Published online Jun 8, 2025. doi: 10.35712/aig.v6.i1.106149
Revised: March 23, 2025
Accepted: May 8, 2025
Published online: June 8, 2025
Processing time: 109 Days and 5.7 Hours
Colorectal cancer (CRC) can be prevented by screening and early detection. Colonoscopy is used for screening, and adenoma detection rate (ADR) is used as a key quality indicator of sufficient colonoscopy. However, ADR can vary sig
To explore the current status of AI assistance colonoscopy in adenoma detection and improving quality of colonoscopy.
This systematic review followed PRISMA guidelines, both PubMed and Web of Science databases were used for articles search. Metanalyses and systematic reviews that assessed AI's role during colonoscopy. English article only published between January 2000 and January 2025 were included. Articles related to non-adenoma indications were excluded. Data extraction was independently per
22 articles met the inclusion criteria, with significant heterogeneity (I2 = 28%-91%) observed in multiple studies. The number of studies per metanalysis ranged from 5 to 33, with higher heterogeneity in analyses involving more than 18 RCTs. AI demonstrated improvement in ADR, with an approximate 20% increase across multiple studies. However, its effectiveness in detecting flat or serrated adenomas remains unproven. Endoscopists with low ADR benefit more from AI-colonoscopies, while expert endoscopists outperformed AI in ADR, adenoma miss rate, and the identification of advanced lesions. No significant change in withdrawal time was observed when comparing AI-assisted colonoscopy to conventional endoscopy.
While AI-assisted colonoscopy has been shown to improve procedural quality, particularly for junior endoscopists and those with lower ADR, its performance decreases when compared to expert endoscopists in real-time clinical practice. This is especially evident in non-randomized studies, where AI demonstrates limited real-world benefits despite its benefit in controlled settings. Furthermore, no meta-analyses have specifically examined AI's impact on the learning experience of fellows and residents. Some experts caution that reliance on AI may prevent trainees from developing essential observational skills, potentially leading to less thorough examinations. Further research is needed to determine the actual benefits of AI-colonoscopy, particularly its role in cancer prevention. As technology advances, improved outcomes are expected, especially in detecting small, flat, and lesions at difficult anatomical locations.
Core Tip: Artificial intelligence (AI) has shown promising potential in improving the adenoma detection rate (ADR) during colonoscopy, particularly for junior endoscopists and those with a lower baseline ADR. However, expert endoscopists continue to outperform AI in real-world settings, especially in detecting flat and serrated lesions. While the implementation of AI-assisted colonoscopy does not significantly impact withdrawal time, its effectiveness in routine clinical practice remains uncertain. Future research should focus on the role of AI-assisted colonoscopy in colorectal cancer prevention, its impact on resident and fellow training, and its ability to enhance the detection of challenging lesions.
- Citation: Aleissa MA, Luca M, Singh JP, Chitragari G, Drelichman ER, Mittal VK, Bhullar JS. Current status of artificial intelligence colonoscopy on improving adenoma detection rate based on systematic review of multiple metanalysis. Artif Intell Gastroenterol 2025; 6(1): 106149
- URL: https://www.wjgnet.com/2644-3236/full/v6/i1/106149.htm
- DOI: https://dx.doi.org/10.35712/aig.v6.i1.106149
High-quality colonoscopy is essential for effective colorectal cancer (CRC) screening and prevention. The United States Multi-Society Task Force on CRC has outlined key recommendations to optimize colonoscopy performance[1]. These include adequate bowel preparation, complete cecal intubation with photographic documentation, and a meticulous withdrawal time of at least 6–10 minutes to maximize adenoma detection rate (ADR)[1,2]. Continuous quality im
Studies have shown that increased ADR correlates with reduced risks of interval CRC, advanced-stage disease, and cancer-related mortality[5,6]. By ensuring the early detection and removal of precancerous lesions, endoscopists with higher ADR significantly lower the likelihood of patients developing or dying from CRC. This highlights ADR as a vital quality measure in colonoscopy[6,7].
Artificial intelligence (AI) has emerged as a supportive tool in colonoscopy by integrating advanced technologies such as computer-aided detection (CADe) and computer-aided diagnosis (CADx). CADe systems utilize deep learning and convolutional neural networks to analyze real-time video feeds, identifying polyps and abnormalities that might unnoticed by naked eye prompting closer inspection[8]. CADx systems complement this by classifying detected lesions based on histological features, helping differentiate benign from malignant lesions[9]. Additionally, AI was developed to support quality assurance by monitoring key performance metrics such as withdrawal time and mucosal visualization[10]. It suggested implementing AI-colonoscopy into existing workflows has the potential to enhance ADR and overall procedural quality[10].
The study aimed to explore the evolving role of AI in colonoscopy by reviewing multiple meta-analyses and addressing its ability to improve ADR. The insights will help define AI's impact on enhancing colonoscopy quality, guide its integration into routine clinical practice, and identify key areas for future research to optimize its clinical effectiveness in preventing CRC.
Comprehensive literature following preferred reporting item for systematic reviews and metanalysis PRISMA. Search was performed using the PubMed and Web of Science databases to identify relevant studies published between January 2000 and January 2025. The search strategy utilized a combination of keywords, including "artificial intelligence", "machine learning", "deep learning", "supervised learning", "unsupervised learning", "colonoscopy", "adenoma", and "polyp". Filters were applied to restrict results to English-language publications. The search focused on systematic reviews and meta-analyses comparing AI-aided colonoscopy with conventional colonoscopy.
Studies were included if they were systematic reviews or meta-analyses examining AI-assisted colonoscopy for adenoma or polyp detection and comparing real-time AI-aided colonoscopy with conventional colonoscopy. Exclusion criteria included studies focusing on non-adenoma indications, those not classified as systematic reviews or meta-analyses, non-English publications, and abstract only studies.
All citations identified during the search were imported into reference management software. Titles and abstracts were screened for relevance, followed by full-text review to confirm inclusion. When multiple reports existed for a single study, the most recent and complete version was included. The selection process was conducted independently by two reviewers, and discrepancies were resolved through discussion or by consulting a third reviewer.
The primary objective was to systematically summarize findings from included meta-analyses to identify how AI help in improving ADR and what is the factors that reduced AI efficiently. No meta-analysis was performed in this study.
Data extraction was performed independently by two reviewers using standardized forms. Extracted information included: (1) Study name, author, and year of publication; (2) Study characteristics, including pooled participant numbers, ADR, and adenoma miss rates; (3) Subgroup analyses, if available, including variations in adenoma size or polyp morphology, and endoscopist experience; and (4) Withdrawal time. Discrepancies were resolved through discussion. A qualitative synthesis of findings was performed, with results summarized in tabular form to highlight study characteristics and outcomes.
Risk of bias in the included studies was not formally assessed due to the nature of the review but was qualitatively considered when interpreting results, particularly regarding heterogeneity and study design.
No statistical methods were employed as this study did not perform a meta-analysis. The findings were qualitatively synthesized, given the variability in study methodologies and outcomes.
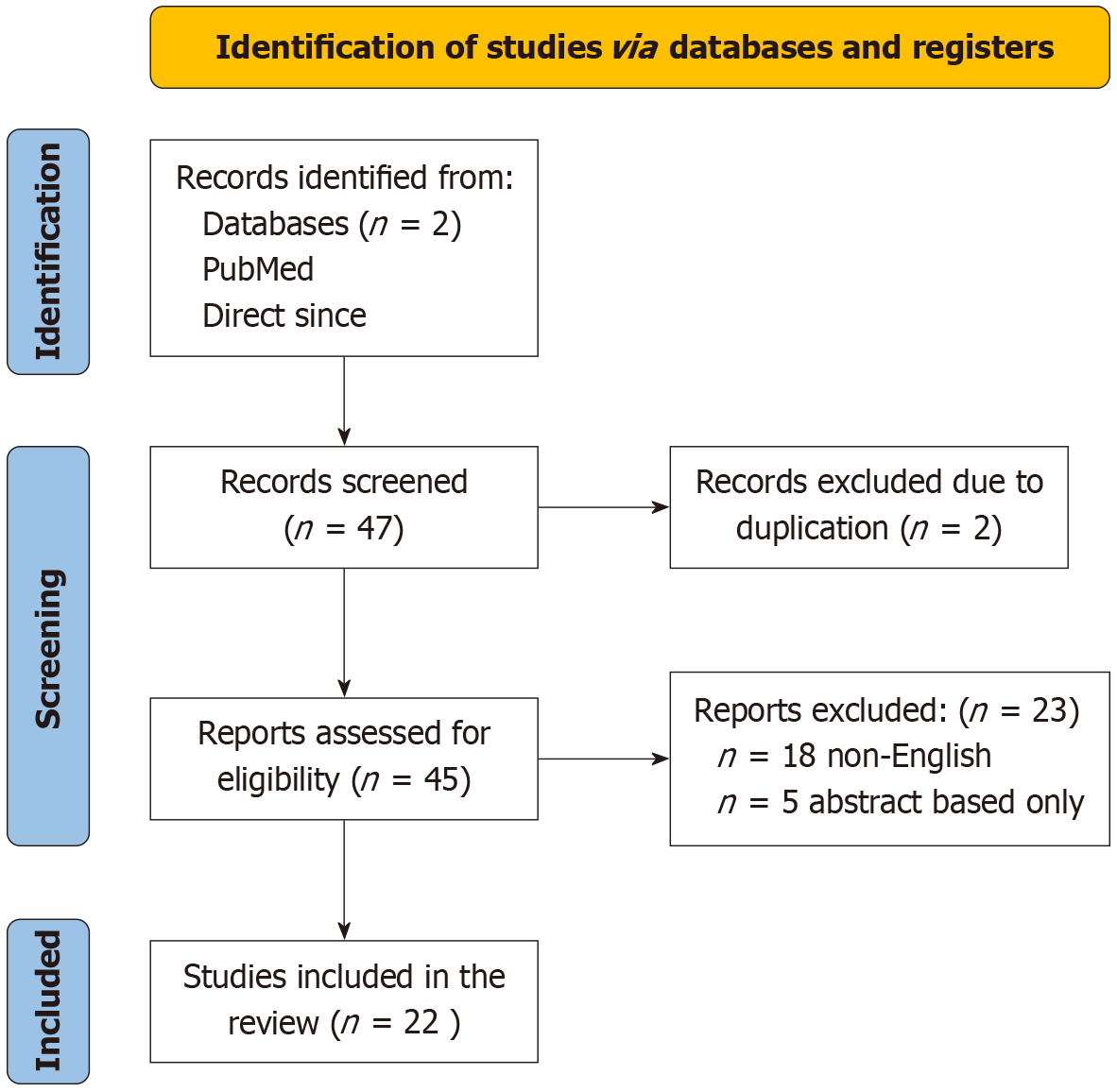
The initial database search identified a total of 47 articles. After removing duplicates, 45 records were screened based on titles and abstracts. 23 full-text articles were assessed for eligibility. Of these, 22 studies met the inclusion criteria and were included in the final analysis. 13 were excluded as they were non-English and 5 of them were abstract only study. The selection process is summarized in the PRISMA flow diagram (Figure 1). Most studies utilized RCT to evaluate AI colonoscopy, with the number of included trials per study ranging from 5 to 33 studies (Table 1).
| Ref. | Study design | Year of publication | Number of study and participants | Type of the study | Overall heterogeneity |
| Soleymanjahi et al[25] | Comparison of CADe-versus conventional colonoscopy performance | 2024 | 33 studies, 27404 participants | Mixed | I² = 74% |
| Makar et al[17] | Impact of CADe systems on key colonoscopy quality indicators | 2024 | 28 RCTs, 23861 participants | RCT | I² = 48% |
| Lee et al[15] | Evaluation of how study characteristics influence outcomes in AI-assisted polyp detection | 2024 | 24 RCTs, 17413 participants | RCT | I² = 53% |
| Patel et al[26] | Assessment of benefits and harms associated with CADe in real-world colonoscopy | 2024 | 8 studies, m9782 participants | Non-RCT | I² = 83% |
| Lou et al[18] | Prospective advantages and disadvantages of AI-assistance systems in colonoscopy | 2024 | 12 studies, 11660 participants | Non-RCT | I² = 87% |
| Barua et al[27] | Comparison of ADR with and without AI utilization | 2023 | 33 RCTs, 27404 participants | RCT | I² = 38.33% |
| Mehta et al[28] | Effectiveness of CADs in early colorectal cancer diagnosis compared to conventional colonoscopy | 2023 | 15 studies, 174602 participants | Mixed | Not mentioned |
| Shiha et al[29] | Effectiveness of CADe in adenoma and polyp detection rates | 2023 | 12 RCTs, 11340 participants | RCT | I2 = 64% |
| Zhang et al[30] | Accuracy measurement of AI-assisted colonoscopy | 2023 | 8 RCTs, 2984 participants | RCT | Moderate to high heterogeneity |
| Nazarian et al[31] | Utilizing CADs for polyp detection and characterization | 2023 | 13 RCTs, 15334 participants | RCT | I2 = 86% |
| Adiwinata et al[32] | Impact of AI colonoscopyon increasing ADR | 2023 | 13 studies, 2958 participants | Mixed | I2 = 57% |
| Vadhwana et al[33] | Assessment of AI colonoscopy in real-time histological prediction | 2023 | 80 studies, 25304 participants | RCT | Moderate to high heterogeneity |
| Hassan et al[34] | Summary of RCTs on CADe systems for colorectal neoplasia detection | 2021 | 28 studies, 29079 participants | Mixed (RCTs and preclinical studies) | I2 = 42.1% |
| Lui et al[35] | AI's role in histology prediction and colorectal polyp detection | 2021 | 10 RCTs, 6629 participants | RCT | I2 = 38.33% |
| Huang et al[36] | Evaluation of AI's impact on colonoscopy outcome metrics | 2021 | 5 studies, 4311 participants | RCT | I2 = 36% |
| Li et al[37] | Evaluation of AI's effect on ADR | 2021 | 26 RCT, 17413 participants | Mixed | I2 = 39.2% |
| Wang et al[38] | AI-assisted polyp detection and classification | 2021 | 6 RCTs, 5058 participants | RCT | I2 = 69% |
| Ashat et al[39] | Determining the statistical significance of AI polyp detection for clinical adoption | 2021 | 6 RCTs, 4996 participants | RCT | I2 = 28% |
| Deliwala et al[40] | Comparison of colorectal cancer detection between standard and AI-assisted colonoscopies | 2021 | 5 RCTs, 4354 participants | RCT | I2 = 70% |
| Hassan et al[41] | Diagnostic accuracy of CADe systems in colorectal neoplasia detection | s2020 | 5 RCTs, 4311 Participants | RCT | I2 = 42% |
| Wei et al[42] | Analysis of CADe's effect on ADR and adenoma detection reproducibility | 2020 | 18 studies, 969318 participants | Mixed | I2 = 91% |
| Mohan et al[43] | Comparison of ADR between CADe assisted colonoscopy and standard colonoscopy | 2020 | 6 RCTs, 4962 participants | RCT | I2 = 56% |
In our analysis, we found that the included studies show moderate to high heterogeneity, with I2 values ranging from 28% to 91%. This heterogeneity indicates the present true heterogeneity of studies rather than a chance. The heterogeneity was categorized as low (0%–40%), moderate (30%–60%), or high (50%–90% or more) based on the I2 statistic[11]. Subgroup analyses were conducted in most meta-analyses to address this issue, based on factors such as polyp size, polyp morphology, and geographic location of the study. We found that withdrawal time exhibited the highest heterogeneity (94%) after extracting data from each study, primarily because of inconsistencies in its reporting across studies. The subgroup analyses conducted within each study were examined separately to further investigate this variability.
AI has demonstrated a consistent positive effect on ADR across multiple meta-analyses, as evidenced by the data presented in Table 1. The pooled ADR improvement with AI-assisted colonoscopy is consistently reported across studies, with relative risk (RR) or odds ratio (OR) values indicating significant enhancements in detection rates. However, the result was noticed to be with heterogenicity. To further understand the data subgroup analyses have been conducted to identify which patient populations and polyp characteristics benefit most from AI systems. When considering polyp size, AI consistently outperforms routine colonoscopy in detecting diminutive lesions (≤ 5 mm). However, the effectiveness of AI diminishes with larger polyps (≥ 10 mm). Regarding polyp location, AI has been shown to outperform routine colonoscopy in detecting distal lesions located beyond splenic flexure. However, AI's performance in detecting proximal lesions is similar to that of routine colonoscopy. In terms of polyp morphology, AI's performance in detecting sessile serrated lesions (SSL) and flat polyps has been less impressive. Subgroup analyses indicate that AI underperforms in detecting these types of lesions compared to routine colonoscopy (Table 2).
| Ref. | Adenoma detection with AI | Adenoma detection without AI | Heterogeneity I2 | Adenoma missing rate | False positive |
| Soleymanjahi et al[25] | 44.7% (RR: 1.21, 95%CI: 1.15-1.28) | 37% | 76% | AI: 16.1%, Conv: 35.3% (RR: 0.47, 95%CI: 0.36-0.60, I2 = 35%) | |
| Makar et al[17] | 20% (RR: 1.20, 95%CI: 1.14-1.27) | Lower than AI aid | 64% | 55% reduction (RR: 0.45, 95%CI: 0.37-0.54, I2 = 22.44%) | 39% increase in total (RR: 1.39, 95%CI: 1.23-1.57, I2 = 1.81%) |
| Lee et al[15] | RR: 1.24, 95%CI: 1.17-1.31 | Lower than AI aid | 53% | RR: 0.44 (95%CI: 0.35-0.56, | |
| Patel et al[26] | 44% (RR: 1.11, 95%CI: 0.97-1.28) | 38% | 83% | ||
| Lou et al[18] | RR 1.24, 95%CI: 115-1.33 | 78.87% | 50.5% decrease (RR: 0.495, 95%CI: 0.390–0.627, I2 = 48.76%) | 12.2% increase in total. Range (7.5%–16.9%) | |
| Barua et al[27] | 29.6% (RR: 1.52, 95%CI: 1.31-1.77) | 19% | 48% | 11.2% increase in total. Range (7.1%-20.1%) | |
| Mehta et al[28] | 37.3% (OR: 1.91, 95%CI: 1.32–2.18) | 30% | |||
| Shiha et al[29] | 33.7% (RR: 1.76, 95%CI: 1.55-2.00) | 23% | 28% | ||
| Zhang et al[30] | 33% (OR: 1.52- 1.72) | ||||
| Nazarian et al[31] | 34% (OR: 1.53, 95%CI: 1.32-1.77) | Lower than AI aid | 45% | ||
| Adiwinata et al[32] | OR: 1.58 (95%CI: 1.37-1.82) | Lower than AI aid | |||
| Vadhwana et al[33] | No improvement | 74% | |||
| Hassan et al[34] | 44.0% (RR: 1.24, 95%CI: 1.16-1.33) | 36% | 70% | 16% decrease (RR: 0.45, 95%CI: 0.35-0.58, I2 = 49%) | 0.52 increase per colonoscopy, Mean Difference 018 polypectomies (95%CI: 0.11-0.26, I2 = 92%) |
| Lui et al[35] | 24.2% (RR: 1.242, 95%CI: 1.159-1.332) | 78% | 50.5% decrease (RR: 0.495, 95%CI: 0.390-0.627, I2 = 48.76%) | 12.20% increase in total | |
| Huang et al[36] | 35.4% (RR: 1.43, 95%CI: 1.33-1.53) | 25% | 36% | 10.5% increase in total. Range (7.1%-17.3%) | |
| Li et al[37] | OR: 1.75, 95%CI: 1.52-2.01 | 39% | |||
| Wang et al[38] | 10% (AUC 0.79, 95%CI: 0.79-0.82) | 90% | |||
| Ashat et al[39] | 33.7% (OR 1.76, 95%CI: 1.55-2.00) | 30% | 28% | ||
| Deliwala et al[40] | 77% (OR: 1.77, 95%CI: 1.50-2.08) | 35% | |||
| Hassan et al[41] | 36.6% (RR: 1.44, 95%CI: 1.27-1.62) | 25% | 42% | ||
| Wei et al[42] | 36.3% (RR: 1.13, 95%CI: 1.01-1.28) | 36% | 64% | ||
| Mohan et al[43] | 32.8% (RR: 1.5, 95%CI: 1.33-1.51) | 21% | 56% | 10.3% increase in total (I2 = 93%) |
In studies where subgroup analysis the mean adenoma missing rate is approximately 16.2%, (15.5%-17.5%) for AI-assisted colonoscopy. This represents a significant improvement compared to the conventional colonoscopy missing rate, which is often around 30% or higher, as seen in other studies[12]. The mean false-positive rate across the studies is approximately 16.87% (10.3%-39%). This wide range reflects variability in how false positives result are measured and reported across studies as well as the difference in AI system used. While AI significantly improves adenoma detection, it also tends to increase false positives, which could lead to unnecessary polypectomies (Table 2).
There are three main indications for colonoscopies which are screening, surveillance, and diagnosis. Patients in average risk of CRC undergo screening colonoscopy. While surveillance colonoscopy is performed for patients at higher risk, such those with a history of CRC or adenomatous polyps; diagnostic colonoscopy is provided for those presenting with symptoms that demand more investigation. Our review shows that for screening colonoscopies, AI-assisted colonoscopies show a better ADR than surveillance colonoscopies. Thus, AI colonoscopies may not provide great benefit for patients who are at higher risk, as these patients often have more advanced or larger lesions that are easier to find even without AI help. Furthermore, endoscopist behavior is an important cofounder factor as endoscopists are likely to spend more withdrawal time examining the colonic mucosa, aware that these patients have a higher probability of harboring polyps. This enhanced focus on detail during traditional colonoscopy could close the performance difference between AI-assisted and non-AI-assisted treatments in these groups. Nevertheless, AI still adds value in high-risk environments by enhancing the identification of minor or more subtle lesions and guaranteeing consistent examination quality among several endoscopists (Tables 3 and 4).
| Ref. | Pooled adenoma detection rate | ADR based on subgroup | |||||||
| Size | Polyp location | Polyp morphology | |||||||
| diminutive lesions (≤ 5 mm) | Small lesions (6–9 mm) | Large lesions (≥ 10 mm), distal | Distal | Proximal cecum | Polypoid | Non polypoid | SSL | ||
| Soleymanjahi et al[25] | RR: 1.21, 95%CI: 1.15–1.28, Heterogeneity: I² = 76% | ||||||||
| Makar et al[17] | RR: 1.20, 95%CI: 1.14-1.27, I² = 64% | 46% increase in detection (IRR: 1.46, 95%CI: 1.19–1.80, P < 0.001, I² = 86.06%) | No significant improvement detection. IRR: 1.11, 95%CI: 0.94–1.31, P = 0.20, I² = 51.23% | No significant improvement detection. IRR: 1.24, 95%CI: 0.94–1.62, P = 0.12, I² = 31.35% | No significant improvement detection. RR: 1.10, P = 0.27 | ||||
| Lee et al[15] | RR: 1.24, 95%CI: 1.17–1.31, I² = 53%, P < 0.001 | ||||||||
| Patel et al[26] | RR: 1.11, 95%CI: 0.97–1.28, I² = 83% | No significant difference. RR: 0.84, 95%CI: 0.59–1.20, I² = 65% | No significant improvement. RR: 1.01, 95%CI: 0.84–1.20, I² = 0% | ||||||
| Lou et al[18] | RR: 1.13, 95%CI: 1.01-1.28, I² = 64% | ||||||||
| Barua et al[27] | RR: 1.242, 95%CI: 1.159–1.332, I² = 78.87% | Largest improvement. RR: 1.27, 95%CI: 1.13–1.42, I² = 62% | Moderate improvement. RR: 1.24, 95%CI: 1.10–1.39, I² = 76% | No significant improvement. RR: 1.09, 95%CI: 0.98–1.21, I² = 84% | Smaller improvement. RR: 1.13, 95%CI: 1.05–1.22, I² = 51% | Significant improvement. RR: 1.19, 95%CI: 1.13–1.24, I² = 63% | |||
| Mehta et al[28] | RR: 1.76, 95%CI: 1.55-2.00 | Largest improvement. OR: 2.07, 95%CI: 1.81–2.36, P < 0.001, I² = 27% | No significant improvement. OR: 14.7, 95%CI: 1.19–1.82, P = 0.004, I² = 0% | Moderate improvement. OR: 1.79, 95%CI: 1.27–2.53, P < 0.001, I² = 12% | Smaller improvement. OR: 1.96, 95%CI: 1.70–2.27, P < 0.001, I² = 0% | Moderate improvement. OR: 1.81, 95%CI: 1.57–2.10, P < 0.001, I² = 22% | |||
| Shiha et al[29] | OR: 1.52-1.72 | Largest improvement. Weighted mean difference = -0.48, 95%CI: -0.81 to -0.15, P = 0.004, I² = 0% | AI detected fewer pedunculated polyps. OR: 0.64, 95%CI: 0.49–0.83, P < 0.001, I² = 0% | ||||||
| Zhang et al[30] | OR: 1.58, 95%CI 1.37-1.82, P = 0.003 | Largest improvement. RR: 1.269, 95%CI: 1.133–1.421, I² = 62.34% | Moderate improvement. RR: 1.238, 95%CI: 1.009–1.520, I² = 75.76% | Moderate improvement. RR: 1.287, 95%CI: 0.984–1.684, I² = 83.66% | Smaller improvement. RR = 1.291, 95%CI: 1.092–1.526, I² = 50.91% | Moderate improvement. RR: 1.187, 95%CI: 1.134–1.242, I² = 9.79% | Smaller improvement. RR = 1.230, 95%CI: 1.050–1.441, I² = 63.37% | Better improvement. RR = 1.419, 95%CI: 1.204–1.671, I² = 57.63% | |
| Nazarian et al[31] | No improvement | ||||||||
| Adiwinata et al[32] | RR 1.24, 95%CI: 1.16-1.33 | Largest improvement (medium differences = 0.167) | No improvement. | No improvement | Small improvement. Improvement (medium differences = 0.105) | Small improvement. (medium | Slight Improvement but statistically not significant | ||
| Vadhwana et al[33] | OR: 1.53, 95%CI 1.32–1.77, P < 0.001, I² = 45.5, P = 0.088 | ||||||||
| Hassan et al[34] | RR: 1.43, 95%CI: 1.33-1.53 | RR: 1.71, 95%CI: 1.45–2.02, P < 0.001, I² = 42% | RR: 1.45, 95%CI: 1.23–1.71, P < 0.001, I²= 50% | RR: 1.73, 95%CI: 1.38–2.17, P < 0.00, I² = 55% | Moderate improvement. RR: 1.70, 95%CI: 1.40-2.06, P < 0.001, I² = 50% | No significant improvement. RR: 1.28, 95%CI: 0.92–1.78, P = 0.48 | No significant improvement. RR: 1.13, 95%CI: 0.86-1.48, P = 0.37, I² = 60% | Significant improvement. RR: 2.00, 95%CI: 1.60 2.50, P < 0.001, I² = 50% | Moderate improvement. RR: 1.75, 95%CI: 1.50–2.04, P < 0.001, I² = 40% |
| Lui et al[35] | OR: 1.75, 95%CI: 1.52–2.01 | ||||||||
| Huang et al[36] | OR: 1.75, 95%CI: 1.36–2.25 | Sensitivity 95%. I² = 96.86% | |||||||
| Li et al[37] | OR: 1.76, 95%CI: 1.55-2.00 | Significantly improved. OR: 2.07, 95%CI: 1.81–2.36, I² = 27% | Improved. OR: 1.47, 95%CI: 1.19–1.82, I² = 0% | Significantly improved. OR: 1.79, 95%CI: 1.27–2.53. Heterogeneity. I² = 12% | Significantly improved. OR: 1.96, 95%CI: 1.70–2.27, I² = 0% | Significantly improved. OR: 1.81, 95%CI: 1.57–2.10, | |||
| Wang et al[38] | OR: 1.77, 95%CI: 1.50–2.08, P < 0.001 | Significant improvement. OR: 1.33, 95%CI: 1.12–1.59, P < 0.001 | No improvement. OR: 0.96, 95%CI: 0.96-1.33, | No improvement. OR: 1.43, 95%CI: .0.87-1.78, | No improvement. OR: 0.19, 95%CI: 1.88-1.43, P = 0.25 | No improvement. OR: 1.00, 95%CI: 0.76–1.32, P = 0.99 | |||
| Ashat et al[39] | RR: 1.44, 95%CI: 1.27-1.62 | Significant improvement. RR: 1.69, 95%CI: 1.48–1.84, I² = 63% | Moderate improvement. RR: 1.44, 95%CI: 1.19–1.75, I² = 4% | Moderate improvement. RR: 1.46, 95%CI: 1.04–2.06, I² = 0% | Moderate improvement. RR: 1.68, 95%CI: 1.50–1.88, I² = 0% | Moderate improvement. RR: 1.59, 95%CI: 1.34–1.88, I² = 55% | Moderate improvement. RR: 1.54, 95%CI: 1.40–1.68, I² = 0% | Moderate improvement. RR: 1.78, 95%CI: 1.47–2.15, I² = 71% | No improvement. RR: 1.52, 95%CI: 1.14–2.02, I² = 0%, P = 0.33 |
| Deliwala et al[40] | 95%CI: 22.2%–37.0% | Significant improvement. Mean difference: = +0.15, 95%CI: 0.12–0.18, P < 0.001, I² = 0.02% | Minimal improvement. Mean difference: +0.03, 95%CI: 0.01–0.05, P = 0.01, I² = 0.04% | No improvement. Mean difference: +0.01. 95%CI: 0.00–0.02, P = 0.76, I² = 0.19% | |||||
| Hassan et al[41] | OR: 1.75, 95%CI: 1.52–2.01, I² = 39.2%, P = 0.160 | Significant improvement. AUC = 0.98, sensitivity = 93.5%, specificity = 90.8% | |||||||
| Wei et al[42] | RR: 1.42, 95%CI: 1.33–1.51, P < 0.00001, I² = 9% | Significant improvement. RR: 1.39, 95%CI: 1.15–1.69, P = 0.0008 | Moderate improvement. RR: 1.56, 95%CI: 1.12–2.19, P = 0.009 | Moderate improvement. RR: 1.56, 95%CI: 1.12–2.19, P = 0.009 | Significant improvement. RR: 1.36, 95%CI: 1.18–1.58, P < 0.0001 | Moderate improvement. RR: 1.75, 95%CI: 1.54–1.98, P = 0.07 | |||
| Mohan et al[43] | RR: 1.5, 95%CI: 1.33-1.51, I² = 32.8% | ||||||||
| Ref. | ADR based on endoscopist | Indication for colonoscopy | ||
| Baseline ADR < 25% | Baseline ADR ≥ 40% | Screening | Surveillance | |
| Soleymanjahi et al[25] | RR: 1.39, 95%CI: 1.18-1.63. Similar improvements in ADR, but data were less stratified | RR: 1.14, 95%CI: 1.08-1.21. (> 1000 colonoscopies): ADR improved by 19%. (RR: 1.19, 95%CI: 1.11–1.27, P < 0.001, I2 = 24.51%) | Significant improvement. RR: 1.21, 95%CI: 1.15-1.28, I2 = 76% | Less improvement. RR: 1.14, 95%CI: 1.05-1.24, I2 = 65% |
| Makar et al[17] | Improvement. RR: 1.23, 95%CI: 1.16–1.32, I2 = 0%, P < 0.001 | Improvement. RR: 1.24, 95%CI: 1.15–1.34, I2 = 45%, P < 0.001 | Improvement. RR: 1.13, 95%CI: 1.07–1.19, I2 = 15%, P < 0.001 | Improvement. RR: 1.33, 95%CI: 1.23–1.45, I2 = 42%, P < 0.001 |
| Lee et al[15] | No significant improvement. RR: 1.01, 95%CI: 0.84–1.20 | 16% increase in ADR compared to standard colonoscopy, but the result was not statistically significant. RR for ADR: 1.16, 95%CI: 0.83–1.62, I2 = 77% | 5% increase in ADR but the result was not statistically significant. RR for ADR: 1.05, 95%CI: 0.92–1.19, I2 = 62% | |
| Lou et al[18] | Significant improvement. RR: 1.42, 95%CI: 1.28–1.58, I2 = 65% | Minimal improvement. RR: 1.12, 95%CI: 1.03–1.22, I2 = 52% | ||
| Deliwala et al[40] | Significantly improved. AUC: 0.97, 95%CI: 0.96–0.98, P < 0.01 | Moderate improved. AUC: 0.90, 95%CI: 0.87–0.93, P < 0.01 | ||
The variability in AI systems used across studies has significantly contributed to the heterogeneity observed in meta-analyses. These differences stem from variations in algorithms, training datasets, and validation protocols, which in turn impact ADR for each system. Due to these inherent differences, multiple meta-analyses have conducted subgroup analyses based on AI systems, revealing that certain programs perform better in specific geographic regions, which are likely due to the locations where they were trained and validated. For instance, GI Genius has shown a substantial improvement in ADR among Western populations, while EndoScreener has demonstrated superior performance in Asian populations. Additionally, our review indicates that the ENDO-AID system by Olympus excels in detecting SSLs, likely due to its extensive training and validation for these lesions alongside adenomas (Table 5).
| Characteristics | GI genius | CAD EYE | EndoScreener | ENDO-AID | SOKUT | Endoangel |
| Developer | Medtronic | Fujifilm | Beijing-based company weiming vision technology | Olympus | Iterative | Wuhan endoangel medical technology co |
| AI function | CADe | CADe + CADx | CADe | CADe + CADx | CADe | CADe + CADx |
| System description | Real-time polyp detection with visual/audio alerts. Integrated with HD colonoscopes | Optimized for Fujifilm scopes. Useful for optical biopsy | Compatible with multiple endoscope brands. High sensitivity for small polyps. Validated in Chinese populations | Focuses on adenoma detection. Trained for sessile adenoma. Validated in United States multicenter trials | Focus in adenoma detection and diagnosis. Can assess bowel preparation quality. Additional benefit in diagnosis gastric neoplasm | |
| Regulatory approval | United States Food and Drug Administration Europe, Select markets in Asia, Australia, and the Middle East | Pharmaceuticals and Medical Devices Agency (Japan). United States Food and Drug Administration | National Medical Products Administration (China) | Europe, Middle East, Africa. United States Food and Drug Administration | United States Food and Drug Administration | National Medical Products Administration (China) |
| Workflow integration | Flexible | Fujifilm-only | Flexible | Olympus-only | Flexible | Flexible |
| Clinical adaption | United State. Europe | Japan | China | Japan. Europe | United State | China |
| ADR improvement | Consistent ADR. Lower non-neoplastic resection. Prolonged withdrawal time. No improvement with SSL | Prioritize large/advanced adenoma. Less sensitive over subtle and small adenoma. No improvement with SSL | Improvement in ADR. Increase non-neoplastic resection | Consistent ADR. Significant SSL benefits | Improvement in ADR. Increase non-neoplastic resection | Real-time detection and withdrawal time monitoring. Alerts for suboptimal technique. Improves withdrawal technique. Reduces miss rates |
Compared with Western studies, Asian studies showed a significantly higher improvement of ADR, with moderate heterogeneity observed in Asian studies (I2 = 44%) and low heterogeneity in Western studies (I2 = 23%). This trend was observed in several meta-analyses in this review and others and raises concern for bias. On further analysis, we found that the studies from Asia were mostly single-centered and controlled where ADR is known to be higher than multicentric, non-randomized studies. Furthermore, AI systems in Asian studies tend to be validated and adapted locally, increasing their success.
The reporting of withdrawal time among the metanalyses is inconsistent, as subgroup analysis revealed heterogeneity of up to 94%. This is attributed to the fact that some studies reported withdrawal times inclusive of biopsy, which is known to prolong withdrawal time, while others excluded biopsy time, and some combined both in their analyses. Nonetheless, subgroup analysis of withdrawal time was reported in 16 out of 22 studies. The mean increase was 20 seconds and was not statistically significant. This suggests that adapting AI-assisted colonoscopy is not linked to prolonged withdrawal times or diminished clinical efficacy of endoscopies.
Out of the 22 reviewed studies, 12 performed subgroup analyses to evaluate the advantages of AI-assisted colonoscopy among expert endoscopists. The definition of a "expert endoscopist" varied among studies. in general, it was defined as endoscopist with baseline ADR exceeding 40% or those who have conducted over 2000 colonoscopies. Conversely, non-expert endoscopists have a baseline ADR below 25%. The findings showed AI have a positive impact of ADR among both groups however, the data consistently indicated better improvement in ADR among non-expert endoscopists, with an average RR of 1.30 compared to expert endoscopists (Tables 3 and 4).
Subgroup analysis showed bowel preparation impact the result of ADR in AI-colonoscopy, where patients with good bowel preparation score have higher ADR compared to those of suboptimal bowel preparation. ADR were higher among patients below 50 years of age. Likewise, individuals with a body mass index (BMI) ≤ 25 exhibited substantial ADR enhancement, whereas those with a BMI > 25 encountered diminished advantages. All these factors are crucial before implementing AI colonoscopy in any practice and also present potential sources for improvement among developers.
This systematic review of meta-analyses highlights the progressive advancements in AI modules over the years, which have significantly influenced study outcomes. Earlier studies, often limited by smaller sample sizes, and limited AI modules demonstrated less robust results compared to recent analyses with larger patients numbers and more advanced AI systems employed. The overall improvement in ADR with AI-colonoscopy ranged from 20% to 40%, reflecting Al's ability to improve the efficiency of colonoscopy screening. However, this result may not be translated into real-time practice[13].
Our findings indicate that the performance of AI systems in colonoscopy varies by geographic region and the indication for the procedure. Studies conducted in Asia have shown greater improvements in ADR compared to those in Europe and North America, a trend also observed in other studies[14]. This variation is likely due to differences in patient demographics. In Asian studies, patients tend to be younger. As a result, these populations typically have a higher baseline prevalence of small adenomas, where AI excels. In contrast, older patients, or symptomatic patients who are more common in Western studies, often present with larger adenomas that are less likely to be missed by endoscopists, reducing AI's added benefit[15].
Despite AI's consistent ability to enhance the detection of diminutive adenomas and those in challenging locations, such as folds or flexures, its effectiveness in detecting SSL remains unclear[16-18]. These lesions are clinically significant yet pose diagnostic challenges even for experienced endoscopists[18]. Not only because it is difficult to visualize as these types of polyps usually have rapid progression to cancer, especially in the right side of the colon[19].
The ability of AI to detect diminutive and small lesions, which are less likely to harbor high-grade dysplasia or malignancy, highlights its potential to impact long-term surveillance strategies. Studies have shown that even non-advanced diminutive adenomas can increase the risk of metachronous cancer, underscoring the importance of their detection[6,8,20]. Data showed implanting AI during colonoscopy increases surveillance by approximately 35% in the United States and 20% in Europe[21]. Moreover, AI offers promising advantages in colonoscopy quality assurance, which was not studied in our review. Some studies demonstrated the use of AI to monitor key performance metrics, such as withdrawal time, and bowel preparation scores which are directly linked to ADR improvements.
A comprehensive cost-benefit analysis is essential when evaluating the implementation of AI-assisted colonoscopy. Such an analysis should extend beyond the initial financial outlay to include potential long-term healthcare savings. The upfront costs of adopting AI include hardware upgrades, software licensing, and clinician training. However, the improved detection of adenomas may lead to a reduction in interval CRC incidence, potentially translating into sig
Given this evidence, AI-assisted colonoscopy may be especially valuable for endoscopists with lower ADR, potentially narrowing the skill gap and promoting greater consistency in ADR across varying levels of clinical expertise. However, to date, no studies have systematically evaluated the effect of AI colonoscopy on the education and training of fellows or residents. It remains unclear whether AI can enhance learning by facilitating lesion recognition or, conversely, whether it may promote overreliance on AI-generated alerts at the expense of developing independent diagnostic skills. In light of these considerations, clinicians who decided to integration AI into their colonoscopy practice should assess several key factors. These include compatibility with existing endoscopic equipment, the specific functionalities required CADe alone or in combination with CADx, overall cost, and the regulatory status of the system in their region.
Our results indicate that AI plays a limited role in improving ADR and AMR when expert endoscopists perform colonoscopy. However, its benefits appear more pronounced among endoscopists with lower baseline ADR. Additionally, studies suggest that AI does not significantly increase procedure time, addressing a common concern and supporting its broader implementation. Given the presence of multiple meta-analyses in this field, conducting an umbrella meta-analysis could provide a more comprehensive understanding and robust conclusions about the overall impact of AI in colonoscopy. Further studies are also needed to address the role of AI in ADR and its role in preventing colon cancer.
| 1. | Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O'Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 558] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1468] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 3. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1133] [Article Influence: 283.3] [Reference Citation Analysis (0)] |
| 4. | Helsingen LM, Kalager M. Colorectal Cancer Screening - Approach, Evidence, and Future Directions. NEJM Evid. 2022;1:EVIDra2100035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Waldmann E, Kammerlander AA, Gessl I, Penz D, Majcher B, Hinterberger A, Bretthauer M, Trauner MH, Ferlitsch M. Association of Adenoma Detection Rate and Adenoma Characteristics With Colorectal Cancer Mortality After Screening Colonoscopy. Clin Gastroenterol Hepatol. 2021;19:1890-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1561] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 7. | Schottinger JE, Jensen CD, Ghai NR, Chubak J, Lee JK, Kamineni A, Halm EA, Sugg-Skinner C, Udaltsova N, Zhao WK, Ziebell RA, Contreras R, Kim EJ, Fireman BH, Quesenberry CP, Corley DA. Association of Physician Adenoma Detection Rates With Postcolonoscopy Colorectal Cancer. JAMA. 2022;327:2114-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 8. | Desai M, Ausk K, Brannan D, Chhabra R, Chan W, Chiorean M, Gross SA, Girotra M, Haber G, Hogan RB, Jacob B, Jonnalagadda S, Iles-Shih L, Kumar N, Law J, Lee L, Lin O, Mizrahi M, Pacheco P, Parasa S, Phan J, Reeves V, Sethi A, Snell D, Underwood J, Venu N, Visrodia K, Wong A, Winn J, Wright CH, Sharma P. Use of a Novel Artificial Intelligence System Leads to the Detection of Significantly Higher Number of Adenomas During Screening and Surveillance Colonoscopy: Results From a Large, Prospective, US Multicenter, Randomized Clinical Trial. Am J Gastroenterol. 2024;119:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 9. | Bang CS, Lee JJ, Baik GH. Computer-Aided Diagnosis of Diminutive Colorectal Polyps in Endoscopic Images: Systematic Review and Meta-analysis of Diagnostic Test Accuracy. J Med Internet Res. 2021;23:e29682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Kim HJ, Parsa N, Byrne MF. The role of artificial intelligence in colonoscopy. Semin Colon Rectal Surg. 2024;35:101007. [DOI] [Full Text] |
| 11. | Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, Gluud C, Devereaux PJ, Wetterslev J. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One. 2012;7:e39471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, Guo L, Meng Q, Yang F, Qian W, Xu Z, Wang Y, Wang Z, Gu L, Wang R, Jia F, Yao J, Li Z, Bai Y. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:1661-1674.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 13. | Mansour NM. Artificial Intelligence in Colonoscopy. Curr Gastroenterol Rep. 2023;25:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Rizkala T, Menini M, Massimi D, Repici A. Role of Artificial Intelligence for Colon Polyp Detection and Diagnosis and Colon Cancer. Gastrointest Endosc Clin N Am. 2025;35:389-400. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Lee MCM, Parker CH, Liu LWC, Farahvash A, Jeyalingam T. Impact of study design on adenoma detection in the evaluation of artificial intelligence-aided colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2024;99:676-687.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Shaukat A, Colucci D, Erisson L, Phillips S, Ng J, Iglesias JE, Saltzman JR, Somers S, Brugge W. Improvement in adenoma detection using a novel artificial intelligence-aided polyp detection device. Endosc Int Open. 2021;9:E263-E270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Makar J, Abdelmalak J, Con D, Hafeez B, Garg M. Use of artificial intelligence improves colonoscopy performance in adenoma detection: a systematic review and meta-analysis. Gastrointest Endosc. 2025;101:68-81.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Lou S, Du F, Song W, Xia Y, Yue X, Yang D, Cui B, Liu Y, Han P. Artificial intelligence for colorectal neoplasia detection during colonoscopy: a systematic review and meta-analysis of randomized clinical trials. EClinicalMedicine. 2023;66:102341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Trovato A, Turshudzhyan A, Tadros M. Serrated lesions: A challenging enemy. World J Gastroenterol. 2021;27:5625-5629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Niv Y. Changing pathological diagnosis from hyperplastic polyp to sessile serrated adenoma: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G, Mandel JS, Bond JH, Van Stolk RU, Summers RW, Rothstein R, Church TR, Cole BF, Byers T, Mott L, Baron JA. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Mori Y, Wang P, Løberg M, Misawa M, Repici A, Spadaccini M, Correale L, Antonelli G, Yu H, Gong D, Ishiyama M, Kudo SE, Kamba S, Sumiyama K, Saito Y, Nishino H, Liu P, Glissen Brown JR, Mansour NM, Gross SA, Kalager M, Bretthauer M, Rex DK, Sharma P, Berzin TM, Hassan C. Impact of Artificial Intelligence on Colonoscopy Surveillance After Polyp Removal: A Pooled Analysis of Randomized Trials. Clin Gastroenterol Hepatol. 2023;21:949-959.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Areia M, Mori Y, Correale L, Repici A, Bretthauer M, Sharma P, Taveira F, Spadaccini M, Antonelli G, Ebigbo A, Kudo SE, Arribas J, Barua I, Kaminski MF, Messmann H, Rex DK, Dinis-Ribeiro M, Hassan C. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 2022;4:e436-e444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 24. | Barkun AN, von Renteln D, Sadri H. Cost-effectiveness of Artificial Intelligence-Aided Colonoscopy for Adenoma Detection in Colon Cancer Screening. J Can Assoc Gastroenterol. 2023;6:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Soleymanjahi S, Huebner J, Elmansy L, Rajashekar N, Lüdtke N, Paracha R, Thompson R, Grimshaw AA, Foroutan F, Sultan S, Shung DL. Artificial Intelligence-Assisted Colonoscopy for Polyp Detection: A Systematic Review and Meta-analysis. Ann Intern Med. 2024;177:1652-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 26. | Patel HK, Mori Y, Hassan C, Rizkala T, Radadiya DK, Nathani P, Srinivasan S, Misawa M, Maselli R, Antonelli G, Spadaccini M, Facciorusso A, Khalaf K, Lanza D, Bonanno G, Rex DK, Repici A, Sharma P. Lack of Effectiveness of Computer Aided Detection for Colorectal Neoplasia: A Systematic Review and Meta-Analysis of Nonrandomized Studies. Clin Gastroenterol Hepatol. 2024;22:971-980.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 27. | Barua I, Vinsard DG, Jodal HC, Løberg M, Kalager M, Holme Ø, Misawa M, Bretthauer M, Mori Y. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 28. | Mehta A, Kumar H, Yazji K, Wireko AA, Sivanandan Nagarajan J, Ghosh B, Nahas A, Morales Ojeda L, Anand A, Sharath M, Huang H, Garg T, Isik A. Effectiveness of artificial intelligence-assisted colonoscopy in early diagnosis of colorectal cancer: a systematic review. Int J Surg. 2023;109:946-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Shiha MG, Oka P, Raju SA, David Tai FW, Ching H, Thoufeeq M, Sidhu R, Mcalindon ME, Sanders DS. Artificial intelligence–assisted colonoscopy for adenoma and polyp detection: an updated systematic review and meta-analysis. iGIE. 2023;2:333-343.e8. [DOI] [Full Text] |
| 30. | Zhang Y, Zhang X, Wu Q, Gu C, Wang Z. Artificial Intelligence-Aided Colonoscopy for Polyp Detection: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Laparoendosc Adv Surg Tech A. 2021;31:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 31. | Nazarian S, Glover B, Ashrafian H, Darzi A, Teare J. Diagnostic Accuracy of Artificial Intelligence and Computer-Aided Diagnosis for the Detection and Characterization of Colorectal Polyps: Systematic Review and Meta-analysis. J Med Internet Res. 2021;23:e27370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Adiwinata R, Tandarto K, Arifputra J, Waleleng BJ, Gosal F, Rotty L, Winarta J, Waleleng A, Simadibrata P, Simadibrata M. The Impact of Artificial Intelligence in Improving Polyp and Adenoma Detection Rate During Colonoscopy: Systematic-Review and Meta-Analysis. Asian Pac J Cancer Prev. 2023;24:3655-3663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Vadhwana B, Tarazi M, Patel V. The Role of Artificial Intelligence in Prospective Real-Time Histological Prediction of Colorectal Lesions during Colonoscopy: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2023;13:3267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Hassan C, Spadaccini M, Mori Y, Foroutan F, Facciorusso A, Gkolfakis P, Tziatzios G, Triantafyllou K, Antonelli G, Khalaf K, Rizkala T, Vandvik PO, Fugazza A, Rondonotti E, Glissen-Brown JR, Kamba S, Maida M, Correale L, Bhandari P, Jover R, Sharma P, Rex DK, Repici A. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy: A Systematic Review and Meta-analysis. Ann Intern Med. 2023;176:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 35. | Lui TKL, Guo CG, Leung WK. Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:11-22.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 36. | Huang D, Shen J, Hong J, Zhang Y, Dai S, Du N, Zhang M, Guo D. Effect of artificial intelligence-aided colonoscopy for adenoma and polyp detection: a meta-analysis of randomized clinical trials. Int J Colorectal Dis. 2022;37:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Li J, Lu J, Yan J, Tan Y, Liu D. Artificial intelligence can increase the detection rate of colorectal polyps and adenomas: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 38. | Wang A, Mo J, Zhong C, Wu S, Wei S, Tu B, Liu C, Chen D, Xu Q, Cai M, Li Z, Xie W, Xie M, Kato M, Xi X, Zhang B. Artificial intelligence-assisted detection and classification of colorectal polyps under colonoscopy: a systematic review and meta-analysis. Ann Transl Med. 2021;9:1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 39. | Ashat M, Klair JS, Singh D, Murali AR, Krishnamoorthi R. Impact of real-time use of artificial intelligence in improving adenoma detection during colonoscopy: A systematic review and meta-analysis. Endosc Int Open. 2021;9:E513-E521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Deliwala SS, Hamid K, Barbarawi M, Lakshman H, Zayed Y, Kandel P, Malladi S, Singh A, Bachuwa G, Gurvits GE, Chawla S. Artificial intelligence (AI) real-time detection vs. routine colonoscopy for colorectal neoplasia: a meta-analysis and trial sequential analysis. Int J Colorectal Dis. 2021;36:2291-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 41. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77-85.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 42. | Wei MT, Fay S, Yung D, Ladabaum U, Kopylov U. Artificial Intelligence-Assisted Colonoscopy in Real-World Clinical Practice: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2024;15:e00671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 43. | Mohan BP, Facciorusso A, Khan SR, Chandan S, Kassab LL, Gkolfakis P, Tziatzios G, Triantafyllou K, Adler DG. Real-time computer aided colonoscopy versus standard colonoscopy for improving adenoma detection rate: A meta-analysis of randomized-controlled trials. EClinicalMedicine. 2020;29-30:100622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |