Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1336
Revised: November 10, 2005
Accepted: December 7, 2005
Published online: March 7, 2006
Selective cyclooxygenase (COX)-2 inhibitors (coxibs) were developed as one of the anti-inflammatory drugs to avoid the various side effects of non-steroidal anti-inflammatory drugs (NSAIDs). However, coxibs also have an ability to inhibit tumor development of various kinds the same way that NSAIDs do. Many experimental studies using cell lines and animal models demonstrated an ability to prevent tumor proliferation of COX-2 inhibitors. After performing a randomized study for polyp chemoprevention study in patients with familial adenomatous polyposis (FAP), which showed that the treatment with celecoxib, one of the coxibs, significantly reduced the number of colorectal polyps in 2000, the U.S. Food and Drug Administration (FDA) immediately approved the clinical use of celecoxib for FAP patients. However, some coxibs were recently reported to increase the risk of serious cardiovascular events including heart attack and stroke. In this article we review a role of COX-2 in carcinogenesis of gastrointestinal tract, such as the esophagus, stomach and colorectum, and also analyze the prospect of coxibs for chemoprevention of gastrointestinal tract tumors.
- Citation: Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: A review and report of personal experience. World J Gastroenterol 2006; 12(9): 1336-1345
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1336
The administration of non-steroidal anti-inflammatory drugs (NSAIDs), one of the most prevalent antipyretics and analgesics, is also known to reduce the risk of cancer development in the gastrointestinal tract organs including the esophagus, stomach and colorectum[1,2]. Vane[3] indicated in 1971 that NSAIDs act upon cyclooxygenase (COX), a rate-limiting enzyme in the arachidonate metabolism. The enzyme catalyzes the biosynthesis of prostaglandin H2, the precursor of derivatives such as prostaglandins, prostacyclin, and thromboxanes. Up to now there have been at least two isoenzymes of COX reported, COX-1 and COX-2. COX-1 is constitutively expressed in many tissues and it controls homeostasis by maintaining physiological levels of prostaglandins, while COX-2, induced by cytokines, mitogens, and growth factors, is responsible for inflammatory reactions and tumor development. Recently, COX-3, was reported to be related with pain and fever, and identified as an alternative splice of COX-1[4].
COX-2 and PGE2 play an important role in tumorigenesis from the development to invasion and metastasis of carcinoma through various mechanisms. COX-2 expression promotes cell proliferation by the activation of EGFR[5] and inhibit apoptosis by up-regulation of bcl-2[6], and suppresses host immune response[7]. Furthermore, COX-2 induces angiogenesis with VEGF and bFGF expression[8], and facilitates a metastatic potential by up-regulation of uPA and MMP-2[9,10]. Theoretically, NSAIDs may be a candidate for chemopreventive agents against tumorigenesis by inhibiting COX-2. In fact, two large-scale randomized, double-blind trials demonstrated that aspirin, a representative of NSAIDs, could prevent colorectal adenoma[11,12].
But the regular use of NSAIDs causes severe adverse effects including gastrointestinal bleeding, a reduction of the renal blood flow, and dysfunction of platelets because they inhibit both COX-1 and COX-2. To avoid these side effects of NSAIDs the development of selective COX-2 inhibitors was gradually aroused after the discovery of COX-2 in the early 1990s[13]. Some drugs were discovered as a result of a search for selective COX-2 inhibitors, others were revealed as being COX-2 selective after the discovery of COX-2. There are three classes of selective COX-2 inhibitors (Table 1), the first one being 1,2-diarylcyclopentenes (so-called tricyclic compounds), such as celecoxib and rofecoxib; the second one being methanesulphonamide compounds, such as NS-398 and nimesulide; and the third one being NSAIDS-derivates, such as meloxicam and etodolac. Some selective COX-2 inhibitors, which demonstrate chemopreventive effects on gastrointestinal cancers in experiments and human studies, are already commercialized as anti-inflammatory drugs, but no drug except for celecoxib is presently allowed for use in chemoprevention. In this paper we review the role of COX-2 in the carcinogenesis of gastrointestinal tract cancers and also discuss the prospect of selective COX-2 inhibitors for chemoprevention of gastrointestinal tract cancers.
| Generic name | Brand name | PhCob | Esophagus | Stomach | Colorectum | ||||||||
| Cancer cell line | CIAc | reflux-induced animal | Human (BEd) | Cancer cell line | CIAc | MIAe | Cancer cell line | CIAc | MIAe | Human (FAPf) | |||
| Tricyclic | |||||||||||||
| Celecoxib | Celebrex | Pfizer | (23) | (46,47) | (75) | (76,77) | (81,82) | (54) | (56) | ||||
| MF-tricyclic | ECa | Merck | (21) | (53,87) | |||||||||
| Rofecoxib | Vioxx | Merck | (24) | (55) | |||||||||
| Tilmacoxib | Japan Tobacco | (20) | (78) | (88,89) | |||||||||
| Valdecoxib Bextra | Pfizer | ||||||||||||
| EtoricoxibArcoxia | Merck | ||||||||||||
| Methanesulphonamide | |||||||||||||
| NS-398 | ECa | Taisho | (18,19,70,71) | (44,45,72) | (49) | (72) | (83) | ||||||
| Nimesulide | Mesulid | Helsinn | (73) | (48) | (84) | (90) | |||||||
| Flosulide | Schering | (70) | |||||||||||
| Others | |||||||||||||
| Nabumetone | Relafen | Glaxo Smith Kline | (85) | (91) | |||||||||
| Meloxicam | Mobic | Boehringer Ingelheim | (79,80) | (86) | |||||||||
| Etodolac | Lodine | Wyeth | (74) | (74) | |||||||||
| Lumiracoxib | Prexige | Novartis | |||||||||||
Recently, the incidence of Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC) has been rapidly increasing in individuals of Western countries, particularly, among white males. The sequence of events leading from gastroesophageal reflux disease (GERD) to EAC is thought to involve the development of inflammation-stimulated hyperplasia and metaplasia, followed by multifocal dysplasia and adenocarcinoma. The up-regulation of COX-2 expression in human tissue of esophagitis, BE and EAC has been demonstrated. The incidence of COX-2 protein expression gradually increases with the development of esophageal lesions, from 75% in metaplasia, to 83% in low-grade dysplasia and up to 100% in high-grade dysplasia and EAC[14]. Combined reflux of the duodenal contents with gastric juice contributes to the development of these diseases[15] and BE patients have higher bile acid levels in the stomach than healthy controls and GERD patients without BE[16]. These observations strongly indicate that duodenal juice including bile is associated with the inflammation-metaplasia-adenocarcinoma sequence. In particular, bile acid is likely to play a pivotal role. Zhang et al[17] reported that COX-2 was expressed in the esophageal mucosa using a duodenogastroesophageal reflux model and bile acids, not only unconjugated but also conjugated ones, induced COX-2 mRNA, followed by COX-2 protein and PGE2 production.
The suppressive effects of a COX-2 inhibitor, NS398, on the epithelium of BE have been demonstrated in two independent in vitro studies[18,19]. An increase in apoptosis and a suppression of cell proliferation are supposed to be responsible for the inhibition of cancer cells in these articles. Furthermore, some selective COX-2 inhibitors have been reported to prevent the development of esophageal cancer using in vivo animal models. N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats was prevented by the administration of another selective COX-2 inhibitor, JTE-522[20]. The study was carried out using a carcinogen-induced rodent model, whereas two studies have been reported using an esophageal reflux model. Buttar et al[21] showed the preventive effect on EAC of MF-tricyclic in a rat model of BE and EAC induced by duodenogastroesophageal reflux. In their report, MF-tricyclic prevented the development of EAC, but did not suppress the prevalence of BE. On the other hand, celecoxib suppressed not only the development of EAC, but also that of BE in our study.
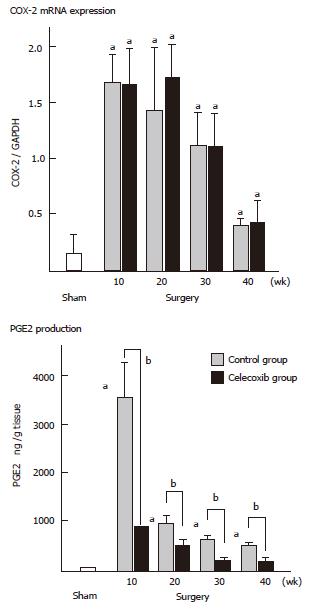
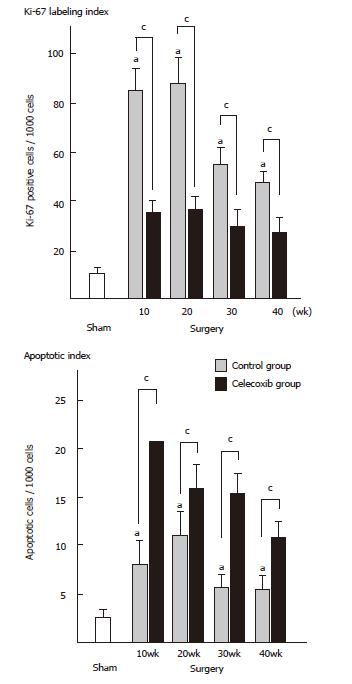
We have investigated the effect of celecoxib on esophageal adenocarcinogenesis by using duodenoesophageal reflux model, established by Miwa and his colleagues [22,23]. Male Fisher 344 rats underwent a duodenoesophageal reflux procedure and were divided into two groups. One group was given commercial chow (control group), while the other group was given experimental chow containing celecoxib (celecoxib group). The animals were sacrificed sequentially, at 10th, 20th, 30th and finally 40th wk after surgery. In the control group, esophagitis, BE and EAC were first observed at 10th wk, 20th wk and 30th wk, respectively. Their incidences sequentially increased and at the 40th wk reached 100%, 89% and 47%, respectively. In the celecoxib group, the esophagitis was mild and the incidence of BE was significantly lower at each week (P < 0.001), in comparison with the control group, and EAC was not identified throughout the experiment (P < 0.05) (Table 2). COX-2 expression was up-regulated at the 10th and 20th wk (P < 0.05, respectively) (Figure 1). PGE2 level and proliferative activity were also up-regulated in both groups, but they were lower in the celecoxib group than in the control group (P < 0.05) (Figures 1 and 2). Apoptosis increased after the celecoxib treatment (P < 0.05) (Figure 2). Celecoxib thus proved to be effective for preventing reflux esophagitis, BE and EAC by suppressing PGE2 production in a rodent model.
Our results showed surges of COX-2 and PGE2 between the beginning and the 20th wk in the control group, thus suggesting that the COX-2 expression played an important role in the early phase of the esophageal carcinogenesis in the inflammation-metaplasia-adenocarcinoma sequence. The fact that the suppression of PGE2 continued throughout the experiment in the celecoxib group may explain that celecoxib suppressed not only the development of EAC, but also that of BE. These data led to perform a clinical chemoprevention study for the patients with BE. Kaur et al[24] administered 25-mg/day rofecoxib to twelve patients with BE for 10 days and reported that COX-2 expression, PGE2 contents and PCNA of epithelium of BE were 3-fold, 2-fold, and 2-fold higher than those of epithelium of normal esophagus, respectively, and all biomarkers decreased after treatment by 77%, 59%, and 62.5%, respectively. Furthermore, a Chemoprevention for Barrett's Esophagus Trial (CBET) was started in 2003 as a phase IIb, multicenter, randomized, double-masked, placebo-controlled study of celecoxib in patients with Barrett's dysplasia[25].
Though the incidence of gastric cancer has recently decreased in the United State of America and Western European countries, it is still a major cause of cancer death in many countries, such as Eastern Asia, Eastern Europe, and Latin America. Gastric cancer develops in a multistep process from normal gastric mucosa to chronic active gastritis, to gastric atrophy and intestinal metaplasia, and finally to dysplasia and cancer[26]. According to recent epidemiologic evidence, it is very likely that Helicobactor pylori (H pylori) plays an important role in this carcinogenic sequence. It is shown that H pylori induces COX-2 mRNA/protein levels with the production of PGE2 in premalignant and malignant lesions[27,28]. A chronic infection of H pylori causes gastritis due to COX-2, iNOS, and other cytokines, but the precise mechanism of H. pylori involvement in gastric carcinogenesis remains to be elucidated. Normal gastric mucosa scarcely expresses COX-2, but the expression of COX-2 increases through the multistep process of gastric carcinogenesis. Sun et al[29] reported the positive rates of COX-2 by immunohistochemistry in superficial gastritis, gastric atrophy, intestinal metaplasia, dysplasia, and cancer to be 10.0 %, 35.7 %, 37.8 %, 41.7 %, and 69.5 %, respectively. In addition to these findings several studies have strongly suggested COX-2 expression to be a relatively early event in the sequence of gastric carcinogenesis[30,31].
Since Ristimaki et al[32] first described an elevated expression of COX-2 in gastric carcinoma in 1997, numerous studies have reported the relationship between COX-2 expression and gastric cancer. According to a review article, COX-2 mRNA is up-regulated in 51% to 76% (median 73 %) of the tumors by Northern blot or RT-PCR, while COX-2 protein is overexpressed in 67% to 83 % (median 73 %) by immunoblotting and 43% to 100 % (median 62 %) by immunohistochemistry[33]. The COX-2 expression is more frequent in intestinal-type than in diffuse-type gastric cancer[34-36], and it also correlates with non-cardia cancer[37], tumor size[38], depth of invasion[36,38,39], lymph node metastasis[38-42], lymphatic invasion[41,42], clinical stage[41-42], and angiogenesis[39,43].
Sawaoka et al[44,45] demonstrated the inhibitory effects of a COX-2 inhibitor, NS-398, on the gastric cell line expressing COX-2 (MKN45) and on its xenograft in nude mice
in vivo. Hu et al[46] examined the chemopreventive effect of indomethacin and celecoxib, using a rat model. They induced gastric cancer by the administration of 100 μg/ml MNNG to Wistar rats for 40 wk and reported the incidence and the tumor multiplicity of gastric cancer of 10 mg celecoxib group to be 18.8 % and 0.19, which was significantly lower than 75.0 % and 1.0 of the control group, but indomethacin did not show any such preventive effect. Curiously, indomethacin strongly inhibited PGE2 production in comparison with celecoxib. They supposed that chemopreventive effects of the celecoxib may not be mediated by the inhibition of the COX-2 activity or prostaglandins production alone and thus carried out another experiment to elucidate the cell kinetics[47]. They indicated that both drugs suppressed cell proliferation, but celecoxib increased the apoptosis of gastric cell in a dose-dependent manner, whereas indomethacin did not effect apoptosis, thus suggesting that celecoxib inhibits gastric carcinogenesis by the COX-2 independent pathway, such as by the inhibition of the NF-1-κB signaling pathway. Furthermore, Nam et al[48] examined the effect of nimesulide on gastric carcinogenesis using an N-methyl-N-nitrosourea (NMU)-induced and an H pylori-infected mouse model, demonstrating that gastric tumors developed in 68.8% of mice given both MNU and H pylori, whereas the tumor incidence in the mice receiving nimesulide in addition to MNU and H pylori was 27.8 %.
More recently COX-2 was proven to have a strong relationship with gastric tumorigenesis in a study using transgenic mice[49]. In the transgenic model expressing both COX-2 and microsomal prostaglandin E synthase (mPGES)-1, the animals developed inflammation-associated hyperplastic gastric tumors in the proximal glandular stomach. In addition, NS-398 treatment for four weeks completely suppressed the gastric hypertrophy, thereby reducing the mucosal thickness in the same model. We previously established a rodent duodenogastric reflux model, in which gastric cancer developed for 50 to 60 wk without any chemical carcinogens[50]. We have now started an experiment to prove the chemopreventive effects of meloxicam on gastric tumors including gastric adenoma and adenocarcinoma using this model and have preliminarily confirmed a suppressive effect on gastric lesions (data not shown).
Colorectal cancer is one of the most popular cancers and its incidence is increasing with high mortality rates in westernized countries. The relationship between the carcinogenesis and COX-2 is most intensively elucidated in both basic and clinical research about colorectal polyps, adenoma, and cancer. Before the discovery of COX-2, numerous studies about inhibitory effects of NSAIDs on intestinal tumorigenesis were performed using chemical carcinogen-induced animal models and Apc gene mutant mouse models[51,52]. The Apc gene plays an important role in colon cancer development. An epoch-making paper was published by Oshima et al[53] in 1996 about the contribution of COX-2 to carcinogenic sequence in Wnt/Apc/Tcf pathway. They induced COX-2 mutations in ApcΔ716 knockout mice, which led to the development of numerous polyps in the intestine. In COX-2-/- ApcΔ716 and COX-2+/- ApcΔ716 mice, the number of polyps dramatically decreased by 86% and 66%, respectively, in comparison to that in the littermate COX-2+/+ ApcΔ716 mice. They also reported in the same paper that MF-tricyclic suppressed number of polyps in ApcΔ716 mice. This is the first report that COX-2 inhibitor reduced the number of intestinal polyps. Following this finding several COX-2 inhibitors have been reported to succeed in polyp reduction in knockout Apc mice (Table 3).
| Drug | Outcomes | Reference | |||||
| Name | Concentration | Term | Animal model | Inhibition rate (%) | P value | Reporter (#) | Year |
| carcinogen-induced rat model | |||||||
| Celecoxib | 1500 ppm | 5-16 wk | F344 rat, AOMa | 40 (ACF) | P < 0.001 | Reddy et al (92) | 1996 |
| NS-398 | 1 mg/kg•bw | 5-11 wk | F344 rat, AOMa | 34 (ACF) | P < 0.05 | Yoshimi et al (83) | 1997 |
| 10 mg/kg•bw | 47 (ACF) | P < 0.01 | |||||
| Celecoxib | 1500 ppm | 5-50 wk | F344rat, AOMa | 93(colontumor) | P<0.00001 | Kawamori et al (81) | 1998 |
| Nimesulide | 200 ppm | 6-30 wk | ICRmouse,AOMa | 36(adenocarcinoma) | NS | Fukutake et al (84) | 1998 |
| 400 ppm | 50(adenocarcinoma) | P < 0.05 | |||||
| Celecoxib | 500 ppm | 5-58 wk | F344 rat, AOMa | 55(adenocarcinoma) | P < 0.001 | Reddyet al (82) | 2000 |
| 1000 ppm | 5-58 wk | 62(adenocarcinoma) | P < 0.001 | ||||
| 1500 ppm | 5-58 wk | 77(adenocarcinoma) | P < 0.0001 | ||||
| 1500 ppm | 22-58 wk | 47(adenocarcinoma) | P < 0.01 | ||||
| Nabumetone | 750 ppm | for18 wk | F344 rat, AOMa | 15 (ACF) | P < 0.05 | Roy et al (85) | 2001 |
| 1500 ppm | 37 (ACF) | P < 0.01 | |||||
| Apc gene mutant mouse model | |||||||
| MF-tricyclic | 3,5 mg/kg•d | 3-11 wk | ApcΔ716 | 52(intestinalpolyp) | P = 0.0037 | Oshima et al (53) | 1996 |
| 14 mg/kg•d | 62 (intestinal polyp) | P < 0.0001 | |||||
| Nimesulide | 400 ppm | 4-15 wk | ApcΔ850 (Min) | 48 (intestinal polyp) | Nakatsugi et al (90) | 1997 | |
| Celecoxib | 150 ppm | 30-80 d | ApcΔ850 (Min) | 48 (intestinal polyp) | P < 0.05 | Jacoby et al (54) | 2000 |
| 500 ppm | 29(intestinalpolyp) | ||||||
| 1500 ppm | 71(intestinalpolyp) | ||||||
| JTE-522 | 0.001 (%) | 4-12 wk | ApcΔ474 | 9 (intestinal polyp) | NS | Sasai et al (88) | 2000 |
| 0.01 (%) | 32 (intestinal polyp) | P < 0.05 | |||||
| Nabumetone | 900 ppm | 5-15 wk | ApcΔ850 (Min) | 50(smallbowelpolyp) | P < 0.05 | Roy et al (91) | 2001 |
| 65(largebowelpolyp) | P < 0.05 | ||||||
| MF-tricyclic | 13 mg/kg/d | 3-7 wk | ApcΔ850 (Min) + | 48 (intestinal polyp) | P < 0.001 | Lal et al (87) | 2001 |
| Msh2-/- | |||||||
| Rofecoxib | 0.0025 (%) | 3-11 wk | ApcΔ716 | 36 (intestinal polyp) | Oshima et al (55) | 2001 | |
| 0.0075 (%) | 55 (intestinal polyp) | ||||||
| JTE-522 | 0.01 (%) | 4-12 wk | ApcΔ474 | 49 (large adenoma) | P < 0.01 | Sunayama et al (89) | 2001 |
| -28 (small adenoma) | NS | ||||||
Both celecoxib and refecoxib, two popular drugs as the first generation of selective COX-2 inhibitors, are now commercially available for orthopedic diseases. Both drugs have been shown to have chemopreventive effects on intestinal polyps in Apc mutant mouse models. Jacoby et al[54] performed two experiments of adenoma prevention (early phase) and regression (late phase) by celecoxib using the Min mice model. They showed that celecoxib decreased not only tumor size and multiplicity in the prevention study, but also caused a decrease in the size of established polyps in the regression study. In the rofecoxib study using ApcΔ716 mice model, the drug successfully decreased the number and size of polyps in a dose-dependent manner[55].
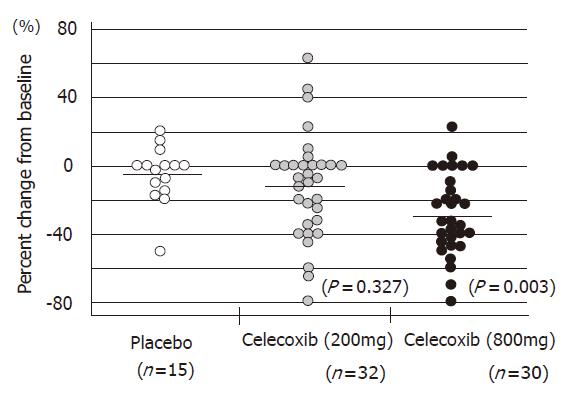
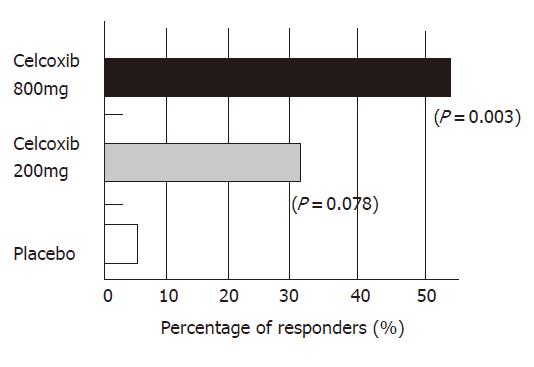
The Apc gene mutation is also responsible for familial adenomatous polyposis (FAP). Some articles have demonstrated the chemopreventive effects of NSAIDs on colorectal polyps of FAP patients[51]. The successful outcomes of selective COX-2 inhibitors in animal models enabled us to start a clinical study of chemoprevention of FAP. Steinbach et al[56] of the University of Texas, Anderson Cancer Center, in Houston, reported that treatment with celecoxib significantly reduced the number of colorectal polyps in patients with FAP in 2000. I also joined this trial, which was performed as a double-blind, placebo-controlled study and was supported by a contract with the U.S. National Cancer Institute, and Searle Pharmaceuticals. All patients underwent total colonoscopy at the beginning and end of the study. All polyps observed by endoscopy were photographed and videotaped. Several members in the study group assessed the number and size of the polyps using these records in a completely blind manner. A statistical analysis was independently carried out by a biomathematician. Seventy-seven FAP patients were randomly assigned to treatment with celecoxib (100 or 400 mg twice daily) or a placebo for six months. Twice daily treatment with 400 mg celecoxib brought a 28% reduction in the number of polyps, a 100-mg dose led to an 11.9% reduction. In contrast, the polyp counts in patients who received placebo fell by only 4.5%. (Figure 3). At least a 25% reduction in polyps was experienced by 53% of the patients in the 400-mg treatment group, compared with 31% of the 100-mg group and 7% of the placebo group (Figure 4). The incidence of adverse events was similar among the groups.
Corresponding to these results, the U.S. Food and Drug Administration (FDA) immediately approved the clinical use of celecoxib for FAP patients, since it was considered to be a potentially useful adjunct to current management by suppressing polyp formation in patients with a residual rectum after colectomy and in patients with an intact colon who are awaiting a colectomy. Several years later the preventive effects on duodenal polyps in FAP patients were established by the same group[57]. Thereafter, three large trials of the chemopreventive effect on the recurrence of neoplastic polyps of the large bowel in patients with a history of colorectal adenoma have been initiated. The APPROVe (Adenomatous Polyp Prevention On Vioxx) was designed to examine the effects of treatment with rofecoxib in April 2000. The APC (Adenoma Prevention with Celebrex)cancer trial and the PreSAP ( Prevention of Spontaneous Adenomatopus Polyps) cancer trial started using celecoxib in December 1999 and March 2001, respectively. Unexpectedly, all the trials now have been stopped because of an observed increased risk in cardiovascular (CV) events.
In spite of the advances and successes of COX-2 inhibitors, recently some pharmaceutical companies have abandoned the development or marketing of such inhibitors. The Vioxx Gastrointestinal Outcomes Research Study (VIGOR study) foreshadowed a current tough situation of COX-2 inhibitors. The VIGOR study was originally designed to assess whether rofecoxib is associated with a lower incidence of clinically important upper gastrointestinal (GI) events (gastroduodenal perforation or obstruction, upper GI bleeding, and symptomatic gastroduodenal ulcers) than is naproxen, a nonselective NSAID, among 8 076 patients with rheumatoid arthritis[58]. As expected, 2.1 confirmed the incidence of GI events per 100 patient-years occurred with rofecoxib, in comparison to 4.5 per 100 patient-year with naproxen (relative risk, 0.5; P <0.001). However, the VIGOR study also showed the relative risk of developing a confirmed adjudicated thrombotic CV event (myocardial infarction, unstable angina, cardiac thrombus, resuscitated cardiac arrest, sudden or unexplained death, ischemic stroke, and transient ischemic attacks) with rofecoxib treatment in comparison to that with naproxen to be 2.38 (P = 0.002). On the other hand, another similar study, the Celecoxib Long-term Arthritis Safety Study (CLASS) yielded different results[59]. The CLASS was conducted to determine whether celecoxib is associated with a lower incidence of significant upper GI toxic effects and other adverse effects in comparison with conventional NSAID, ibuprofen or diclofenac. For all 8 059 patients enrolled in the CLASS, the annualized incidence rates of upper GI ulcer complications alone and combined with symptomatic ulcers of celecoxib vs NSAIDs were 0.76% vs 1.45% (P = 0.09) and 2.08% vs 3.54% (P = 0.02), respectively, whereas there was no significant difference in the CV event (myocardial infarction, stroke, and death) rates between celecoxib and NSAIDs. It was later reported that the adjusted odds ratio for myocardial infarction (MI) among celecoxib users, relative to persons who did not use NSAIDs, was 0.43 in comparison with 1.16 among rofecoxib users, and the use of rofecoxib was associated with a significantly higher odds of MI in comparison wtih the use of celecoxib (adjusted odds ratio for rofecoxib vs celecoxib, 2.72, P = 0.01) in a study comparing rofecoxib with celecoxib regarding the risk of MI incidence[60].
Merck withdrew rofecoxib from the market in September, 2004 because of an increased risk of serious CV events, including heart attack and stroke, among study patients taking rofecoxib compared to patients receiving placebo (the APPROVe). Japan Tobacco Incorporation has already declined to develop JT-522 for clinical use after phase II study in September, 2003. Regarding celecoxib, in an APC cancer trial, Pfizer demonstrated an increased CV risk over placebo, while the PreSAP cancer trial revealed no greater cardiovascular risk than the placebo. The outcomes of two trials were completely different, but Pfizer nevertheless decided to stop them. The US FDA issued a Public Health Advisory, which stated that the long-term use of NSAIDs and selective COX-2 inhibitors might increase the risk of severe CV events (myocardial infarction, strokes, etc) at the beginning of 2005. According to the conclusions of an advisory panel, Pfizer decided to withdraw valdecoxib from the market in April, 2005. Recently, Shaya et al[61] performed an observational cohort study to examine the CV risk of COX-2 inhibitors compared with nonspecific NSAIDs except naproxen in Maryland Medicaid enrollees, a high-risk population. But they did not find that COX-2 inhibitors increased CV risk over nonnaproxen NSAIDs. Whether or not selective COX-2 inhibitors really increase the risk of CV events compared with other NSAIDs remains unknown and still controversial.
COX-1 is constitutively expressed in most tissues and cells, such as the kidney, stomach, platelets, and vascular endothelium, while COX-2 expression is induced in fibroblasts, endothelial cells, monocytes, and ovarian follicles[62,63]. Accordingly, COX-1 alone is expressed in platelets. Ironically, because the selective COX-2 inhibitors hardly suppress COX-1 inducing thromboxane A2, which activates aggregation of platelets, CV risk might be increased among the users of COX-2 inhibitors[64]. In this sense, drugs belonging to the intermediate class of COX-1/COX-2 inhibitors (moderately selective COX-2 inhibitors), such as meloxicam and etodolac, might be reassessed in the near future. But it is very beneficial for most patients that selective COX-2 inhibitors undoubtedly reduce GI disorders about in half compared with NSAIDs[58,59]. Physicians should select COX-2 inhibitors or NSAIDs, after carefully considering which events are most important for each patient, namely GI or CV events. Recently, COX-2 inhibitors have been found to have new pharmacological advantages. Pyo et al[65] reported that NS-398 enhanced the effect of radiation on the COX-2 expressing cells. It was also shown that COX-2 inhibitors had a synergistic antitumor effect in combination with several chemotherapeutic agents, including gemcitabine or 5FU in pancreatic cancer[66], and paclitaxel and carboplatin in non-small-cell lung cancer[67]. Furthermore, the combination of celecoxib and an angiotensin-converting enzyme inhibitor enhanced the antitumor effect through insulin-like growth factor I receptor pathway[68] and low doses of celecoxib was useful for chemoprevention of intestinal polyps in omega-3 polyunsaturated fatty acid-rich diet [69]. These facts are very encouraging to both researchers and clinicians regarding COX-2 inhibitors, thus offering hope for their eventual use in the future.
S- Editor Wang J L- Editor Zhu LH E- Editor Wu M
| 1. | Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97-102. [PubMed] [Cited in This Article: ] |
| 2. | Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322-1327. [PubMed] [Cited in This Article: ] |
| 3. | Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5183] [Cited by in F6Publishing: 4891] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 4. | Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926-13931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1165] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 5. | Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 638] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 6. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1569] [Cited by in F6Publishing: 1553] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 7. | Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961-968. [PubMed] [Cited in This Article: ] |
| 8. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1650] [Cited by in F6Publishing: 1634] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 9. | Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun. 2002;299:886-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336-3340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 997] [Cited by in F6Publishing: 1029] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 11. | Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 1003] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 12. | Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 794] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 13. | Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst. 1998;90:1529-1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Morris CD, Armstrong GR, Bigley G, Green H, Attwood SE. Cyclooxygenase-2 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990-996. [PubMed] [Cited in This Article: ] |
| 15. | Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995;222:525-31; discussion 531-3. [PubMed] [Cited in This Article: ] |
| 16. | Stein HJ, Barlow AP, DeMeester TR, Hinder RA. Complications of gastroesophageal reflux disease. Role of the lower esophageal sphincter, esophageal acid and acid/alkaline exposure, and duodenogastric reflux. Ann Surg. 1992;216:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 196] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Zhang F, Altorki NK, Wu YC, Soslow RA, Subbaramaiah K, Dannenberg AJ. Duodenal reflux induces cyclooxygenase-2 in the esophageal mucosa of rats: evidence for involvement of bile acids. Gastroenterology. 2001;121:1391-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767-5772. [PubMed] [Cited in This Article: ] |
| 19. | Buttar NS, Wang KK, Anderson MA, Dierkhising RA, Pacifico RJ, Krishnadath KK, Lutzke LS. The effect of selective cyclooxygenase-2 inhibition in Barrett's esophagus epithelium: an in vitro study. J Natl Cancer Inst. 2002;94:422-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Li Z, Shimada Y, Kawabe A, Sato F, Maeda M, Komoto I, Hong T, Ding Y, Kaganoi J, Imamura M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by JTE-522, a selective COX-2 inhibitor. Carcinogenesis. 2001;22:547-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 23. | Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, Ohta T, Koichi M. A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis. 2005;26:565-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kaur BS, Khamnehei N, Iravani M, Namburu SS, Lin O, Triadafilopoulos G. Rofecoxib inhibits cyclooxygenase 2 expression and activity and reduces cell proliferation in Barrett's esophagus. Gastroenterology. 2002;123:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Heath EI, Canto MI, Wu TT, Piantadosi S, Hawk E, Unalp A, Gordon G, Forastiere AA. Chemoprevention for Barrett's esophagus trial. Design and outcome measures. Dis Esophagus. 2003;16:177-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 27. | Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva AM, Del Vecchio Blanco C, Bruni CB, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560-28563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, Chan FK. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Sun WH, Yu Q, Shen H, Ou XL, Cao DZ, Yu T, Qian C, Zhu F, Sun YL, Fu XL. Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol. 2004;10:2809-2813. [PubMed] [Cited in This Article: ] |
| 30. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] [Cited in This Article: ] |
| 32. | Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] [Cited in This Article: ] |
| 33. | Saukkonen K, Rintahaka J, Sivula A, Buskens CJ, Van Rees BP, Rio MC, Haglund C, Van Lanschot JJ, Offerhaus GJ, Ristimaki A. Cyclooxygenase-2 and gastric carcinogenesis. APMIS. 2003;111:915-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923-1931. [PubMed] [Cited in This Article: ] |
| 35. | Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. |
Ratnasinghe D, Tangrea JA, Roth MJ, Dawsey SM, Anver M, Kasprzak BA, Hu N, Wang QH, Taylor PR; Expression of cyclooxygenase-2 in human adenocarcinomas of the gastric cardia and corpus. |
| 38. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 39. | Li HX, Chang XM, Song ZJ, He SX. Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:674-677. [PubMed] [Cited in This Article: ] |
| 40. | Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250-253. [PubMed] [Cited in This Article: ] |
| 41. | Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 43. | Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003;37:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Sawaoka H, Kawano S, Tsuji S, Tsujii M, Murata H, Hori M. Effects of NSAIDs on proliferation of gastric cancer cells in vitro: possible implication of cyclooxygenase-2 in cancer development. J Clin Gastroenterol. 1998;27 Suppl 1:S47-S52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;274:G1061-G1067. [PubMed] [Cited in This Article: ] |
| 46. | Hu PJ, Yu J, Zeng ZR, Leung WK, Lin HL, Tang BD, Bai AH, Sung JJ. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Yu J, Tang BD, Leung WK, To KF, Bai AH, Zeng ZR, Ma PK, Go MY, Hu PJ, Sung JJ. Different cell kinetic changes in rat stomach cancer after treatment with celecoxib or indomethacin: implications on chemoprevention. World J Gastroenterol. 2005;11:41-45. [PubMed] [Cited in This Article: ] |
| 48. | Nam KT, Hahm KB, Oh SY, Yeo M, Han SU, Ahn B, Kim YB, Kang JS, Jang DD, Yang KH. The selective cyclooxygenase-2 inhibitor nimesulide prevents Helicobacter pylori-associated gastric cancer development in a mouse model. Clin Cancer Res. 2004;10:8105-8113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669-1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Miwa K, Hasegawa H, Fujimura T, Matsumoto H, Miyata R, Kosaka T, Miyazaki I, Hattori T. Duodenal reflux through the pylorus induces gastric adenocarcinoma in the rat. Carcinogenesis. 1992;13:2313-2316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II). J Natl Cancer Inst. 1998;90:1609-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 365] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 52. | Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8:1021-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1733] [Cited by in F6Publishing: 1658] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 54. | Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040-5044. [PubMed] [Cited in This Article: ] |
| 55. | Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733-1740. [PubMed] [Cited in This Article: ] |
| 56. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1815] [Cited by in F6Publishing: 1680] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 57. | Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 58. | Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520-158, 2 p following 1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2752] [Cited by in F6Publishing: 2503] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 59. | Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2166] [Cited by in F6Publishing: 2025] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 60. | Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Strom BL. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Shaya FT, Blume SW, Blanchette CM, Weir MR, Mullins CD. Selective cyclooxygenase-2 inhibition and cardiovascular effects: an observational study of a Medicaid population. Arch Intern Med. 2005;165:181-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | DeWitt D, Smith WL. Yes, but do they still get headaches. Cell. 1995;83:345-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157-33160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 365] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 64. | Vane JR. Biomedicine. Back to an aspirin a day. Science. 2002;296:474-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Pyo H, Choy H, Amorino GP, Kim JS, Cao Q, Hercules SK, DuBois RN. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7:2998-3005. [PubMed] [Cited in This Article: ] |
| 66. | Milella M, Gelibter A, Di Cosimo S, Bria E, Ruggeri EM, Carlini P, Malaguti P, Pellicciotta M, Terzoli E, Cognetti F. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer. 2004;101:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah K, Pasmantier MW. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645-2650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 68. | Yasumaru M, Tsuji S, Tsujii M, Irie T, Komori M, Kimura A, Nishida T, Kakiuchi Y, Kawai N, Murata H. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 2003;63:6726-6734. [PubMed] [Cited in This Article: ] |
| 69. | Reddy BS, Patlolla JM, Simi B, Wang SH, Rao CV. Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022-8027. [PubMed] [Cited in This Article: ] |
| 70. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] [Cited in This Article: ] |
| 71. | Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 332] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 72. | Tsuji S, Kawano S, Sawaoka H, Takei Y, Kobayashi I, Nagano K, Fusamoto H, Kamada T. Evidences for involvement of cyclooxygenase-2 in proliferation of two gastrointestinal cancer cell lines. Prostaglandins Leukot Essent Fatty Acids. 1996;55:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Baoping Y, Guoyong H, Jieping Y, Zongxue R, Hesheng L. Cyclooxygenase-2 inhibitor nimesulide suppresses telomerase activity by blocking Akt/PKB activation in gastric cancer cell line. Dig Dis Sci. 2004;49:948-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 74. | Noda M, Tatsumi Y, Tomizawa M, Takama T, Mitsufuji S, Sugihara H, Kashima K, Hattori T. Effects of etodolac, a selective cyclooxygenase-2 inhibitor, on the expression of E-cadherin-catenin complexes in gastrointestinal cell lines. J Gastroenterol. 2002;37:896-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Saukkonen K, Tomasetto C, Narko K, Rio MC, Ristimäki A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003;63:3032-3036. [PubMed] [Cited in This Article: ] |
| 76. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. [PubMed] [Cited in This Article: ] |
| 77. | Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045-6051. [PubMed] [Cited in This Article: ] |
| 78. | Tomozawa S, Nagawa H, Tsuno N, Hatano K, Osada T, Kitayama J, Sunami E, Nita ME, Ishihara S, Yano H. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br J Cancer. 1999;81:1274-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Goldman AP, Williams CS, Sheng H, Lamps LW, Williams VP, Pairet M, Morrow JD, DuBois RN. Meloxicam inhibits the growth of colorectal cancer cells. Carcinogenesis. 1998;19:2195-2199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Hussey HJ, Tisdale MJ. Effect of the specific cyclooxygenase-2 inhibitor meloxicam on tumour growth and cachexia in a murine model. Int J Cancer. 2000;87:95-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 81. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] [Cited in This Article: ] |
| 82. | Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, Seibert K, Rao CV. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293-297. [PubMed] [Cited in This Article: ] |
| 83. | Yoshimi N, Kawabata K, Hara A, Matsunaga K, Yamada Y, Mori H. Inhibitory effect of NS-398, a selective cyclooxygenase-2 inhibitor, on azoxymethane-induced aberrant crypt foci in colon carcinogenesis of F344 rats. Jpn J Cancer Res. 1997;88:1044-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Fukutake M, Nakatsugi S, Isoi T, Takahashi M, Ohta T, Mamiya S, Taniguchi Y, Sato H, Fukuda K, Sugimura T. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase-2, on azoxymethane-induced colon carcinogenesis in mice. Carcinogenesis. 1998;19:1939-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Roy HK, Karolski WJ, Ratashak A. Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int J Cancer. 2001;92:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Brown WA, Skinner SA, Malcontenti-Wilson C, Misajon A, DeJong T, Vogiagis D, O'Brien PE. Non-steroidal anti-inflammatory drugs with different cyclooxygenase inhibitory profiles that prevent aberrant crypt foci formation but vary in acute gastrotoxicity in a rat model. J Gastroenterol Hepatol. 2000;15:1386-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Lal G, Ash C, Hay K, Redston M, Kwong E, Hancock B, Mak T, Kargman S, Evans JF, Gallinger S. Suppression of intestinal polyps in Msh2-deficient and non-Msh2-deficient multiple intestinal neoplasia mice by a specific cyclooxygenase-2 inhibitor and by a dual cyclooxygenase-1/2 inhibitor. Cancer Res. 2001;61:6131-6136. [PubMed] [Cited in This Article: ] |
| 88. | Sasai H, Masaki M, Wakitani K. Suppression of polypogenesis in a new mouse strain with a truncated Apc(Delta474) by a novel COX-2 inhibitor, JTE-522. Carcinogenesis. 2000;21:953-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Sunayama K, Konno H, Nakamura T, Kashiwabara H, Shoji T, Tsuneyoshi T, Nakamura S. The role of cyclooxygenase-2 (COX-2) in two different morphological stages of intestinal polyps in APC(Delta474) knockout mice. Carcinogenesis. 2002;23:1351-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Nakatsugi S, Fukutake M, Takahashi M, Fukuda K, Isoi T, Taniguchi Y, Sugimura T, Wakabayashi K. Suppression of intestinal polyp development by nimesulide, a selective cyclooxygenase-2 inhibitor, in Min mice. Jpn J Cancer Res. 1997;88:1117-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Roy HK, Karoski WJ, Ratashak A, Smyrk TC. Chemoprevention of intestinal tumorigenesis by nabumetone: induction of apoptosis and Bcl-2 downregulation. Br J Cancer. 2001;84:1412-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566-4569. [PubMed] [Cited in This Article: ] |