Published online Apr 26, 2015. doi: 10.13105/wjma.v3.i2.125
Peer-review started: June 16, 2014
First decision: August 7, 2014
Revised: November 17, 2014
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 26, 2015
Processing time: 315 Days and 23.6 Hours
AIM: To evaluate whether red meat intake is related to the risk of endometrial cancer (EC) using meta-analysis.
METHODS: We searched PubMed, EMBASE, and the Cochrane Library up to June 2013, using common keywords related to red meat and EC. Case-control studies and cohort studies comparing the risk of endometrial cancer among categories by the amount of intake were included. Eleven case-control studies and five cohort studies met our criteria. We performed a conventional and a dose-response meta-analysis of case-control studies using the DerSimonian-Laird method for random-effects. For cohort studies we performed a conventional meta-analysis. Publication bias was evaluated using Egger’s test.
RESULTS: In the meta-analysis of 11 case-control studies including 5419 cases and 12654 controls, higher red meat consumption was associated with an increased risk of EC [summary relative risk (SRR) = 1.43, 95%CI: 1.15-1.79; I2 = 73.3% comparing extreme intake categories). In a dose-response analysis, for red meat intake of 100 g/d, SRR was 1.84 (95%CI: 1.64-2.05). In contrast, in the meta-analysis of five prospective studies including a total of 2549 cases among 247746 participants, no significant association between red meat intake and EC risk (SRR = 0.97, 95%CI: 0.85-1.11; I2 = 4.9% comparing extreme intake categories) was observed.
CONCLUSION: Our meta-analysis found a significant linear association between red meat intake and EC risk based on case-control studies but this was not confirmed in prospective studies.
Core tip: By conducting a dose-response meta-analysis, we found a significant linear association between red meat intake and endometrial cancer risk based on case-control studies. However this association was not confirmed in prospective studies. In our paper, we argue that those findings are attributable to methodological difference between retrospective case-control studies and prospective studies.
- Citation: Ju W, Keum N, Lee DH, Kim YH, Kim SC, Ding EL, Cho E. Red meat intake and the risk of endometrial cancer: Meta-analysis of observational studies. World J Meta-Anal 2015; 3(2): 125-132
- URL: https://www.wjgnet.com/2308-3840/full/v3/i2/125.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i2.125
Endometrial cancer (EC) is estimated to be the fourth most common cancer in females in the United States in 2013[1]. Risk factors for EC include obesity, postmenopausal hormone replacement therapy (HRT), type II diabetes, tamoxifen use, and conditions related to unopposed estrogen such as chronic anovulation and estrogen-only HRT[2-4]. In particular, obesity measured by body-mass index (BMI) has been a well-established risk factor present in almost 50% of women with EC[5]. Recently, red meat intake has received an increasing attention as a potential risk factor for EC[6-8].
An harmful effect of red meat intake has been most studied with colorectal cancer (CRC)[9]. A meta-analysis of 26 cohort studies found an approximately 20% increased risk of colorectal cancer with higher red meat intake[10]. Given that CRC and EC share similar risk factors such as obesity, diabetes, and low physical activity, it has been hypothesized that high red meat intake may increase the risk of EC. This hypothesis is further supported by several mechanisms. Heterocyclic amines generated by overcooking or N-nitroso compounds from proteins have been suggested to act as carcinogens[11]. Iron component in red meat may increase the risk of EC by damaging DNA through oxidative stress[12].
While considerable observational studies have been conducted to examine the effect of red meat intake on EC risk, the epidemiologic relationship remains inconclusive. World Cancer Research Fund (WCRF) panel concluded that there was limited evidence suggesting red meat as a risk factor for EC[13]. While past meta-analysis suggested evidence for a significant inverse association (SRR = 1.59, 95%CI: 1.24-2.05; I2 = 50.2%, comparing extreme intake categories) and quantified that 100 g/d intake was significantly associated with an approximately 60% increased risk of EC (SRR = 1.6, 95%CI: 1.26-2.03)[14], this meta-analysis included only one prospective study[15]. Several prospective studies have been published afterward[6,7,16,17]. Therefore, we aim to conduct an up-to date dose-response meta-analysis of red meat consumption and the risk of EC based on case-control studies and prospective cohort studies.
We searched PubMed, EMBASE, and the Cochrane Library up to June 2013, using common keywords related to red meat and EC. The keywords were combined as follows: “meat products” as a Medical Subject Headings (MeSH) term or “animal protein” or “red meat” or “meat” and “endometrial neoplasm” as a MeSH term or “uterine cancer” or “corpus cancer” or “endometrial carcinoma” or “uterine carcinoma” or “corpus carcinoma” We also reviewed the bibliographies of relevant articles to locate additional publications. The language of publication was restricted to English.
We included observational studies that met all of the following inclusion criteria: studies with human subjects, measured outcomes with pathologic confirmation, RR(s) of red meat intake for EC, and statistical information sufficient enough to restore CI(s) for RR(s). If data were duplicated or shared in more than one study, the most comprehensive study with the greatest number of cases was included in the analysis.
Based on the pre-determined selection criteria, two of the authors (Ju W, Keum N) independently reviewed all studies retrieved from the databases and bibliographies. Two authors screened titles for initial selection and reviewed abstracts/tables of the initially selected articles to identify relevant studies. The reference lists of articles included in our analysis and studies included in the previous meta-analyses were also reviewed for additional papers. Inconsistency between researchers was resolved through discussion based on full articles or in consultation with the third author (Lee DH).
From each study, the following information was extracted: author, year of publication, study design, cohort name, country of study, study period, age range at baseline, types of exposure (red meat, all type of meat except fish), intake range (g/d, g/wk, servings/wk recent), the most fully adjusted measures of association [odds ratio (OR), rate ratio(RR), or hazard ratio (HR)], 95%CI, the number of cases, and total number of participants or person-time in each exposure categories. For the quality assessment of each study, exclusion criteria (hysterectomy, prior endometrial cancer), use of validated questionnaire, variables adjusted for confounding were extracted. For case-control studies, information on the types of controls (population vs hospital) was additionally extracted.
Both conventional and linear dose-response meta-analyses were performed. We assumed a linear dose-response relation in two points. First the previous studies[18,19] with CRC showed linear dose-response association up to 100 g/d intake. Second even in non-linearity model, over 100 g/d did not showed a reduced risk but a blunted slope of increasing risk, which means additional harmful effect of the same direction. In the conventional meta-analysis, random effects model was used to calculate the summary OR for the highest vs lowest intake and red meat and the 95%CI. Heterogeneity was assessed using Q test and I2. Potential sources of heterogeneity were explored using meta-regression based on a priori selected variables. The quality of respective studies was evaluated by performing meta-regression in relation to proper definition of exclusion criteria, types of controls, use of validated questionnaire, type of exposure, adjustment for at least BMI, parity, and menopausal status. Potential publication bias was visually checked using funnel plot and statistically assessed with Egger’s regression asymmetry test. Sensitivity analyses were performed by omitting each study at a time.
For the dose-response meta-analysis, a subset of studies included in the conventional meta-analysis was used if they satisfy the following criteria: availability of red meat intake in objectively quantifiable units; having at least three categories of red meat intake including the reference category; availability of number of cases, either number of participants or person-time, and 95%CI for each exposure category, Aggregate method assuming random effects model was used to calculate the SRR of EC associated with 100 g/d intake of red meat and 95%CI. For every study, the mean level of red meat intake in each category was assigned to the corresponding measure of association. In order to calculate the category-specific mean intake for the open-ended highest category, the length of the adjacent interval was assumed; for the open-ended lowest category, 0 g/d was set as a lower limit.
All statistical analyses were conducted using STATA 12 software package (StataCorp, College Station, TX) and based on 2-tailed α set at P≤ 0.05 for statistical significance. In dose-response meta-analysis we used “Generalized Least Squares for trend estimation of summarized dose-response data” using the GLST command in STATA, which considers the correlation among exposure categories by approximating covariance with GL method.
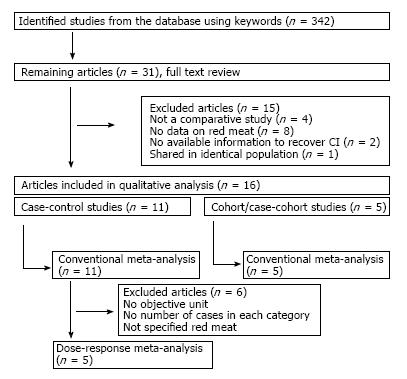
Figure 1 shows how we identified relevant studies. Initial search identified a total of 342 articles, of which 311studies were excluded for not satisfying the pre-determined selection criteria. We reviewed the full texts of the remaining 31 articles and further excluded 15 articles for the following reasons: not a comparative study (n = 4); no data on red meat (n = 8); no information to recover CI (n = 2); shared in identical population (n = 1). Finally, a total of 16 studies (11 case-control studies, 5 prospective studies) were included in our meta-analyses[6,7,15,16,20-30]. and their characteristics are summarized in Tables 1 and 2, respectively.
| Ref. | Location | Study base, subjects | Nutrient | Measurement (unit) | Reference year | Adjustment factors | OR(95%CI) | Meta-analysis | |
| Con-ventional | Dose-response | ||||||||
| Shu et al[20] | China | Population; 268 cases, 278 controls | Red meat | 1 liang (50 g) | 10 yr prior to interview | Age, number of pregnancies, BMI, caloric intake | 2.5 | √ | √ |
| Potischman et al[21] | United States | Population; 399 cases, 296 controls | Red meat | Times/wk | Past few years | Age, BMI, estrogen use, oral contraceptive, number of births, current smoking, education, total calories | 1.3 (0.8-2.4) | √ | √ |
| Levi et al[22] | Swiss | Hospital; 274 cases, 572 controls | Beef | Subjective score | Year before the occurrence of symptoms | Study center, age | 2.26 (1.57-3.24) | √ | |
| Goodman et al[23] | United States (Hawaii) | Population; 332 cases, 511 controls | Red meat | g | Year prior to diagnosis | Age, ethnicity, pregnancy history, oral contraceptive, diabetes, BMI, total calories | 2 (1.1-3.7) | √ | √ |
| McCann et al[24] | United States | Population; 232 cases, 639 controls | Red meat | Times/mo | 2 yr prior to interview | Age, education, BMI, diabetes, hypertension, smoking, age at menarche, parity, oral contraceptive, menopausal status, estrogen | 0.8 (0.5-1.4) | √ | √ |
| Tavani et al[25] | Italy | Hospital; 750 cases, 4770 controls | Red meat | Portions/wk | 2 yr preceding diagnosis | Age, year of recruitment, education, smoking, alcohol, fat, fruit, vegetables | 1.5 (1.2-1.8) | √ | √ |
| Littman et al[26] | United States | Population; 679 cases, 944 controls | All meat | Servings/d | 5 yr prior to diagnosis | Age, residence, total energy intake, unopposed estrogen, smoking, BMI | 1 (0.75-1.4) | √ | |
| Terry et al[27] | Sweden | Hospital; 709 cases, 2887 controls | All meat | Quartile | 1 yr before diagnosis | Age, BMI, smoking, physical activity, diabetes, fatty fish consumption, total food consumption | 1.3 (1.0-1.8) | √ | |
| Dalvi et al[28] | United States | Population; 488 cases, 461 controls | Western diet | Quintile | 1 yr preceding diagnosis | Age, race, age at menarche, oral contraceptive, parity, daily calorie intake, physical activity, menopause, hormone therapy, BMI | 1.5 (0.77-3.0) | √ | |
| Xu et al[29] | China | Population; 1204 cases, 1212 controls | Red meat | 1 liang (50 g) | Past 5 yr | Age, menopause, diabetes, alcohol, BMI, physical activity, total energy intake, other meat | 1.3 (1.0-1.8) | √ | √ |
| Petridou et al[30] | Greece | Hospital 84 cases 84 controls | All meat | Frequency/mo | 1 yr preceding onset of disease | Education, BMI, pregnancy, total energy intake | 0.78 (0.53-1.16) | √ | |
| Ref. | Location | Cohort | Studydesign | Population | Nutrient | Intake unit | Reference year | Adjustment factors | RR (95%CI) |
| Zheng et al[15] | United States | Iowa Women’s Health Study | Cohort | 23070 total 216 cases | Total meat | g/d | Baseline | Age, age at menopause, parity, hormone therapy, total energy intake | 1.1 |
| Kabat et al[16] | Canada | National Breast Screening Study | Cohort | 426 cases 33722 non-cases | Red meat | g/d | Baseline | Age, BMI, menopause, parity, age at menarche, estrogen use, oral contraceptive, total calories, raw vegetable, alcohol intake, physical activity, education | 0.86 (0.61-1.22) |
| van Lonkhuijzen et al[6] | Canada | Canadian Study of Diet, Lifestyle, and Health | Case-cohort | 56837 total 221 cases 3697 non-cases | Red meat | g/d | Baseline | Age, BMI, age at menarche, number of live birhts, breastfeeding, oral contraceptive, exercise, average calorie, vegetable intake, postmenopausal status, hormone therapy | 1.62 (0.86-3.08) |
| Genkinger et al[7] | Sweden | Swedish Mammography Cohort | Cohort | 60895 total 720 cases | Red meat | g/wk | Baseline | Age, energy, BMI, parity, and education | 1.06 (0.68-1.66) |
| Arem et al[17] | United States | NIH-AARP Diet and Health Study | Cohort | 72796 total 966 cases | Red meat | g/1000 kcal | Baseline | Age, BMI, smoking, total energy intake, age at menarche, age at first child’s birth, parity, age at menopause, hormone therapy, oral contraceptive, diabetes, physical activity | 0.91 (0.77-1.08) |
The 11 case-control studies included a total of 5419 cases and 12654 controls. The year of publication of the included studies ranged between 1993 and 2009. The countries where the studies were conducted were as follows: United States (n = 5), China (n = 2), Greece (n = 1), Italy (n = 1), Sweden (n = 1), and Switzerland (n = 1).
Both conventional and linear dose-response meta-analyses were performed.
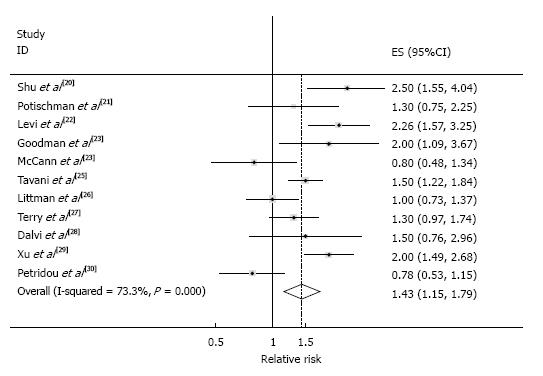
Conventional meta-analysis: The SRR comparing the “highest” with the “lowest” categories of red meat intake was 1.43 (95%CI: 1.15-1.79), with considerable heterogeneity (I2 = 73.3%, Pheterogeneity < 0.001), which are shown in Figure 2. In this random-effects meta-analysis, z-value for the overall effect was 3.18 and P-value for test of effect size was 0.001.
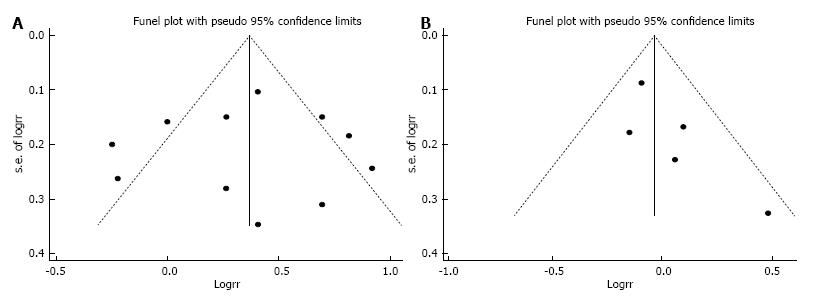
None of a priori selected factors such as type of exposure (red meat vs all type of meat) and publication year, and BMI adjustment was a significant source of heterogeneity. Publication bias was not evident with Funnel plot showing a symmetric dispersion of studies (Figure 3A) and with Egger’s test non-significant (P = 0.911, intercept: -0.20, 95%CI: -4.21-3.80).
Meta-regression for assessing the quality of individual studies showed that methodological components such as exclusion criteria, types of controls, validation of dietary questionnaire, and confounding adjustment did not significantly modify the relationship between red meat intake and EC.
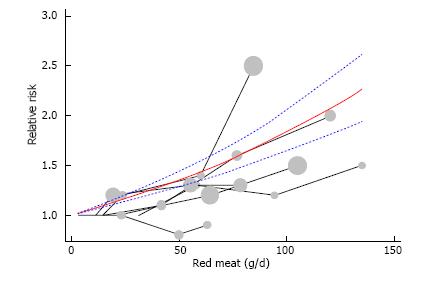
Dose-response meta-analysis: Six out of the eleven studies were eligible for the dose-response meta-analysis, including a total of 3364 cases and 10916 controls. Figure 4 illustrates a significant linear dose-response relationship between red meat intake and EC risk. For each 100 g/d increase of red meat intake, SRR was 1.84 (95%CI: 1.64-2.05), with no significant evidence for heterogeneity (I2 = 21.7%, Pheterogeneity = 0.21). In this dose-response meta-analysis, z-value for the overall effect was 10.75 and P-value for test of effect size was less than 0.001.
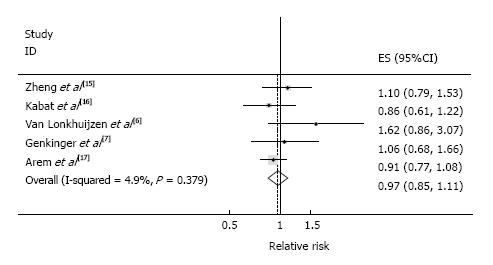
Four cohort studies and one case-cohort study were identified and included a total 549 cases among 247746 participants. Only conventional meta-analysis was conducted because the five studies did not provide all the necessary information needed for linear dose-response meta-analysis. The SRR of EC for the highest vs lowest category of red meat intake was 0.97 (95%CI: 0.85-1.11), with little heterogeneity (I2 = 4.9%, Pheterogeneity = 0.38) (Figure 5). In this random-effects meta-analysis, z-value for the overall effect was 0.44 and P-value for test of effect size was 0.66. No publication bias was indicated by Funnel plot inspection (Figure 3B) and Egger’s test (P = 0.142, intercept = 1.61, 95%CI: -0.98-4.20).
In this conventional and dose-response meta-analysis of observational studies, we found inconsistent results with retrospective case-control studies suggesting a significant increase in EC risk approximately by 84% associated with 100 g/d intake of red meat while with prospective observational studies indicating no such association.
Conventional meta-analysis which dichotomizes continuous exposures as highest vs lowest categories and collapses intake categories regardless of intake level ignores absolute intake and is not optimal to elucidate a dose-response relationship between dietary intake and disease outcomes. This approach may be particularly problematic in populations with wide intake range where cutoffs of intake categories are substantially different. A highest dosage in one study could be a reference dosage in another study, which means that the SRR from conventional meta-analysis might not give proper weights proportional to the dose-related risk. Dose-response meta-analysis has its practical importance than conventional meta-analysis of comparing extreme intake groups by providing summary estimates per absolute amount of intake, which can be easily incorporated in cancer prevention strategy and dietary policy.
Our findings based on 11 retrospective case-control studies are consistent with results from the previous meta-analysis of 7 case-control studies. The SRRs of 1.43 (95%CI: 1.15-1.79) for the highest vs lowest category of red meat intake and of 1.84 (95%CI: 1.64-2.05) per 100 g/d increment in the intake were similar to corresponding SRRs of 1.59 (95%CI: 1.24-2.05) and 1.60 (95%CI: 1.26-2.03) in the previous meta-analysis[14].
Despite those cumulative evidences arising from case-control studies suggests positive associations, most prospective studies have not supported an increased risk of EC associated with red meat intake. In 2000, Trichopoulou et al[31] summarized the nutritional etiology of various forms of cancer in their review, but did not find an evidence for a positive relationship between red meat intake and EC. In 2007, the Panel of WCRF concluded that although evidence for harmful effects of red meat and processed meat on EC risk was stronger than it had been in the mid-1990s, overall evidence remained suggestive, at most[13]. The most recent meta-analysis performed by Bandera et al[14] did not reported a pooled RR for cohort studies because they included only one cohort study by Zheng et al[15], which found no significant association (OR, 1.10, 95%CI: 0.79-1.52). Our updated meta-analysis of five prospective studies still suggests that red meat intake was not significantly associated with the risk of EC (SRR, 0.97, 95%CI: 0.85-1.11; I2 = 4.9%). The discrepant results between retrospective case-control and prospective observational studies can be partially explained by several issues related to the measurement of red meat intake. First, retrospective vs prospective nature of measurement is an important consideration. In retrospective case-control studies, red meat intake was assessed after diagnosis of EC and thus, participants’ knowledge about disease status could lead to differential measurement error. For instance, since cases are more sensitive to their dietary intake than controls in general, it is entirely possible that cases over-report their red meat intake, which could lead to the observed positive association between red meat intake and EC risk. In contrast, in prospective studies, red meat intake was assessed prior to the diagnosis of EC and thus, measurement errors are likely to be random with respect to disease status. Random measurement error of dichotomous exposure mostly attenuates a measure of association toward the null and thus, could partially account for the null association observed in our meta-analysis of cohort studies.
Second, difference in reference year for exposure measurement relates to differential assumption regarding etiologic window of red meat intake in affecting EC risk, which could lead to inconsistent results. In case-control studies, participants were asked to recall red meat intake during 1-5 years before the assessment. This inherently assumes that recent red meat intake is relevant to current EC risk. In cohort studies, baseline assessment of red meat intake is usually assumed to represent a long-term diet and participants were followed-up for 7 to 21 years. Thus, long-term red meat intake was assumed to modulate EC risk. Thus, it is possible that case-control studies and prospective observational studies addressed different questions regarding the red meat intake-EC relationship and thus, reached different conclusions.
Our study has several limitations. First we only investigated a role of red meat intake. Animal derived fat or processed meat has also been reported as risk factors of EC[32-35]. We focused on red meat in the context of consumers’ intuition contrasting red meat from white meat or fish as people usually classify red meat as one of representative category when they shop at a market or order at a restaurant. Second we often used rather arbitrary intake as a representative intake of corresponding category when the mean or median intakes were not provided in dose-response meta-analysis. Since the representative dosage should not be missed in each range for dose-response meta-analysis, such an extrapolation can be accepted as technically inevitable.
Nonetheless this study has strength in that it provides updated evidence regarding the relationship between red meat intake and EC risk by incorporating recently published prospective studies.
In summary, our meta-analysis found a significant linear association between red meat intake and EC risk based on case-control studies but this was not confirmed in prospective studies. This discrepancy seems to be attributable to the differences in robustness against biases and reference year of assessment of red meat intake between the retrospective and prospective studies. When the implication of the current study is addressed, however, it should be considered that the quality of evidence from cohort studies be higher because it is more likely to represent the real world situation.
The incidence of endometrial cancer (EC) is increasing as the life styles become westernized globally. EC is estimated to be the fourth most common cancer in females in the. The association between red meat intake and the risk of EC is currently unclear.
While past meta-analysis suggested evidence for a significant inverse association and quantified that 100 g/d intake was significantly associated with an approximately 60% increased risk of EC, this meta-analysis included only one prospective study, which could not be sufficient at this time because several prospective studies have been published afterward.
The aim of the current study was to conduct an up-to date dose-response meta-analysis of red meat consumption and the risk of EC based on case-control studies and prospective cohort studies.
This meta-analysis found a significant linear association between red meat intake and EC risk based on case-control studies but this was not confirmed in prospective studies. More results from prospective studies with long-term follow up are in need to confirm the association between red meat intake and the risk of EC.
EC is a carcinoma originated from the inner mucous membrane of mammalian uterus, which is also referred as uterine cancer or corpus cancer.
This review article is well written and will contribute to the clinical practice of the readers.
P- Reviewer: Itamochi H, Trkulja V, Yokoyama Y, Zhang YJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, Koenig KL, Shore RE, Kim MY, Levitz M, Mittal KR, Raju U, Banerjee S. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer. 2001;84:975-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531-1543. [PubMed] |
| 4. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5276] [Article Influence: 239.8] [Reference Citation Analysis (0)] |
| 5. | Parslov M, Lidegaard O, Klintorp S, Pedersen B, Jønsson L, Eriksen PS, Ottesen B. Risk factors among young women with endometrial cancer: a Danish case-control study. Am J Obstet Gynecol. 2000;182:23-29. [PubMed] |
| 6. | van Lonkhuijzen L, Kirsh VA, Kreiger N, Rohan TE. Endometrial cancer and meat consumption: a case-cohort study. Eur J Cancer Prev. 2011;20:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Genkinger JM, Friberg E, Goldbohm RA, Wolk A. Long-term dietary heme iron and red meat intake in relation to endometrial cancer risk. Am J Clin Nutr. 2012;96:848-854. [PubMed] |
| 8. | Lanou AJ, Svenson B. Reduced cancer risk in vegetarians: an analysis of recent reports. Cancer Manag Res. 2010;3:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Norat T, Riboli E. Meat consumption and colorectal cancer: a review of epidemiologic evidence. Nutr Rev. 2001;59:37-47. [PubMed] |
| 10. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 11. | Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J Natl Cancer Inst. 2006;98:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Kabat GC, Rohan TE. Does excess iron play a role in breast carcinogenesis? An unresolved hypothesis. Cancer Causes Control. 2007;18:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, Jackson A, James W, Kolonel L, Kumanyika S, Leitzmann C. Food, nutrition, physical activity, and the prevention of cancer: a global perspective, the American Institute for Cancer Research. Available from: http: //health-equity.pitt.edu/868/. |
| 14. | Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control. 2007;18:967-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Zheng W, Kushi LH, Potter JD, Sellers TA, Doyle TJ, Bostick RM, Folsom AR. Dietary intake of energy and animal foods and endometrial cancer incidence. The Iowa women’s health study. Am J Epidemiol. 1995;142:388-394. [PubMed] |
| 16. | Kabat GC, Miller AB, Jain M, Rohan TE. Dietary iron and haem iron intake and risk of endometrial cancer: a prospective cohort study. Br J Cancer. 2008;98:194-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Arem H, Gunter MJ, Cross AJ, Hollenbeck AR, Sinha R. A prospective investigation of fish, meat and cooking-related carcinogens with endometrial cancer incidence. Br J Cancer. 2013;109:756-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6:e20456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 534] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 19. | Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98:241-256. [PubMed] |
| 20. | Shu XO, Zheng W, Potischman N, Brinton LA, Hatch MC, Gao YT, Fraumeni JF. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People’s Republic of China. Am J Epidemiol. 1993;137:155-165. [PubMed] |
| 21. | Potischman N, Swanson CA, Brinton LA, McAdams M, Barrett RJ, Berman ML, Mortel R, Twiggs LB, Wilbanks GD, Hoover RN. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239-250. [PubMed] |
| 22. | Levi F, Franceschi S, Negri E, La Vecchia C. Dietary factors and the risk of endometrial cancer. Cancer. 1993;71:3575-3581. [PubMed] |
| 23. | Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, Kolonel LN. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077-5085. [PubMed] |
| 24. | McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control. 2000;11:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, Negri E. Red meat intake and cancer risk: a study in Italy. Int J Cancer. 2000;86:425-428. [PubMed] |
| 26. | Littman AJ, Beresford SA, White E. The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control. 2001;12:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case-control study in Sweden. Nutr Cancer. 2002;42:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Dalvi TB, Canchola AJ, Horn-Ross PL. Dietary patterns, Mediterranean diet, and endometrial cancer risk. Cancer Causes Control. 2007;18:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Xu WH, Dai Q, Xiang YB, Zhao GM, Zheng W, Gao YT, Ruan ZX, Cheng JR, Shu XO. Animal food intake and cooking methods in relation to endometrial cancer risk in Shanghai. Br J Cancer. 2006;95:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Petridou E, Kedikoglou S, Koukoulomatis P, Dessypris N, Trichopoulos D. Diet in relation to endometrial cancer risk: a case-control study in Greece. Nutr Cancer. 2002;44:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869-873. [PubMed] |
| 32. | Cui X, Rosner B, Willett WC, Hankinson SE. Dietary fat, fiber, and carbohydrate intake in relation to risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:978-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074-4080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, Pettinger M, Lane DS, Lessin L, Yasmeen S. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila). 2011;4:1903-1911. [PubMed] |