer. 2002;94:2867-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, Nakanishi Y, Shimoda T. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753-760. [PubMed] [Cited in This Article: ] |
| 18. | Wang XL, Fang JP, Tang RY, Chen XM. Different significance between intratumoral and peritumoral lymphatic vessel density in gastric cancer: a retrospective study of 123 cases. BMC Cancer. 2010;10:299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Arigami T, Natsugoe S, Uenosono Y, Arima H, Mataki Y, Ehi K, Yanagida S, Ishigami S, Hokita S, Aikou T. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer. 2005;93:688-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Davessar K, Pezzullo JC, Kessimian N, Hale JH, Jauregui HO. Gastric adenocarcinoma: prognostic significance of several pathologic parameters and histologic classifications. Hum Pathol. 1990;21:325-332. [PubMed] [Cited in This Article: ] |
| 21. | Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS, Lee JH, Kim YS. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Zhao HC, Qin R, Chen XX, Sheng X, Wu JF, Wang DB, Chen GH. Microvessel density is a prognostic marker of human gastric cancer. World J Gastroenterol. 2006;12:7598-7603. [PubMed] [Cited in This Article: ] |
| 23. | Maehara Y, Orita H, Okuyama T, Moriguchi S, Tsujitani S, Korenaga D, Sugimachi K. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245-247. [PubMed] [Cited in This Article: ] |
| 24. | Wang D, Stockard CR, Harkins L, Lott P, Salih C, Yuan K, Buchsbaum D, Hashim A, Zayzafoon M, Hardy RW. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem. 2008;83:179-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Burgdorf WH, Mukai K, Rosai J. Immunohistochemical identification of factor VIII-related antigen in endothelial cells of cutaneous lesions of alleged vascular nature. Am J Clin Pathol. 1981;75:167-171. [PubMed] [Cited in This Article: ] |
| 26. | Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 425] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679-1684. [PubMed] [Cited in This Article: ] |
| 29. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [Cited in This Article: ] |