Published online Jun 18, 2025. doi: 10.13105/wjma.v13.i2.100483
Revised: March 23, 2025
Accepted: April 16, 2025
Published online: June 18, 2025
Processing time: 302 Days and 12.6 Hours
Ventriculoperitoneal shunt (VPS) catheter insertion is one of the most widely accepted surgical procedures for hydrocephalus. Migration of the end of the distal VPS catheter into the scrotum is one of the rare complications of VPS catheter insertion.
To review the demographics, clinical characteristics, and outcomes of the surgical procedures provided for the cases of migration of the distal VPS catheter into the scrotum.
This is a systematic review of the published cases, and the literature search was performed from 1974 to June 30, 2024, to retrieve the relevant manuscripts. The cases were grouped into two. Group A included cases of migration of the distal VPS catheter into the scrotum, detected in children. Group B included the cases of migration of the distal VPS catheter into the scrotum, which was detected in adults and older people.
One hundred-twenty cases of migration of the distal end of VPS catheter into the scrotum were included in this study, a systematic literature review. Group A included n = 112 cases, and group B included n = 8 cases only. Three-fourths of the cases involved the right scrotum. Bilateral involvement was rare. The indication for initial VPS insertion was congenital hydrocephalus in four-fifths of the group A cases. The majority were infants at the time of initial shunt insertion. Four-fifths of the group A cases were 24 months-old or younger at the time of clinical diagnosis and treatment was provided for migration of the distal VPS catheter into the scrotum. In children, the interval from VPS insertion or shunt revision if any, to the diagnosis of the complication mentioned above was 12 months or less in four-fifths of the cases. The repositioning of migrated distal VPS catheter into the peritoneal cavity and herniotomy was preferred for the surgical procedure, and it was performed for two-thirds of group A cases.
Migration of the distal part of the VPS catheter into the scrotum is a rare complication of cerebrospinal fluid diversion via VPS catheter insertion. It was most frequent in children, and the right side of the scrotum was most often involved.
Core Tip: The present manuscript is a systematic review and was carried out to review the demographics, clinical features, and outcome of the surgical therapy executed for the migration of the distal ventriculoperitoneal shunt (VPS) catheter into the scrotum. This study included n = 120 cases (n = 112 children and n = 8 adults and older individuals) treated for the complication, mentioned above, and published from 1974 to June 30, 2024. Migration of the distal VPS catheter to the right scrotum was most frequent/common, both in children and adults, and older people. In children, the majority of cases (four-fifths) were diagnosed and treated for above mentioned VPS complication during the first 24 months of their life or earlier. Additionally, the complication was detected within the first 12 months after the initial VPS insertion, in four-fifths of the cases. Clinically, the cases presented with the chief complaint of scrotal swelling of varying duration. Two-thirds of the children underwent surgical repositioning of the migrated distal VPS catheter into the peritoneal cavity and herniotomy. The systematic literature review revealed two deaths among the adults/older patients and none among the children treated for migration of the distal VPS catheter into the scrotum.
- Citation: Ghritlaharey RK. Why the migration of peritoneal shunt catheter into the scrotum occurs more in children: A systematic literature review. World J Meta-Anal 2025; 13(2): 100483
- URL: https://www.wjgnet.com/2308-3840/full/v13/i2/100483.htm
- DOI: https://dx.doi.org/10.13105/wjma.v13.i2.100483
The cerebrospinal fluid (CSF) diversion from the ventricular system to the peritoneal cavity through a ventriculoperitoneal shunt (VPS)/peritoneal shunt catheter insertion is one of the most frequently performed surgical procedures for the treatment of hydrocephalus[1-3]. However, VPS catheter insertion is technically a simple procedure associated with several complications and requires revision procedures[2-5]. The shunt complications are most frequently observed during the first 12 months after the initial shunt insertion, and it is also evidenced more in children than adults[5-7]. Migration of the distal shunt catheter into the hollow viscus (gastrointestinal tract, urinary bladder, and uterus) with or without extrusion through the natural orifices is one of the known complications of CSF shunt insertion[8-11]. Migration of the distal shunt catheter to the heart[12], pulmonary vasculature[13], retroperitoneal area, through the umbilicus, and other body parts, has also been reported in the literature[14-17]. Migration of the distal end of the peritoneal catheter into the scrotum is a rare complication of CSF shunt catheter insertion[16,17]. For reporting this manuscript, the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines are followed[18]. This report is a literature review of n = 120 cases published from 1974 to June 2024 related to the treatment of migration of the distal end of a peritoneal shunt catheter into the scrotum[19-113].
An electronic database of PubMed, PubMed Central (PMC), Scopus, Web of Science, Cochrane Library, ResearchGate, and Google Scholar were searched to obtain the published/available literature for the distal end of the VPS/CSF shunt catheter migration into the scrotum. Ramani et al[19] in 1974 first reported a case of migration of a distal end of a peritoneal shunt catheter into the scrotum, in a 6-month-old child. The literature search was made from 1974 to June 30, 2024, to retrieve the relevant manuscripts. The keywords applied for the online search were “migration of distal VPS catheter into the scrotum”, “scrotal migration of distal VPS catheter”, “migration of distal peritoneal shunt catheter”, “migration of distal VPS catheter”, “migration of the CSF shunt catheter” and “rare/unusual migration of distal shunt catheter”. The cases/case reports with incomplete desired details were excluded from the review. Conference proceedings with cases/case reports for migration of a distal end of a VPS into the scrotum were also excluded. Cases diagnosed/treated for a migration of the distal end of a lumbar-peritoneal and a theco-peritoneal shunt catheter into the scrotum were excluded. Cases/case reports presented with extrusion of a distal end of the VPS catheter through the inguinal skin, but without migration to the scrotum were also excluded. Cases of migration of the distal CSF shunt catheter within the inguinal canal, but without migration to the scrotum were also excluded.
The guidelines of PRISMA are followed for reporting this systematic review. This is a single-author manuscript, therefore the selection of the literature, the extraction of the desired detail, paper writing, and others were carried out by the author alone. The titles, abstracts, and full texts of the manuscripts were accessed before the final selection of the manuscripts for the present review.
The details extracted from the included manuscripts were the patient’s age at the time of primary VPS insertion, the causes of hydrocephalus (indication for the primary VPS insertion), the patient’s age at the time of diagnosis of the VPS complication mentioned above, and the interval from VPS insertion/last VPS revision if any, to the diagnosis of the migration of the distal end of a peritoneal shunt catheter into the scrotum. The other details extracted from the manuscripts were the clinical characteristics, radiological diagnostic modalities used, and the surgical procedures’ outcomes.
The migration of the distal VPS catheter into the scrotum is grouped into two groups, group A and group B. Group A included the cases of migration of the distal VPS catheter into the scrotum, detected/diagnosed and treated in children. Group B included the cases of migration of the distal VPS catheter into the scrotum, detected/diagnosed and treated in adults and older people.
The surgical therapy executed for the cases of migration of the distal VPS catheter into the scrotum are grouped as below: (1) A: Reposition of migrated distal VPS catheter into the peritoneal cavity and inguinal herniotomy; (2) B: Reposition (spontaneous) of migrated distal VPS catheter into the peritoneal cavity and inguinal herniotomy; (3) C: Removal of part of migrated distal VPS catheter, repositioning of the remaining peritoneal catheter into the peritoneal cavity and inguinal herniotomy; (4) D: Removal of part of migrated distal VPS catheter, repositioning of the remaining peritoneal catheter into the peritoneal cavity without inguinal herniotomy; (5) E: Removal of migrated/abandoned distal VPS catheter with repositioning of working distal VPS catheter into the peritoneal cavity and inguinal herniotomy; (6) F: Removal of migrated/abandoned distal VPS catheter and inguinal herniotomy; (7) G: Removal of the entire VPS catheter alone; (8) H: Removal of the entire VPS catheter and inguinal herniotomy; (9) I: Removal of entire VPS catheter with re-VPS insertion/VPS revision and inguinal herniotomy; (10) J: Surgical therapy not required for the migrated distal VPS catheter; (11) K: Surgical therapy not done for the migrated distal VPS catheter; (12) L: Surgical therapy details not available for the migrated distal VPS catheter; and (13) M: Other surgical procedures for the migrated distal VPS catheter.
An ethical committee approval was not required for this manuscript, as is a systematic literature review of the already published cases/case reports.
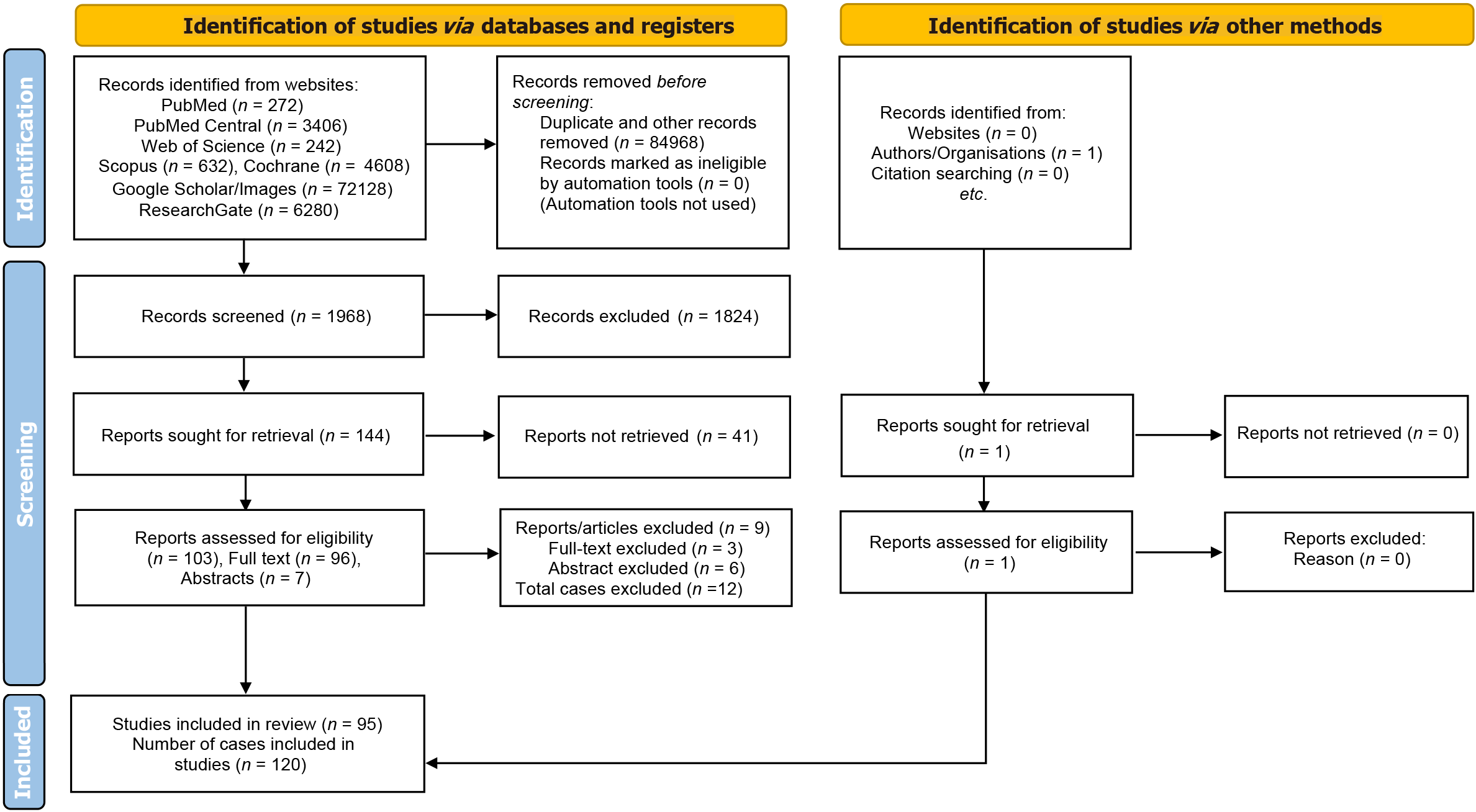
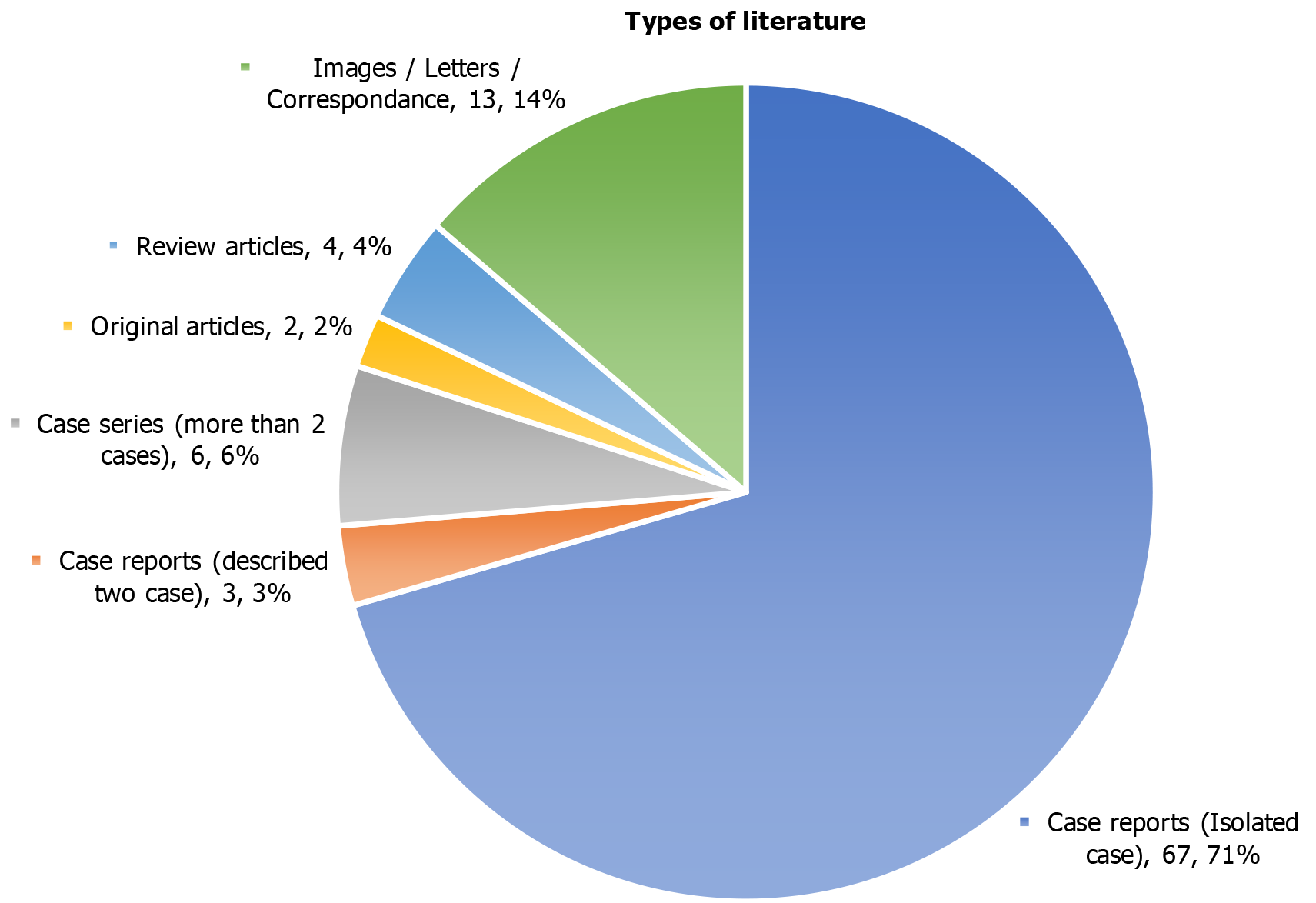
One hundred twenty cases of the migration of the distal end of a VPS catheter into the scrotum were included in the study, a systematic review, and were retrieved from the n = 95 manuscripts. PRISMA flow diagram for the database search, screening, and selection of the literature/manuscripts for the present systematic review are presented in Figure 1. Group A (infants and children below the age of 18 years) included a total of n = 112 cases, detailed in Table 1[19-105]. Group B (adults and older people) included n = 8 cases, detailed in Table 2[106-113]. The year-wise details for the manuscripts included in the present review are provided in Table 3. More than half (n = 67) of the cases were published as isolated cases and are detailed in Figure 2. The year-wise details for the cases included in the present review are provided in Table 4. A total of 9 articles, including n = 12 cases, of migration of the distal VPS catheter into the scrotum, were excluded from the review, and detailed in Table 5[114-122].
| Number | Ref. | Indication for VPS insertion | Age VPS insertion | Age scrotal migration | Interval (months) | VPS (revision) | Site1 (right/left) | Operative procedures executed | Outcome |
| 1 | Ramani et al[19], 1974 | Hydrocephalus (congenital) | Neonate (12 days) | 6 months | 5 months | No | Right | A | R |
| 2 | Levey et al[20], 1977 | Hydrocephalus with NTD (congenital) | Neonate (13 days) | 1 month | 6 days | Yes | Right | A | R |
| 3 | Redman et al[21], 1978 | Hydrocephalus (congenital?) | N/A | 6 months | N/A | No | Right | A | R |
| 4 | Bristow et al[22], 1978 | Hydrocephalus (congenital) | 10 months | 10 months | 1 day | No | Right | C | R |
| 5 | Crofford et al[23], 1983 | Hydrocephalus (post haemorrhagic) | Neonate (15 days) | 6 months | 5 months | No | Right | A | R |
| 6 | Crofford et al[23], 1983 | Hydrocephalus (congenital) | Neonate (1 month) | 3 months | 2 months | No | Right | A | R |
| 7 | Crofford et al[23], 1983 | Hydrocephalus (congenital) | 3 months | 4 months | 1 month | No | Right | A | R |
| 8 | Crofford et al[23], 1983 | Hydrocephalus (brain tumor) | 48 months | 50 months | 2 months | No | Left | B | R |
| 9 | Fuwa et al[24], 1984 | Hydrocephalus (congenital) | Neonate (22 days) | 12 months | 11 months | Yes | Left | I | R |
| 10 | Ram et al[25], 1987 | Hydrocephalus (post infective) | 6 months | 36 months | 30 months | No | Right | C | R |
| 11 | Kwok et al[26], 1989 | Hydrocephalus (congenital) | Neonate (1 month) | 6 months | 1 week | Yes | Bilateral | E | R |
| 12 | Calvário et al[27], 1990 | Hydrocephalus (congenital) | Neonate (1 month) | 24 months | 23 months | No | Right | A | R |
| 13 | Göçer et al[28], 1990 | Hydrocephalus (congenital) | 2 months | 10 months | 8 months | No | Right | C | R |
| 14 | Selcuklu[29], 1991 | Hydrocephalus with NTD (congenital) | Neonate | 8 months | 7 months | No | Right | B | R |
| 15 | Ammar et al[30], 1991 | Hydrocephalus (congenital) | 5 months | 7 months | 2 months | No | Left | G | R |
| 16 | Jamjoom et al[31], 1992 | Hydrocephalus (congenital) | Neonate (3 weeks) | 14 months | 2 months | Yes | Right | E | R |
| 17 | Wong[32], 1994 | Hydrocephalus (congenital) | 6 months | 7 months | 1 month | No | Right | A | R |
| 18 | Goh et al[33], 1997 | Hydrocephalus (congenital) | Neonate (1 day) | 4 months | 4 months | No | Right | B | R |
| 19 | Oktem et al[34], 1998 | Hydrocephalus (congenital) | 10 months | 16 months | 6 months | No | Right | A | R |
| 20 | Oktem et al[34], 1998 | Hydrocephalus (congenital) | 2.5 months | 8 months | 5 months | No | Right | A | R |
| 21 | Oktem et al[34], 1998 | Hydrocephalus (congenital) | Neonate (9 days) | 4 months | 4 months | No | Right | A | R |
| 22 | Oktem et al[34], 1998 | Hydrocephalus (congenital) | 2.5 months | 2.5 months | 1 day | No | Left | A | R |
| 23 | Porras Estrada et al[35], 1999 | Hydrocephalus (congenital) | Neonate (1 day) | 11 months | 5 months | Yes | Left | A | R |
| 24 | Ozveren et al[36], 1999 | Hydrocephalus with NTD (congenital) | Neonate (3 days) | 4 days | 1 day | No | Right | A | R |
| 25 | Silver et al[37], 2000 | Hydrocephalus (congenital) | 6 months | 36 months | N/A | Yes | Right | F | R |
| 26 | Ward et al[38], 2001 | Hydrocephalus (post infective) | 11 months | 18 months | 7 months | No | Right | A | R |
| 27 | Gupta[39], 2003 | Hydrocephalus (congenital) | Neonate | 10 months | 9 months | No | Right | A | R |
| 28 | Henriques et al[40], 2003 | Hydrocephalus (post-haemorrhagic) | Neonate | 5 months | 4 months | No | Right | A | R |
| 29 | Walsh et al[41], 2004 | Hydrocephalus (congenital) | 5 months | 11 months | 6 months | No | Right | L | N/A |
| 30 | de Aquino et al[42], 2006 | Hydrocephalus with NTD (congenital) | Neonate (1 day) | 14 months | 14 months | No | Right | L | R |
| 31 | de Aquino et al[42], 2006 | Hydrocephalus (congenital) | Neonate (11 days) | 1 month | 20 days | No | Right | L | R |
| 32 | Moon et al[43], 2007 | Hydrocephalus (congenital) | 4 months | 12 years | 4 months | Yes | Left | C | R |
| 33 | Karaosmanoglu et al[44], 2008 | Hydrocephalus with NTD (congenital) | N/A | 14 months | N/A | No | Right | G? | R |
| 34 | Kayaoğlu et al[45], 2008 | Hydrocephalus with NTD (congenital) | Neonate (10 days) | 5 months | 4 months | No | Right | C | R |
| 35 | Agarwal et al[46], 2009 | Hydrocephalus (congenital?) | 8 months | 24 months | 7 months | Yes | Right | A | R |
| 36 | Rahman et al[47], 2009 | Hydrocephalus (brain tumor) | 48 months | 49 months | 1 month | No | Right | C | R |
| 37 | de Quintana-Schmidt et al[48], 2010 | Hydrocephalus (congenital) | Neonate | 2 months | 1.6 month | No | Left | A | R |
| 38 | Kita et al[49], 2010 | Hydrocephalus (brain tumor) | 60 months | 64 months | 4 months | No | Left | A | R |
| 39 | Ho et al[50], 2011 | Hydrocephalus (congenital) | N/A | 14 years | 12 months | Yes | Left | F | R |
| 40 | Potineni et al[51], 2012 | Hydrocephalus (congenital) | 2 months | 2 months | 1 day | No | Left | A | R |
| 41 | Mohammadi et al[52], 2012 | Hydrocephalus (congenital) | 7 months | 12 months | 5 months | No | Right | A | R |
| 42 | Alladi et al[53], 2012 | Hydrocephalus with NTD (congenital) | Neonate | 20 months | 19 months | No | Left | J | R |
| 43 | Gupta et al[54], 2012 | Hydrocephalus (congenital) | 6 months | 24 months | 18 months | No | Right | A | R |
| 44 | Kundal et al[55], 2012 | Hydrocephalus (congenital) | 6 months | 24 months | 18 months | No | Right | A | R |
| 45 | Rivero-Garvía et al[56], 2013 | Hydrocephalus (post infective) | 12 months | 72 months | 60 months | No | Right | A | R |
| 46 | Elizabeth et al[57], 2013 | Hydrocephalus (post-haemorrhagic) | 2 months | 14 months | 12 months | No | Right | A | R |
| 47 | Ghasemi and Ashouri[58], 2013 | Hydrocephalus (congenital) | 2 months | 9 months | 7 months | No | Right | A | R |
| 48 | Shahizon et al[59], 2013 | Hydrocephalus (congenital) | N/A | 14 years | 7 months | Yes | Left | F | R |
| 49 | Panda et al[60], 2013 | Hydrocephalus (congenital) | 18 months | 60 months | 42 months | No | Left | A | R |
| 50 | Ojo et al[61], 2013 | Hydrocephalus (congenital) | 10 months | 15 months | 5 months | No | Left | A | R |
| 51 | Erikci et al[62], 2014 | Hydrocephalus (congenital) | 2 months | 48 months | 46 months | No | Left | A | R |
| 52 | Erikci et al[62], 2014 | Hydrocephalus with NTD (congenital) | Neonate (6 days) | 2 months | 2 months | No | Right | A | R |
| 53 | Shankar et al[63], 2014 | Hydrocephalus (congenital) | Neonate (1 month) | 12 months | 11 months | No | Right | A | R |
| 54 | Basaran et al[64], 2014 | Hydrocephalus (congenital) | Neonate (12 days) | 6 months | 5 months | No | Right | C | R |
| 55 | Kumar and Jagannathan[65], 2015 | Hydrocephalus (congenital) | 3 months | 11 months | 8 months | No | Left | A | R |
| 56 | Bayindir et al[66], 2015 | Hydrocephalus with NTD (congenital) | 3 months | 18 months | 2 months | Yes | Right | A | R |
| 57 | Sarangi et al[67], 2016 | Hydrocephalus (post infective) | 12 months | 48 months | 36 months | No | Left | G | R |
| 58 | Singh et al[68], 2016 | Hydrocephalus (congenital) | Neonate | 8 months | 7 months | No | Right | A | R |
| 59 | Singh et al[68], 2016 | Hydrocephalus with NTD (congenital) | 7 months | 14 months | 7 months | No | Right | A | R |
| 60 | Ricci et al[69], 2016 | Hydrocephalus (congenital?) | 24 months | 10 years | N/A | Yes | Left | I | R |
| 61 | Hung et al[70], 2017 | Hydrocephalus (post-haemorrhagic) | 3 months | 5 months | 2 months | No | Right | A | R |
| 62 | Javadi et al[71], 2017 | Hydrocephalus (congenital) | 8 months | 10 months | 2 months | No | Right | A | R |
| 63 | Kumar et al[72], 2017 | Hydrocephalus with NTD (congenital) | Neonate (14 days) | 48 months | 47 months | No | Right | A | R |
| 64 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Right | A | R |
| 65 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Right | A | R |
| 66 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Right | A | R |
| 67 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Right | A | R |
| 68 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Right | A | R |
| 69 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Left? | A | R |
| 70 | Fernandez-Ibieta et al[73], 2017 | Hydrocephalus (congenital) | Neonate | 7.5 months (mean) | 6.5 months (mean) | No | Left | D | R |
| 71 | Bawa et al[74], 2017 | Hydrocephalus with NTD (congenital) | 3 months | 7 months | 4 months | No | Right | A | R |
| 72 | Bawa et al[74], 2017 | Hydrocephalus (congenital) | 48 months | 51 months | 3 months | No | Right | A | R |
| 73 | Bawa et al[74], 2017 | Hydrocephalus (congenital) | 10 months | 15 months | 5 months | No | Right | A | R |
| 74 | Bawa et al[74], 2017 | Hydrocephalus (congenital) | 48 months | 52 months | 4 months | No | Right | I | R |
| 75 | Paterson et al[75], 2018 | Hydrocephalus (congenital?) | 11 months | 13 months | 1 week | Yes | Right | C | R |
| 76 | Deora et al[76], 2018 | Hydrocephalus (congenital) | 3 months | 12 months | 9 months | No | Right | A | R |
| 77 | Shah et al[77], 2018 | Hydrocephalus (post infective) | 13 months | 30 months | 17 months | No | Right | A | R |
| 78 | Nawaz et al[78], 2018 | Hydrocephalus (post infective) | 2 months | 6 months | 4 months | No | Right | A | R |
| 79 | Burhan et al[79], 2018 | Hydrocephalus (congenital?) | N/A | 24 months | N/A | No | Right | A | R |
| 80 | Ezzat et al[80], 2018 | Hydrocephalus (congenital) | 2 months | 3 months | 1 month | No | Left | A | R |
| 81 | Ezzat et al[80], 2018 | Hydrocephalus (congenital) | 2 months | 3 months | 1 month | No | Right? | A | R |
| 82 | Ezzat et al[80], 2018 | Hydrocephalus (congenital) | Neonate (1 month) | 2 months | 1 month | No | Right? | A | R |
| 83 | Soufiany et al[81], 2018 | Hydrocephalus (congenital) | Neonate (1 month) | 6 months | 7 days | Yes | Bilateral | E | R |
| 84 | Soufiany et al[81], 2018 | Hydrocephalus (N/A) | 56 months | 60 months | 4 months | No | Right | A | R |
| 85 | Dharmajaya[82], 2018 | Hydrocephalus (congenital) | 5 months | 12 months | 7 months | No | Right | A | R |
| 86 | Rajendran et al[83], 2019 | Hydrocephalus (congenital) | 3 months | 36 months | 33 months | No | Right | A | R |
| 87 | Shedrov et al[84], 2019 | Hydrocephalus (congenital) | 2 months | 9 years | 48 months | Yes | Right | F | R |
| 88 | Shedrov et al[84], 2019 | Hydrocephalus (post hemorrhage) | 3 months | 24 months | 21 months | No | Right | A | R |
| 89 | Abdoli et al[85], 2019 | Hydrocephalus (congenital?) | 23 months | 24 months | 10 days | No | Right | A | R |
| 90 | Abdoli et al[85], 2019 | Hydrocephalus (congenital) | 2 months | 12 months | 10 months | No | Right | A | R |
| 91 | Abdoli et al[85], 2019 | Hydrocephalus (congenital) | 5 months | 17 months | 12 months | No | Right | A | R |
| 92 | Abdoli et al[85], 2019 | Hydrocephalus (congenital) | 8 months | 12 months | 4 months | No | Left | A | R |
| 93 | Chan et al[86], 2019 | Hydrocephalus (congenital) | Neonate (16 days) | 18 months | 17 months | No | Right | H | R |
| 94 | Prates Junior et al[87], 2020 | Hydrocephalus (congenital) | Neonate (3 days) | 1.6 months | 1 month | No | Left | I | R |
| 95 | Gupta and Gupta[88], 2020 | Hydrocephalus (congenital) | Neonate (1 month) | 11 months | 3 months | Yes | Right | A | R |
| 96 | Khaliq et al[89], 2021 | Hydrocephalus with NTD (congenital) | Neonate | 12 months | 11 months | No | Right | A | R |
| 97 | Agarwal et al[90], 2021 | Hydrocephalus (post infective) | 15 months | 18 months | 3 months | No | Right | D | R |
| 98 | Javed et al[91], 2021 | Hydrocephalus (congenital) | 5 months | 5 months | 4 days | No | Right | A | R |
| 99 | Hauser et al[92], 2021 | Hydrocephalus with NTD (congenital) | Neonate (3 weeks) | 23 months | 22 months | No | Right | B | R |
| 100 | Ahmed et al[93], 2021 | Hydrocephalus (congenital) | Neonate (1 month) | 8 months | 7 months | No | Left | A | R |
| 101 | Alkhudari et al[94], 2022 | Hydrocephalus (post-haemorrhagic) | Neonate? | 6 months | 5 months? | No | Right | J | R |
| 102 | Taha et al[95], 2022 | Hydrocephalus with NTD (congenital) | Neonate (1 month) | 3 months | 2 months | No | Right | A | R |
| 103 | Topp et al[96], 2023 | Hydrocephalus with NTD (congenital) | Neonate (6 days) | 16 months | 16 months | No | Right | A | R |
| 104 | Tzeng et al[97], 2023 | Hydrocephalus (post-haemorrhagic) | Neonate? | 2 months? | 1 month | No | Right | K | R |
| 105 | Chettahi et al[98], 2023 | Hydrocephalus (congenital) | Neonate (1 month) | 60 months | 59 months | No | Right | A | R |
| 106 | Joseph et al[99], 2023 | Hydrocephalus (congenital) | Neonate (12 days) | 6 months | 1 month | Yes | Left | K | ?? |
| 107 | Chanchlani et al[100], 2023 | Hydrocephalus (congenital) | 2 months | 2 months | 5 days | No | Right | A | R |
| 108 | Fitra and Asrizal[101], 2023 | Hydrocephalus (congenital) | Neonate (1 month) | 1.5 months | 2 weeks | No | Left | J | R |
| 109 | Kiyak and Cansaran[102], 2023 | Hydrocephalus with NTD (congenital) | 1.5 months | 4 months | 2 months | No | Left | B | R |
| 110 | Darwish et al[103], 2023 | Hydrocephalus (congenital) | Neonate (1 month) | 6 months | 5 months | No | Right | A | R |
| 111 | Castrillo et al[104], 2023 | Hydrocephalus (congenital) | Neonate (1 month) | 3 months | 2 months | No | Right | B | R |
| 112 | Javeed et al[105], 2024 | Hydrocephalus with NTD (congenital) | 6.5 months | 7 months | 13 days | No | Right | A | R |
| Number | Ref. | Indication for VPS insertion | Age VPS insertion | Age scrotal migration | Interval | VPS (revision) | Site (right/left) | Operative procedures executed | Outcome |
| 1 | Rehm et al[106], 1997 | Hydrocephalus (brain tumor) | 46 years | 50 years | 4 years | No | Right | G | Death |
| 2 | Korfias et al[107], 2013 | Hydrocephalus (arachnoid cyst) | 29.6 years | 30 years | 6 months | No | Left | A | R |
| 3 | Lee et al[108], 2015 | Hydrocephalus (NPH) | 65 years | 65 years | 7 days | No | Right | D | R |
| 4 | Foster et al[109], 2019 | Hydrocephalus (post haemorrhagic) | Infant? (N/A) | 27 years | Days? | Yes | Right | B | R |
| 5 | Perret et al[110], 2021 | Hydrocephalus (N/A) | 16 years | 22 years | 6 years | No | Right | I | R |
| 6 | Khoudir et al[111], 2022 | Hydrocephalus (NPH) | 75 years | 75.4 years | 4 months | No | Right | C | R |
| 7 | Ndongo Sonfack et al[112], 2022 | Hydrocephalus (NPH) | 73 years | 73 years | 2 days | Yes | Right | A | R |
| 8 | Vicaría and Unzué[113], 2022 | Hydrocephalus (NPH) | 80 years | 80.2 years | 47 days | No | Right | G | Death |
| Publication (year-wise) | Group A | Group B | Total (A+B) | Standard deviation | Mean | 95%CI (margin of error) |
| 1974–1980 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1981–1985 | 2 | 0 | 2 | 1 | 1 | 1-1.386 (± 138.59%) |
| 1986–1990 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1991–1995 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1996–2000 | 5 | 1 | 6 | 2 | 3 | 3-2.772 (± 92.40%) |
| 2001–2005 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 2006–2010 | 8 | 0 | 8 | 4 | 4 | 4-5.544 (± 138.59%) |
| 2011–2015 | 17 | 2 | 19 | 7.5 | 9.5 | 9.5-10.394 (± 109.42%) |
| 2016–2020 | 22 | 1 | 23 | 10.5 | 11.5 | 11.5-14.552 (± 126.54%) |
| 2021–2024 | 17 | 4 | 21 | 6.5 | 10.5 | 10.5-9.009 (± 85.80%) |
| Total | 87 | 8 | 95 | 39.5 | 47.5 | 47.5-54.744 (± 115.25%) |
| Publication (year-wise) | Group A cases | Group B cases | Total (A+B) | Standard deviation | Mean | 95%CI (margin of error) |
| 1974–1980 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1981–1985 | 5 | 0 | 5 | 2.5 | 2.5 | 2.5-3.465 (± 138.59%) |
| 1986–1990 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1991–1995 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 1996–2000 | 8 | 1 | 9 | 3.5 | 4.5 | 4.5-4.851 (± 107.79%) |
| 2001–2005 | 4 | 0 | 4 | 2 | 2 | 2-2.772 (± 138.59%) |
| 2006–2010 | 9 | 0 | 9 | 4.5 | 4.5 | 4.5-6.237 (± 138.59%) |
| 2011–2015 | 18 | 2 | 20 | 8 | 10 | 10-11.087 (± 110.87%) |
| 2016–2020 | 39 | 1 | 40 | 19 | 20 | 20-26.333 (± 131.66%) |
| 2021–2024 | 17 | 4 | 21 | 6.5 | 10.5 | 10.5-9.009 (± 85.80%) |
| Total | 112 | 8 | 120 | 52 | 60 | 60-72.068 (± 120.11%) |
| Ref. | Number of cases | Literature retrieved | Reason for exclusion |
| Cooper et al[114], 1976 | 1 | Abstract | Full text not retrieved |
| Villarejo et al[115], 1980 | 1 | Abstract | Full text not retrieved |
| Kobayashi et al[116], 1987 | 2 | Abstract | Full text not retrieved |
| Albala et al[117], 1989 | 1 | Abstract | Full text not retrieved |
| Celik et al[118], 2005 | 1 | Full text | Incomplete case details |
| Aly et al[119], 2015 | 3 | Full text | Incomplete case details |
| Vagholkar et al[120], 2016 | 1 | Full text | Incomplete case details |
| Montasr[121], 2020 | 1 | Abstract | Incomplete case details |
| Wu et al[122], 2024 | 1 | Abstract | Full text not retrieved |
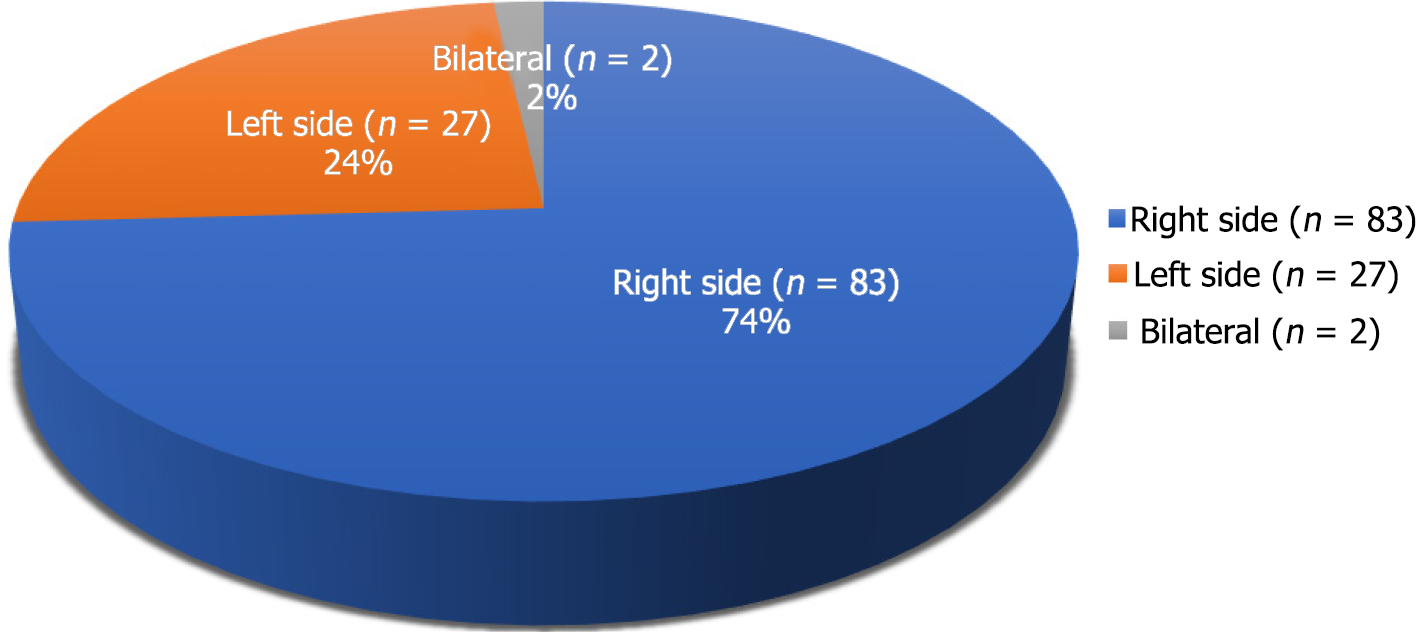
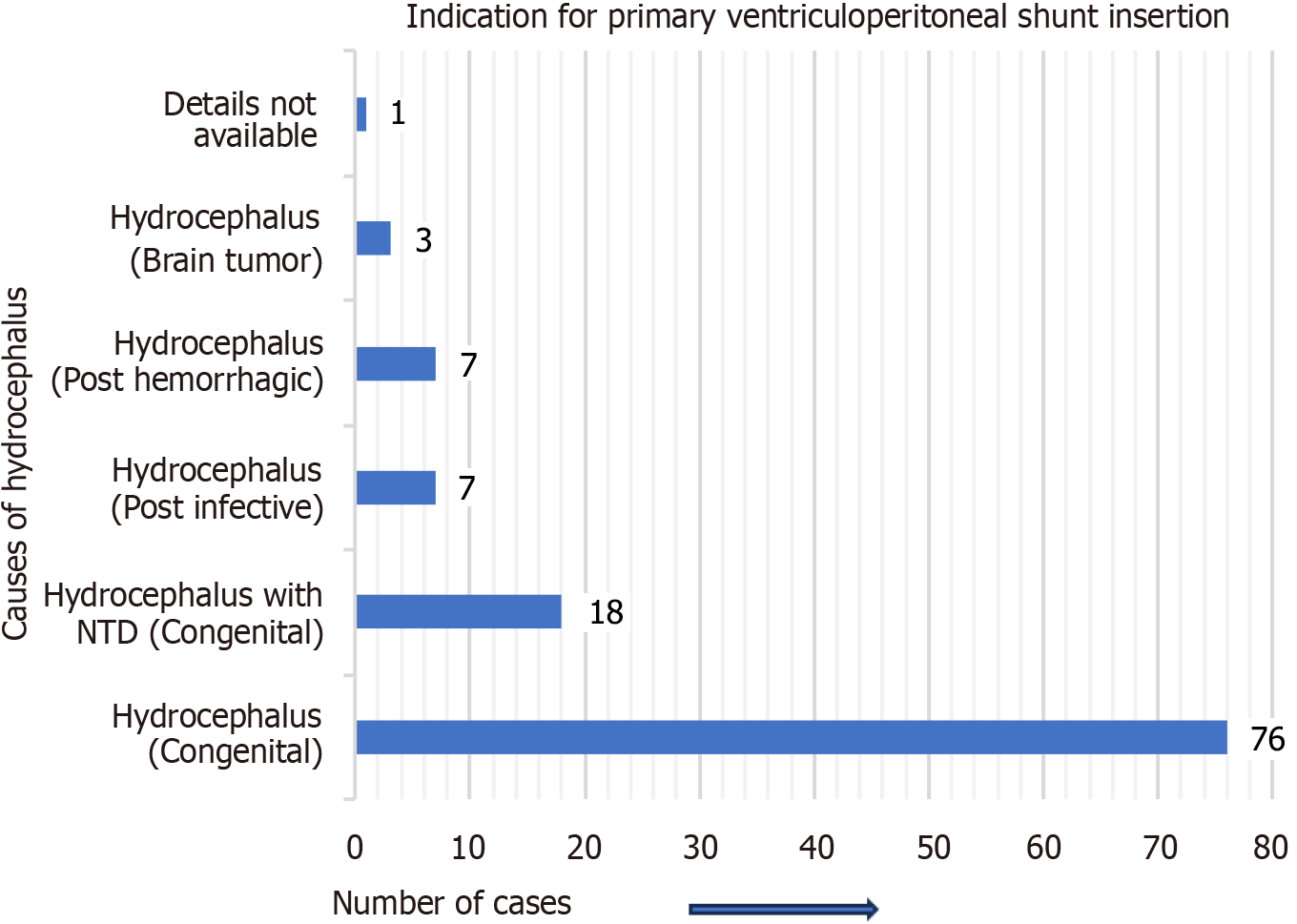
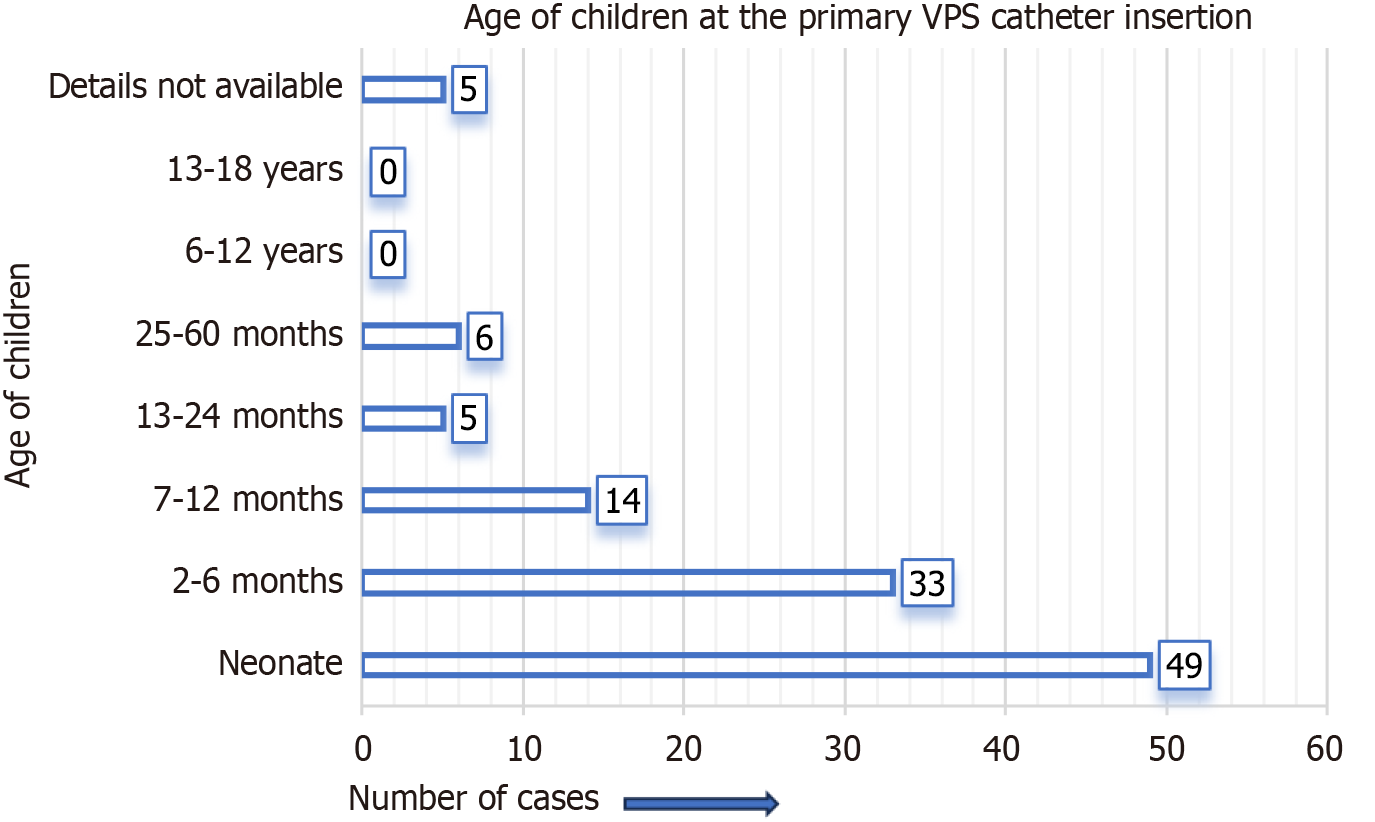
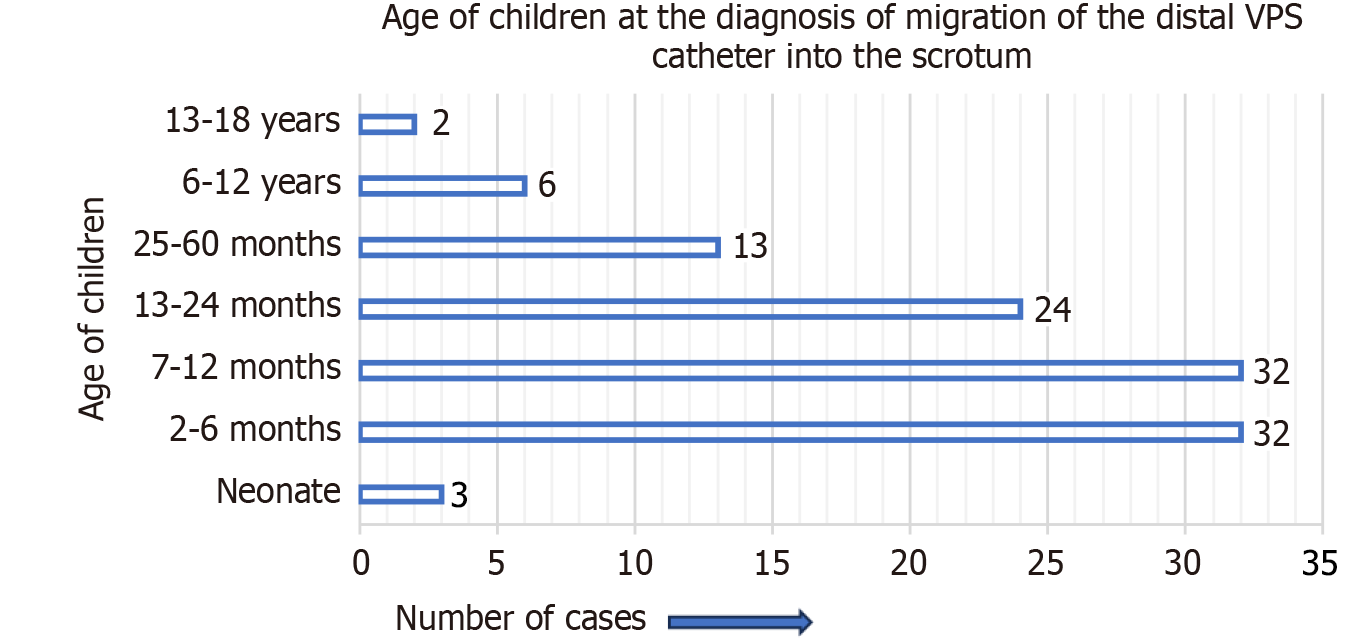
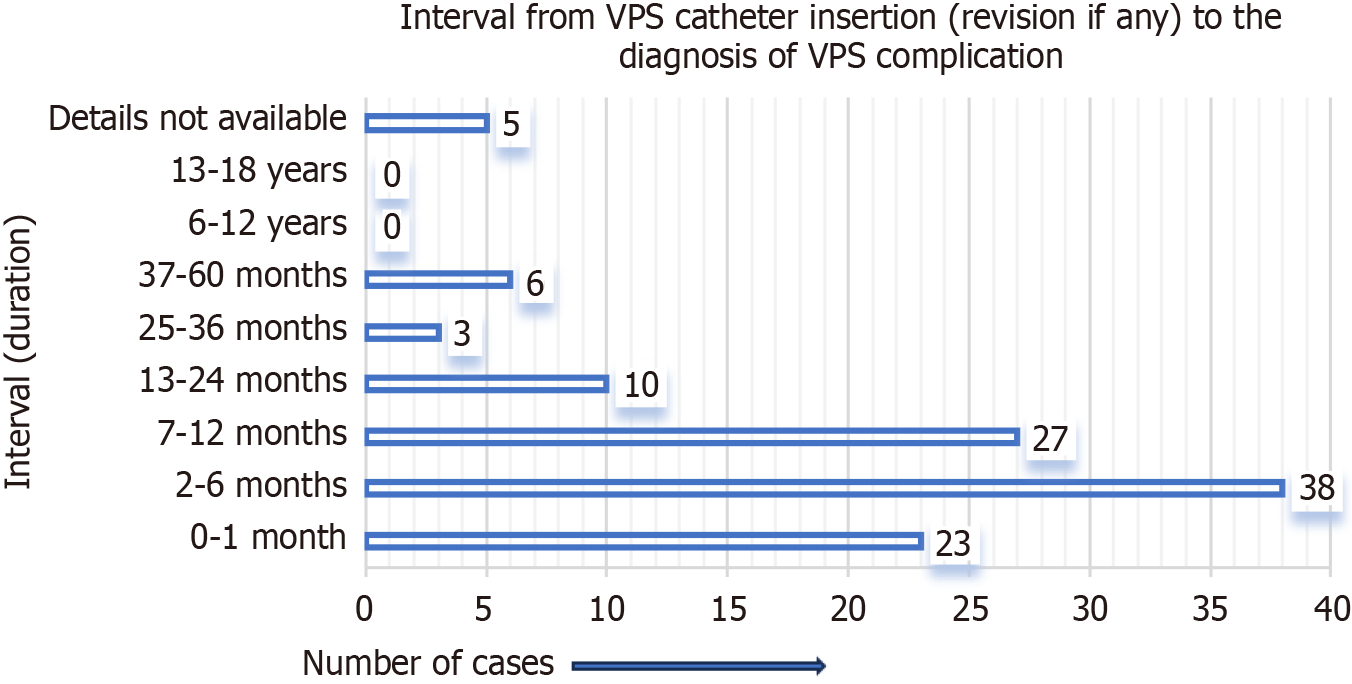
Figure 3 represents the site of migration of the distal VPS catheter into the scrotum in children. It was most frequently observed on the right side of the scrotum, not only in group A cases but also in group B cases. For group A cases (infants and children), the indications (causes of hydrocephalus) for the initial/primary VPS catheter insertion are detailed in Figure 4. In four-fifths of the group A cases, the indication for the primary VPS insertion was congenital hydrocephalus. For group A cases, the age of children at the time of primary VPS insertion is detailed in Figure 5. In, more than four-fifths of children (n = 96), the initial VPS catheters were implanted during infancy. For group A cases, the age of children at the time of diagnosis and the treatment provided for the scrotal migration of the distal VPS catheter are provided in Figure 6. In four-fifths of the children, their age was ≤ 24 months at the time of clinical diagnosis and treatment carried out for the VPS complication mentioned above. For group A cases, the interval from the primary VPS insertion or last VPS revision if any, to the diagnosis of the above-described CSF shunt complication is detailed in Figure 7. In more than three-fourths (n = 78) of children, this VPS complication was detected within 12 months after the initial VPS insertion/last shunt revision.
For group B (adults and older people) cases, the mean age at the time of diagnosis of the VPS complication mentioned was 52.7 years and ranged from 22 years to 80 years. For group B cases, the indication for initial VPS catheter placement was normal pressure hydrocephalus (NPH) for fifty percent of the cases. For group B cases, the interval was 6 months or less for three- fourths (n = 6) cases and ranged from 2 days to 6 months.
The chief complaint for the entire group A and group B cases was the scrotal swelling of variable duration, which occurred within a day to a few months. In 40% (n = 45) of children, the duration of the symptoms ranged from a day to 30 days. Clinical examination of the entire group A and group B cases revealed that a tube-like structure (distal VPS catheter) was detected in the scrotum. None of the group A and group B cases had the classical features of meningitis. Clinical features of peritonitis were also absent in children, while one of group B (80-year-old) cases had features of peritonitis and scrotal abscess.
A plain Roentgenogram of the abdomen including the inguinoscrotal area, confirmed the presence of a distal VPS catheter into the scrotum in all the cases. Various other diagnostic modalities ordered before the operative therapy for VPS complication mentioned above were cranial computed tomography (CT) scan n = 16 for group A cases and n = 4 for group B cases and CT scan of the abdomen including the scrotum n = 2 for group A cases and n = 3 for group B cases.
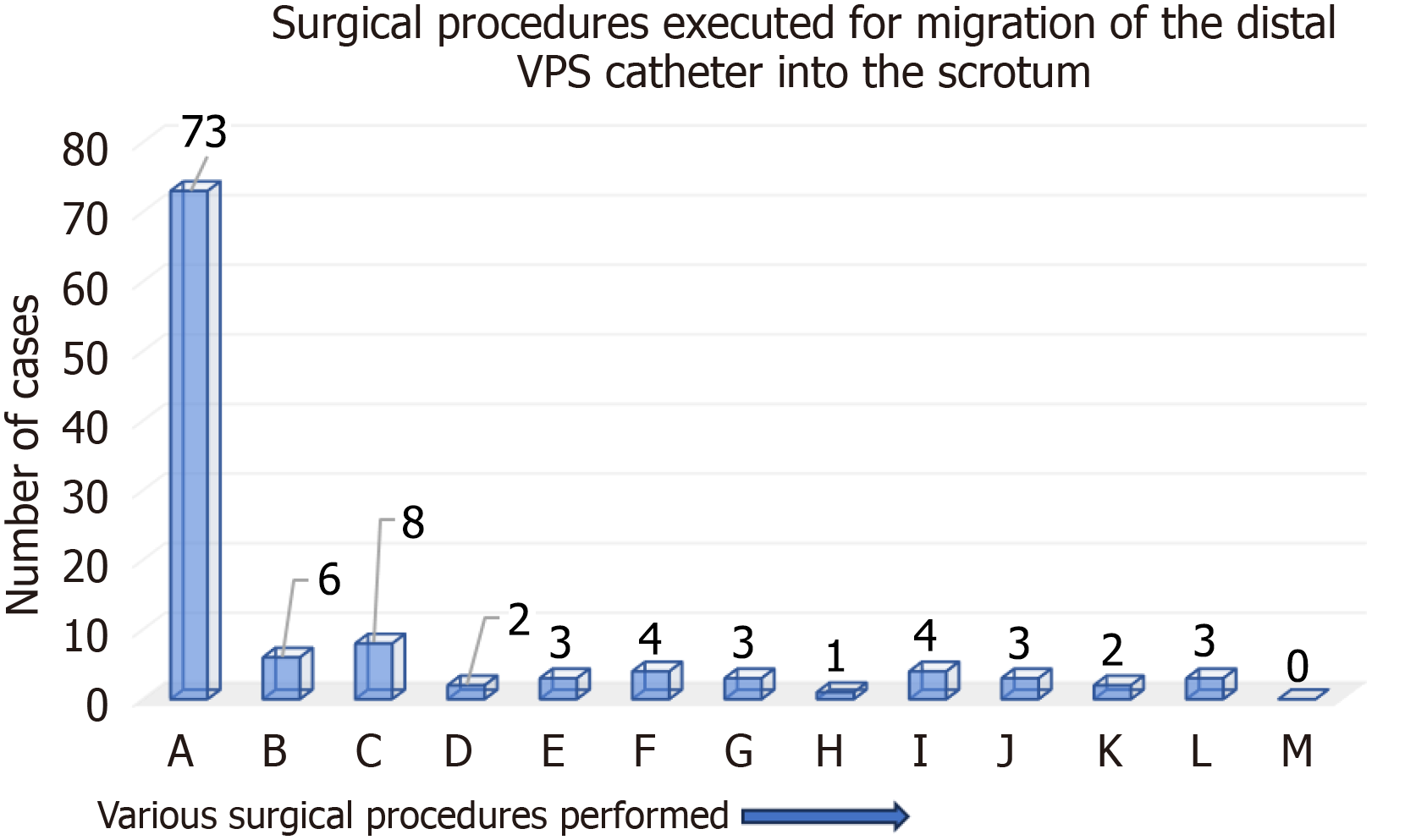
The various surgical procedures performed for the treatment of the above-mentioned VPS complication for group A cases are detailed in Figure 8. In two-thirds (n = 73) of children, repositioning of migrated distal VPS catheter back into the peritoneal cavity and inguinal herniotomy were carried out. For group B cases, the surgical procedures performed by the authors were not uniform. Two of the cases were managed by the removal of their entire VPS catheter, and another n = 2 of the cases was managed by repositioning of migrated distal VPS catheter back into the peritoneal cavity and inguinal herniotomy. A comparison of the results obtained for groups A and B is detailed in Table 6. This present systematic review revealed n = 2 deaths among group B and none from group A cases.
| Details | Description | Group A | Group B | Total (A+B) | Standard deviation | Mean | 95%CI (margin of error) |
| Cases | Total | 112 (93.33) | 8 (6.66) | 120 (100) | 52 | 60 | 60-72.068 (± 120.11%) |
| Location | Right side | 83 (69.16) | 7 (5.83) | 90 (75) | 38 | 45 | 45-52.665 (± 117.03%) |
| Left side | 27 (22.5) | 1 (0.83) | 28 (23.33) | 13 | 14 | 14-18.017 (± 128.69%) | |
| Bilateral | 2 (1.66) | 0 (00) | 2 (1.66) | 1 | 1 | 1-1.386 (± 138.59%) | |
| Indication (ventriculoperitoneal shunt insertion) | Congenital pathologies | 94 (78.33) | 0 (00) | 94 (78.33) | 47 | 47 | 47-65.139 (± 138.59%) |
| Post-hemorrhagic | 7 (5.83) | 1 (0.83) | 8 (6.66) | 3 | 4 | 4-4.158 (± 103.94%) | |
| Brain tumor | 3 (2.5) | 2 (1.66) | 5 (4.16) | 0.5 | 2.5 | 2.5-0.693 (± 27.72%) | |
| Normal pressure hydrocephalus | 0 (00) | 4 (3.33) | 4 (3.33) | 2 | 2 | 2-2.772 (± 138.59%) | |
| Interval | 0-12 months | 88 (73.33) | 6 (5) | 94 (78.33) | 41 | 47 | 47-56.823 (± 120.90%) |
| 13-24 months | 10 (8.33) | 0 (00) | 10 (8.33) | 5 | 5 | 5-6.93 (± 138.59%) | |
| Surgical procedure executed | Group A | 73 (60.83) | 2 (1.66) | 75 (62.5) | 35.5 | 37.5 | 37.5-49.2 (± 131.20%) |
| Group B | 6 (5) | 1 (0.83) | 7 (5.83) | 2.5 | 3.5 | 3.5-3.465 (± 98.99%) | |
| Group C | 8 (6.66) | 1 (0.83) | 9 (7.5) | 3.5 | 4.5 | 4.5-4.851 (± 107.79%) | |
| Mortality | Postoperative | 0 (00) | 2 (1.66) | 2 (1.66) | 1 | 1 | 1-1.386 (± 138.59%) |
VPS insertion is preferred over ventriculoatrial shunt insertion for the treatment of hydrocephalus, not only in infants and children but also in adults and older people[123]. VPS catheter insertion is associated with various complications ranging from infection, shunt malfunction, shunt obstruction, shunt disconnection[123-125], and shunt catheter migration to the different body parts[126], and through natural orifices, and are more frequently documented in children than adults[8-11]. Many VPS complications[14-17] may require shunt revisions[123-126].
Ninety-five manuscripts were identified and included in the present systematic review of the management of migration of distal VPS catheters into the scrotum and extracted from the literature published from 1974 to June 2024[19-113]. Among a total of n = 95 manuscripts, only n = 8 was published and are available in languages other than English[35,40,43,48,56,58,84,104]. For n = 8 of the above manuscripts the abstracts are available in English, and the other parts of the manuscripts were translated into English using the “Google Translate” for extracting the desired information. The remaining n = 87 manuscripts are published and available in English[19-34,36-39,41,42,44-47,49-55,57,59-83,85-103,105-113]. Ninety-four of the manuscripts included in this review are full-text articles. Only one of the manuscripts included in this review is the abstract of a case report[32].
Further review of the literature included for the systematic review, revealed that more than two-thirds (n = 67) of manuscripts, which included a total of n = 67 cases of the migration of distal VPS catheter into the scrotum, were published as case report and detailed the isolated cases[19-22,24,25,27-33,35,36,38-40,43,45-47,49-54,56,58,60,61,64-67,69,70,72,75,76,78,82,83,86,89-91,93-106,108,110-113]. Six manuscripts, which included n = 24 cases of VPS complication described above, were published as case series[23,34,55,73,74,85]. Manuscripts that included more than two cases of VPS complications described above were considered/counted as case series[23,34,73,74,85]. Only one of the cases was extracted from a manuscript published as a case series[55]. Three of the articles, which included details for n = 6 cases, described two cases of the VPS complication in each manuscript and were also published as case reports[62,68,84]. Two of the literature that were published and available as original articles, which included n = 4 cases of VPS complication described[79,80]. Four of the manuscripts contained details of n = 6 cases and were published as review articles[42,81,87,92]. The remaining n = 13 of the manuscripts, which included n = 13 cases, were published and available under the headings of medical images[41,57,59,63,109], letters to the editor[44,48,71,77,88,107], and correspondence or commu
A total of 95 articles were included in the systematic review of which 92% (n = 87) of the manuscripts were related to the VPS catheter migration into the scrotum, which occurred in children. A further literature review confirmed that two-thirds (n = 63) of these manuscripts were published in the past 14 years, from 2011 to June 2024. Although the first case of migration of the distal VPS catheter into the scrotum was reported in 1974, in a child, there were only n = 23 such cases reported in children between 1974 and 2005. This VPS complication is rarer in adults and older individuals, with only n = 8 cases published as of June 2024. A deeper review of the literature found that two-thirds (n = 81, group A = 74, and group B = 7) of the migration of distal VPS catheters into the scrotum were reported/published during the past 14 years from 2011 to June 2024. Half (n = 61) of the cases (both groups) were published during the past 9 years, from 2016 to June 2024. In children, only n = 25 such cases were reported/published between 1974 and 2000. Nine manuscripts, including n = 12 cases of the VPS complication described above, were excluded from this review due to incomplete case information or inability to retrieve the full texts of the articles[114-122].
In children, migration of distal VPS catheter to the right side of the scrotum was more common and evidenced in three-fourths (n = 83) of cases. The shunt catheter was migrated into the left side of the scrotum in one-fourths (n = 27) of children[23,24,30,34,35,43,48-51,53,59-62,65,67,69,73,80,85,87,93,99,101,102]. Migration of VPS catheter to both sides of the scrotum (bilateral migration) was rare and only observed in two children[26,81]. In adults and older people, the shunt catheter was migrated into the right side of the scrotum in n = 7 cases[106,108-113]. In one case, the shunt catheter was migrated into the left side of the scrotum[107].
The indications for primary VPS catheter insertion in children were most frequently for congenital hydrocephalus while in adults and older people, it was NPH in half of the cases. For group A cases (children), the indications (causes of hydrocephalus) for the initial/primary VPS catheter insertion in order of frequency were; congenital hydrocephalus/hydrocephalus of congenital origin (n = 76; 67.8%), hydrocephalus associated with neural tube defects (n = 18; 16.07%), post-infective hydrocephalus (n = 7; 6.25%), hydrocephalus developed after the ventricular hemorrhage (n = 7; 6.25%), hydrocephalus developed due to brain tumor (n = 3), and for one child the cause was not mentioned/not available. For group B cases, the indications (causes of hydrocephalus) for the primary VPS catheter insertion were NPH in half (n = 4) of the cases, followed by the hydrocephalus secondary to the brain tumor (n = 1), arachnoid cyst (n = 1), post-hemorrhagic hydrocephalus (n = 1), and the cause for hydrocephalus was not known for one case.
The initial/primary VPS catheter insertions were carried out during infancy in more than four-fifths (n = 96) of children. Of the above, at n = 49 occasions it was implanted during the neonatal period. In n = 5 children their age was 13 months to 24 months at the time of VPS catheter insertion, and for n = 6 children their age ranged from 25 months to 60 months, at the time of VPS catheter insertion. The mean age at initial VPS catheter insertion was 55.28 years for group B cases.
In more than four-fifths (n = 91) of children (group A), their age was 0 day to 24 months at the time of diagnosis and the treatment provided for the presence of the distal VPS catheter into the scrotum. In n = 67 (60%) children, their age was 0 day to 12 months. For group B cases, the mean age at the time of diagnosis of VPS complication mentioned above was 52.7 years, ranging from 22 years to 80 years[106-113].
For group A cases (children), in more than three-fourths (n = 88) cases the interval from the primary VPS insertion/last VPS revision if any, to the diagnosis of the migration of the VPS catheter into the scrotum was ≤ 12 months. In one-fourths (n = 23) of children, the VPS complication occurred within a month of VPS insertion. The VPS complication described was not frequent after 36 months of shunt implantation/shunt revision, and it was documented only in n = 6 cases[56,60,62,72,84,98]. For group B cases, the interval ranged from 2 days to 6 years. In n = 6 of group B cases, it was ≤ 6 months[107-109,111-113]. In n = 2 of group B cases, it occurred 4 years and 6 years after the VPS catheter insertion[106,110].
For the entire group A and group B cases, the chief complaint was the scrotal swelling after the VPS catheter insertion or VPS revision, if any, and scrotal swelling occurred within a day to a few months. In 40% (n = 45) of children, the symptom’s duration ranged from 1 day to 30 days. In the entire group A and group B cases, the clinical examination revealed that the distal VPS catheter was detected/palpated in the involved side of the scrotum. The classical signs of meningitis were not detected in any of the group A and group B cases. Clinical signs of peritonitis were also absent in children, while one of group B (80-year-old) cases had features of peritonitis and scrotal abscess. For confirmation of the clinical diagnosis, a plain Roentgenogram of the abdomen including the inguinoscrotal area/X-Ray series was mostly carried out for the cases, and that confirmed the presence of a distal VPS catheter into the involved scrotum in all the cases. Additionally, before surgical therapy, cranial CT scan was ordered for n = 16 children, and n = 4 of group B cases. CT scan of the abdomen including the inguinoscrotal area was ordered for n = 2 children, and n = 3 of group B cases.
In children, for migration of distal VPS catheter into the scrotum, the preferred surgical procedure was the repositioning of the migrated distal VPS catheter back into the peritoneal cavity and inguinal herniotomy, and it was carried out for two-thirds (n = 73) cases. In n = 8 children, the part of the distal shunt catheter was removed (cut short), the remaining peritoneal catheter was repositioned back into the peritoneal cavity, and an inguinal herniotomy was carried out[22,25,28,43,45,47,64,75]. In two group B cases, the repositioning of migrated distal VPS catheter back into the peritoneal cavity, and inguinal herniotomy were carried out[107,112]. In another n = 2 group B case, the entire VPS catheter was removed without inguinal herniotomy[106,113].
VPS catheter disconnection/fracture was documented in n = 8 cases, seven among children and one in a 22-year-old person[24,26,50,53,59,69,81,110]. In two children, there was the migration of the VPS shunt catheters into both sides of the scrotum, on one side it was an abandoned/disconnected peritoneal catheter, and on another side, it was a working distal VPS catheter. For both children, the abandoned/disconnected peritoneal catheter was removed, the working peritoneal catheter repositioned back into the peritoneal cavity, and bilateral inguinal herniotomies were carried out[26,81]. In another two children, the entire VPS catheter (including the disconnected peritoneal catheter) was removed and re-VPS insertion/immediate VPS revision, and inguinal herniotomy were carried out[24,69]. In a 22-year-old person, the VPS catheter was removed, and immediate VPS revision and inguinal hernia repair were carried out for the migrated VPS catheter[110].
The present systematic review revealed two deaths among group B cases[106,113]. A 46-year-old person was diagnosed with an intraventricular tumor (ependymoma) causing hydrocephalus and was operated upon (tumor excision and VPS catheter insertion). Because of severe brain damage, he was in a persistent vegetative condition. Four years after the above surgical procedures, his distal VPS catheter migrated into the right scrotum and extruded/eroded out through the scrotal skin, and was treated by removal of the entire VPS catheter. A new VPS catheter was inserted once the CSF culture became sterile. He died of poor general condition during the treatment[106]. A VPS catheter was inserted for NPH in an 80-year-old person. His distal shunt catheter was migrated into the right scrotum and treated by removal of the entire VPS catheter. He was also diagnosed with a scrotal abscess, developed peritonitis, and died within a few hours during the management[113]. There were no deaths among the n = 112 children, treated for migration of the VPS shunt catheter into the scrotum.
Following are the possible factors responsible for the migration of the distal VPS catheter into the scrotum more in infants and young children, than the older children, adults, and older people. The shunt catheter also migrated more often into the right scrotum: (1) Persistent processus vaginalis; (2) Infants and children younger than 24 months; (3) Long length of an intra-abdominal part of the peritoneal part of the VPS catheter; (4) Increasing intra-abdominal pressure due to diversion of CSF into the peritoneal cavity; and (5) VPS revision in the past.
The present review also revealed that the persistence of processus vaginalis (either as patent process vaginalis or as an inguinal hernia) was evidenced during the operative procedures carried out for migration of the distal VPS catheter into the scrotum in two-thirds of infants, and half of the younger children (13-24 months of age). This finding further supports the theory that persistent processus vaginalis is one of the important factors responsible for the VPS complication described above. In general, processus vaginalis remains patent in 80% of the children at birth, and the patency decreases with the advancing age[127]. Twenty percent of male children may have a patent processus vaginalis at the age of 24 months. Chen et al[128] in 2011 reported their findings that children who received VPS for the treatment of hydrocephalus at the age ≤ 5 years, were more prone to the development of inguinal hernia, more so during the first two years after the VPS catheter insertion[128]. In a Cohort study, Wu et al[129] included n = 1568 children aged 0 day to 5 years who received VPS catheters and were followed for the development of an inguinal hernia. They also concluded that an inguinal hernia is more likely to develop in neonates after VPS surgery than in infants, toddlers, and preschool-aged children[129].
In 60% (n = 67) of the cases, the VPS complication occurred during infancy (0-12 months), and in another one-fourths (n = 24) of cases, their age was 13-24 months at the time of diagnosis/treatment of migration of the distal VPS catheter into the scrotum. These findings correlate to the findings of patent processus vaginalis more during infancy, than older age. The insertion of a long length of the peritoneal part of the VPS catheter into the peritoneal cavity may be one of the factors responsible for the scrotal migration of the VPS catheter, as this complication was documented more in children than adults.
Diversion of CSF into the peritoneal cavity via a VPS catheter insertion may lead to accumulation of CSF into the peritoneal cavity. Excess CSF in a small peritoneal cavity in neonates and infants may further elevate the intra-abdominal pressure. The presence of CSF in the peritoneal cavity may lead to or help in increasing intra-abdominal pressure and may prevent obliteration of processus vaginalis or result in patent processus vaginalis. In a retrospective study of n = 430 children who received VPS catheters for the treatment of hydrocephalus and were followed for the development of inguinal hernia and hydrocele. The authors concluded that the raised intra-abdominal pressure may be the reason for the high occurrence of inguinal hernia and hydrocele after the insertion of the VPS catheter in these children[130].
It is not clear whether history of prior shunt revision, is also a factor responsible for the migration of shunt catheter into the scrotum. Although, the present literature review documented that n = 17; 15.1% of children had shunt revision before the shunt migration[20,24,26,31,35,37,43,46,50,59,66,69,75,81,84,88,99]. In the present review of n = 112 children diagnosed and treated for migration of shunt catheter into the scrotum, n = 83; 74% of the cases the migration was into the right scrotum, n = 27; 24% of shunt catheters migrated into the left side of the scrotum, and only two children had migration of VPS shunt catheters into both the scrotum. The above findings are probably related to the fact that in boys, approximately 60% of inguinal hernias are right-sided, 30% of them involve the left side and only 10% are bilateral inguinal hernias[127].
Comparing the results obtained from group A cases to group B cases revealed that, migration of the distal VPS catheter into the scrotum was significantly more frequently reported in children than adults and older individuals. Results further support that the younger the age of children the higher the chances of shunt catheter migration into the scrotum, as four-fifths (n = 91) of children were ≤ 24 months old, at the time of diagnosis of the VPS complication mentioned above. Hydrocephalus of congenital pathologies was the indication for primary VPS catheter insertion in four-fifths (n = 94) of children, while the most common indication for VPS catheter insertion in adults and older people was NPH and it was documented in fifty percent of the group B cases. In three-fourths of group A and group B cases, the aforementioned VPS complication was diagnosed within 12 months of shunt catheter insertion. The most preferred surgical therapy offered in children was repositioning of the migrated VPS catheter back into the peritoneal cavity and inguinal herniotomy, and it was opted by the various authors for n = 73 (65%) children. The above said surgical technique was opted only for two of the cases for group B cases. A further literature review documented n = 2 deaths, both the cases belong to group B, and there was no mortality among group A cases. One of the patients died of poor general condition, while another one died of peritonitis, caused by VPS complications. Both the cases died of either poor general condition or peritonitis, and none of them died of surgical techniques opted /surgical procedures executed.
The present study is a systematic review of already published literature and has limitations. Nine manuscripts (n = 3 full-text, and n = 6 abstract) including n = 12 cases of migration of the distal VPS catheter into the scrotum were excluded from the review, due to either incomplete case information or unavailability of the full text of the articles[114-122]. A limited number of literature/articles were retrieved through PubMed and PMC search. Therefore, other websites such as Scopus, Web of Science, Google Scholar, and ResearchGate were also utilized for the retrieval of manuscripts.
Migration of the distal VPS catheter into the scrotum is one of the rare complications of CSF diversion executed by the VPS catheter insertion for hydrocephalus. It was more frequently documented in infants and young children, although reported also in older children, adults, and older people. The migration of the shunt catheter into the right scrotum was more often, not only in children but also in adults and older people. In children, four-fifths of children’s age was 0-24 months, at the time of diagnosis and the treatment provided for the VPS complication described above. This VPS complication was diagnosed/occurred within ≤ 12 months after the primary VPS insertion or last VPS revision if any, in more than three-fourths of children. Two-thirds of children were treated by repositioning the migrated distal VPS catheter back into the peritoneal cavity and inguinal herniotomy.
| 1. | Hochstetler A, Raskin J, Blazer-Yost BL. Hydrocephalus: historical analysis and considerations for treatment. Eur J Med Res. 2022;27:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Greuter L, Ruf L, Guzman R, Soleman J. Open versus laparoscopic ventriculoperitoneal shunt placement in children: a systematic review and meta-analysis. Childs Nerv Syst. 2023;39:1895-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 3. | Ho YJ, Chiang WC, Huang HY, Lin SZ, Tsai ST. Effectiveness and safety of ventriculoperitoneal shunt versus lumboperitoneal shunt for communicating hydrocephalus: A systematic review and meta-analysis with trial sequential analysis. CNS Neurosci Ther. 2023;29:804-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Vlasak A, Okechi H, Horinek D, Albright AL. Pediatric Ventriculoperitoneal Shunts Revision Rate and Costs in High-Volume sub-Saharan Department. World Neurosurg. 2019;130:e1000-e1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Gonzalez DO, Mahida JB, Asti L, Ambeba EJ, Kenney B, Governale L, Deans KJ, Minneci PC. Predictors of Ventriculoperitoneal Shunt Failure in Children Undergoing Initial Placement or Revision. Pediatr Neurosurg. 2017;52:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Merkler AE, Ch'ang J, Parker WE, Murthy SB, Kamel H. The Rate of Complications after Ventriculoperitoneal Shunt Surgery. World Neurosurg. 2017;98:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014;81:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Ghritlaharey RK. Systematic Literature Review of the Management of Transanal Extrusion of Distal Ventriculoperitoneal Shunt Catheter. MJDRDYPV. 2022;15:629-659. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (3)] |
| 9. | Ghritlaharey RK. Migration of the distal ventriculoperitoneal shunt catheter into the stomach with or without trans-oral extrusion: A systematic literature review and meta-analysis. World J Clin Pediatr. 2023;12:331-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (6)] |
| 10. | Ghritlaharey RK. Systematic Review of Migration of Distal Ventriculoperitoneal Shunt Catheter into the Urinary Bladder with or Without Per-Urethral Extrusion. MJDRDYPV. 2022;15:840-853. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Ghritlaharey RK. Management of trans-vaginal extrusion of the distal ventriculoperitoneal shunt catheter: a systematic literature review from 1973 to 2021. Int J Res Med Sci. 2021;9:3416. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 12. | Hobbs E, Thompson DNP, Muthialu N, Silva AHD. Intracardiac migration of distal catheter-a rare complication of VP shunt insertion: case report and literature review. Childs Nerv Syst. 2024;40:587-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 13. | Li W, Li Y, Sun Y, Chen L. Migration of a Distal Ventriculoperitoneal Shunt Catheter Into the Pulmonary Vasculature: a Report of an Unusual Case and a Review of the Literature. J Craniofac Surg. 2019;30:e243-e244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Pant N, Singh S, Singh G, Kumar A, Rai RK, Rawat J, Wakhlu A. The wandering ventriculoperitoneal shunt and the scope of its salvage. Childs Nerv Syst. 2021;37:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Ghritlaharey RK. Extrusion of the distal ventriculoperitoneal shunt catheter through the umbilicus: a systematic literature review from 1973 to 2021. Int Surg J. 2021;8:3654. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Allouh MZ, Al Barbarawi MM, Asfour HA, Said RS. Migration of the distal catheter of the ventriculoperitoneal shunt in hydrocephalus: A Comprehensive Analytical Review from an Anatomical Perspective. Clin Anat. 2017;30:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Harischandra LS, Sharma A, Chatterjee S. Shunt migration in ventriculoperitoneal shunting: A comprehensive review of literature. Neurol India. 2019;67:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2603] [Cited by in RCA: 4469] [Article Influence: 1117.3] [Reference Citation Analysis (33)] |
| 19. | Ramani PS. Extrusion of abdominal catheter of ventriculoperitoneal shunt into the scrotum. Case report. J Neurosurg. 1974;40:772-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Levey SH, Cooper P, Schiffman D. Simulated testicular torsion in a neonate: complication of ventriculoperitoneal shunt. Urology. 1977;9:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Redman JF, Seibert JJ. Abdominal and genitourinary complications following ventriculoperitoneal shunts. J Urol. 1978;119:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 22. | Bristow DL, Buntain WL, James HL. Ventriculoperitoneal (VP) shunt migration causing an acute scrotum: a case report of Doppler evaluation. J Pediatr Surg. 1978;13:538-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 23. | Crofford MJ, Balsam D. Scrotal migration of ventriculoperitoneal shunts. AJR Am J Roentgenol. 1983;141:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Fuwa I, Matsukado Y, Itoyama Y, Yokota A. Migration of a dissected peritoneal shunt catheter into the scrotum. Brain Dev. 1984;6:336-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Ram Z, Findler G, Guttman I, Cherniak R, Knoller N, Shacked I. Ventriculoperitoneal shunt malfunction due to migration of the abdominal catheter into the scrotum. J Pediatr Surg. 1987;22:1045-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kwok CK, Yue CP, Wen HL. Bilateral scrotal migration of abdominal catheters: a rare complication of ventriculoperitoneal shunt. Surg Neurol. 1989;31:330-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Calvário JS, Paglioli Neto E. Hydrocele following placement of a ventriculoperitoneal shunt: case report. Arq Neuropsiquiatr. 1990;48:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Göçer AI, Bağdatoğlu H, Çetinalp E, Uzuneyüpoğlu Z, Karadayi A. An unusual complication of the ventriculo-peritoneal shunt: Migration of the distal end into the scrotum through the inguinal canal. Turk Neurosurg. 1990;1:176-177. |
| 29. | Selcuklu A. Migration of the peritoneal catheter of a ventriculoperitoneal shunt into the scrotum. Turk Neurosurg. 1991;2:52-53. |
| 30. | Ammar A, Ibrahim AW, Nasser M, Rashid M. CSF hydrocele--unusual complication of V-P shunt. Neurosurg Rev. 1991;14:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Jamjoom A, Ur-Rahman N, Jamjoom ZA, Jawad A, Fadley F. Unique complications of cerebrospinal fluid shunts in children--a report of two cases. Neurochirurgia (Stuttg). 1992;35:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Wong CW. Scrotal migration of a ventriculo-peritoneal shunt: report of a case. J Formos Med Assoc. 1994;93:640-641. [PubMed] |
| 33. | Goh KY, Ng AC, Lee KH, Poon WS. From hydrocephalus to hydrocele. Hong Kong Med J. 1997;3:105-106. [PubMed] |
| 34. | Oktem IS, Akdemir H, Koç K, Menkü A, Tucer B, Selçuklu A, Turan C. Migration of abdominal catheter of ventriculoperitoneal shunt into the scrotum. Acta Neurochir (Wien). 1998;140:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Porras Estrada L, Fernández Portales I, Cabezudo Artero J, Rodríguez-sánchez J, Lorenzana Honrado L, Vigo F. Migración bilateral de catéter ventriculoperitoneal a bolsa escrotal. Neurocirugía. 1999;10:164-170. [DOI] [Full Text] |
| 36. | Ozveren MF, Kazez A, Cetin H, Ziyal IM. Migration of the abdominal catheter of a ventriculoperitoneal shunt into the scrotum--case report. Neurol Med Chir (Tokyo). 1999;39:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (3)] |
| 37. | Silver RI, Docimo SG. A ventriculoperitoneal shunt masquerading as a paratesticular tumor. J Pediatr Surg. 2000;35:1407-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 38. | Ward JF, Moquin RR, Maurer ST. Expanding the differential diagnosis of the acute scrotum: ventriculoperitoneal shunt herniation. Urology. 2001;58:281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Gupta R. Migrated ventriculo-peritoenal shunt in the inguinal hernial sac. Indian J Surg. 2003;65:186-187. |
| 40. | Henriques JG, Pinho AS, Pianetti G. [Complication of ventriculoperitoneal shunting: inguinal hernia with scrotal migration of catheter. Case report]. Arq Neuropsiquiatr. 2003;61:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Walsh AR, Kombogiorgas D. Coiled ventricular-peritoneal shunt within the scrotum. Pediatr Neurosurg. 2004;40:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | de Aquino HB, Carelli EF, Borges Neto AG, Pereira CU. Nonfunctional abdominal complications of the distal catheter on the treatment of hydrocephalus: an inflammatory hypothesis? Experience with six cases. Childs Nerv Syst. 2006;22:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Moon SB, Lee SC, Jung SE. An incidentally detected ventriculoperitoneal shunt catheter in the scrotum. J Korea Assoc Pediatr Surg. 2007;13:212-216. |
| 44. | Karaosmanoglu D, Metin Y, Akata D, Haliloglu M. An unusual cause of hydrocele: malpositioned ventriculoperitoneal shunt in the scrotum. J Ultrasound Med. 2008;27:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Kayaoğlu ÇR, Şengül G, Sezer A, Sili M, Çoban M, Aydın İH. Migration of abdominal catheter of ventriculoperitoneal shunt into the scrotum: A case report. J Nervous Syst Surgery. 2008;1:181-184. |
| 46. | Agarwal T, Pandey S, Niranjan A, Jain V, Mishra S, Agarwal V. Unusual complication of ventriculoperitoneal shunt surgery. J Pediatr Neurosci. 2009;4:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Rahman N, Lakhoo K. Patent processus vaginalis: a window to the abdomen. Afr J Paediatr Surg. 2009;6:116-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | de Quintana-Schmidt C, Laria PC, Folch MT, Calderón EM, Rodríguez RR. [Scrotal migration of ventriculoperitoneal shunts]. An Pediatr (Barc). 2010;73:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Kita D, Hayashi Y, Kinoshita M, Ohama K, Hamada J. Scrotal migration of the peritoneal catheter of a ventriculoperitoneal shunt in a 5-year-old male. Case report. Neurol Med Chir (Tokyo). 2010;50:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Ho CC, Jamaludin WJ, Goh EH, Singam P, Zainuddin ZM. Scrotal mass: a rare complication of ventriculoperitoneal shunt. Acta Medica (Hradec Kralove). 2011;54:81-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Potineni LB, Hartin CW Jr, Gemme S, Caty MG, Bass KD. Laparoscopic assessment of a migrated ventriculoperitoneal shunt into an inguinal hernia. J Laparoendosc Adv Surg Tech A. 2012;22:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Mohammadi A, Hedayatiasl A, Ghasemi-Rad M. Scrotal migration of a ventriculoperitoneal shunt: a case report and review of literature. Med Ultrason. 2012;14:158-160. [PubMed] |
| 53. | Alladi A, Ramareddy R. Scrotal migration of ventriculoperitoneal catheter and hydrocele resolving spontaneously. IJNS. 2012;1:172-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Gupta M, Digra NC, Sharma N, Goyal S, Agrawal A. Migration of the peritoneal catheter of a ventriculoperitoneal shunt into the scrotum. S Afr J CH. 2012;6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Kundal VK, Gajdhar M, Sharma C, Agrawal D, Kundal R. Wandering distal end of ventriculo-peritoneal shunt: our experience with five cases and review of literature. J Nepal Paediatr Soc. 2012;32:266-269. |
| 56. | Rivero-Garvía M, Barbeito Gaído JL, Morcillo J, Márquez Rivas J. [Shunt dysfunction secondary to peritoneal catheter migration to the scrotum]. Arch Argent Pediatr. 2013;111:e14-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 57. | Elizabeth KE, Devakumar VK, Famesh A. Unusual Migration of VP Shunt. Indian Pediatr. 2013;50:435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Ghasemi A, Ashouri S. A case of hydrocele secondary to ventriculoperitoneal shunt. J Babol Univ Med Sci. 2013;15:84-87. |
| 59. | Shahizon AM, Hanafiah M, Hing EY, Julian MR. Migration of a fractured ventriculoperitoneal shunt into the scrotum: a rare complication. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Panda SS, Singh A, Bajpai M, Sharma N. Shunt in scrotum: unusual complication in operated cases of hydrocephalus. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 61. | Ojo OA, Elebute O, Kanu OO, Popoola OA. Unusual complication of ventriculoperitoneal shunt. Romanian Neurosurg. 2013;20:375-378. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Erikci V, Ganiüsmen O, Hoşgör M. Complications of ventriculoperitoneal shunt in hydrocephalic children. Ann Pediatr Surg. 2014;10:50-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Shankar B, Narayanan R, Paruthikunnan SM, Kulkarni CD. Scrotal migration of ventriculoperitoneal shunt. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Basaran R, Senol M, Efendioglu M, Mustafa ON, Nejat IS, Kaner T. Scrotal migration as an unusual complication of ventriculoperitoneal shunt case report. J Neurol Sci (Turk). 2014;31:408-412. |
| 65. | Kumar S, Jagannathan M. From hydrocephalus to hernia. J Health Spec. 2015;3:41. [DOI] [Full Text] |
| 66. | Bayındır P, Çolak E, Baş KK, Alper H. Remarkable Imaging Findings of a Migrated Ventriculoperitoneal Shunt: Report of a Case. jpr. 2015;2:161-163. [DOI] [Full Text] |
| 67. | Sarangi G, Mohanty S, Rout S. Late onset scrotal migration of the distal catheter of a ventriculoperitoneal shunt in a 4-year-old male with post meningitic hydrocephalus-a case report and review of literature. Int Surg J. 2016;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Singh S, Pant N, Kumar P, Pandey A, Khan TR, Gupta A, Rawat J. Migration of Ventriculoperitoneal Shunt into a Hernia Sac: An Unusual Complication of Ventriculoperitoneal Shunt Surgery in Children. Pediatr Neurosurg. 2016;51:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 69. | Ricci C, Velimirovic BM, Fitzgerald TN. Case report of migration of 2 ventriculoperitoneal shunt catheters to the scrotum: Use of an inguinal incision for retrieval, diagnostic laparoscopy and hernia repair. Int J Surg Case Rep. 2016;29:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Hung SW, Chen KC, Wu CC, Wang TL, Chor-Ming Lin A. A 5-Month-Old Infant with Right Scrotum Swelling; a Case Report. Emerg (Tehran). 2017;5:e48. [PubMed] |
| 71. | Javadi SA, Naderi F. Tip of Peritoneal Catheter of Ventriculo-Peritoneal Shunt in Scrotum. Arch Neurosci. 2017;4:e58389. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Kumar P, Panda SS, Kumar S, Jain N. Appendix along with ventriculo-peritoneal shunt tubing as content in hernia sac: A rare presentation. Orissa J Paediatr. 2017;16:46-48. |
| 73. | Fernandez-Ibieta M, Villamil V, Martinez-Lage J. Migration of a ventriculoperitoneal shunt catheter through the processus vaginalis in infants: how serious inguinal hernia and hydrocele might be. Acta Neurochir (Wien). 2017;159:1417-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 74. | Bawa M, Garge S, Garg R, Narasimha Rao KL. Scrotal Migration of Tubing: An Unusual Complication after Ventriculo-peritoneal Shunt. Asian J Neurosurg. 2017;12:738-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 75. | Paterson A, Ferch R. Infant with recurrent ventriculoperitoneal shunt migration to right scrotum. J Clin Neurosci. 2018;51:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Deora H, Sharma A, Rao KVLN, Somanna S, V V. Needle in a haystack: migration of ventriculoperitoneal shunt into scrotum of infant. Egypt J Neurosurg. 2018;33:9. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Shah I, Mullanfiroze K. Scrotal displacement of ventriculoperitoneal shunt. Indian J Med Spec. 2018;9:104. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 78. | Nawaz A, Chaudhry MBH, Mirza WA. Cerebrospinal fluid hydrocele caused by scrotal migration of a ventriculoperitoneal shunt. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Burhan B, Serdar KB, Abdurrahman A, Edip AM, Ebuzer D. Abdominal Complications of Ventriculoperitoneal Shunt in Pediatric Patients: Experiences of a Pediatric Surgery Clinic. World Neurosurg. 2018;118:e129-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Ezzat AAM, Soliman MAR, Hasanain AA, Thabit MA, Elshitany H, Kandel H, Abdel-Bari SH, Ghoul AMF, Abdullah A, Alsawy MFM, Ghaleb AA, Al Menabbawy A, Marei AA, El Razik BA, Schroeder HWS, Marx S, Zohdi A, El Refaee E. Migration of the Distal Catheter of Ventriculoperitoneal Shunts in Pediatric Age Group: Case Series. World Neurosurg. 2018;119:e131-e137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Soufiany I, Hijrat KA, Soufiany S, Chen L. Mechanisms and Major Sites of Distal Catheter Migration in Ventriculoperitoneal Shunting Maneuvers: A Review Article. Brain Science Advances. 2018;4:141-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Dharmajaya R. Scrotal migration of ventriculoperitoneal shunt: a case report at Haji Adam Malik hospital, Medan. IOP Conf Ser: Earth Environ Sci. 2018;125:012223. [DOI] [Full Text] |
| 83. | Rajendran B. Herniotomy Alone as Treatment for Scrotal Migration of Ventriculoperitoneal Shunt. JHNSS. 2019;3. [DOI] [Full Text] |
| 84. | Shedrov DN, Shelkoshveev DK, Pisareva MV, Morozov EV. Acute scrotal complications of Ventriculoperitoneal Shunting in pediatric practice. Case report and literature review. Vestn urol. 2019;7:66-71. [DOI] [Full Text] |
| 85. | Abdoli A, Ghorbanpour M, Fayyazi A, Saatian MR. Distal Catheter Migration into Scrotum as a Rare Complication of Ventriculoperitoneal Shunt in Pediatrics; A Case Series. Front Emerg Med. 2019;4:e95. |
| 86. | Chan AC, Woo PY, Au Y, Chan K, Wong H. Scrotal migration of a ventriculoperitoneal shunt. Surg Pract. 2019;23:161-166. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 87. | Prates Junior AG, Medeiros Carrera Macedo FA, De Oliveira Vasconcelos e Sá E, Ribeiro Pinto AL. Migration of Ventriculoperitoneal Shunt Catheter into the Scrotum: A Case Report and Literature Review. Archpedneurosurg. 2020;2:e282020. [DOI] [Full Text] |
| 88. | Gupta R, Gupta AK. Scrotal Migration of Two Ventriculoperitoneal Shunts Presenting as Hydrocele. J Indian Assoc Pediatr Surg. 2020;25:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Khaliq A, Ali M, Azam F, Ayub M. Migration of ventriculoperitoneal shunt to scrotum via patent processus vaginalis. J Saidu Med Coll Swat. 2021;11:56-57. |
| 90. | Agarwal M, Pandey S, Kumar P, Gupta L. Shunt in scrotum: unusual shunt complication in an operated case of post TBM hydrocephalus. Roneuro. 2021;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Assad Javed M, Ahmad Khan N, Us-saba A, Sana H. Shunt Migration into Scrotum: A Case Report. Pak J Neurol Surg. 2021;25:194-198. [DOI] [Full Text] |
| 92. | Hauser T, Auer C, Ludwiczek J, Senker W, Rauch PR, Kargl S, Gruber A. Treatment Options for Scrotal Migration of Ventriculoperitoneal Shunts: Case Illustration and Systematic Review of 48 Cases. Oper Neurosurg (Hagerstown). 2021;21:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Ahmed F, Derwish W, Al-wageeh S, Ghabisha S, Al-shami E, Al-naggar K, Obaid G. Migration of a Ventriculo-peritoneal Shunt into the Scrotum. J Pediat Surg Case R. 2021;73:102010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Alkhudari A, Galal M, Wagley Z, Sabbah BN, Houdane A, Aljabr A. A case of spontaneous resolution of a scrotal ventriculoperitoneal shunt migration. Radiol Case Rep. 2022;17:3620-3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Taha MM, Almenshawy HA, Ezzat M, Elbadawy MK. Migration of Distal End of VP Shunt into the Scrotum: A Management Review. Surg J (N Y). 2022;8:e245-e248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Topp G, Entezami P, Ambati S, Szewczyk B, Adamo MA. Cerebrospinal Fluid Leakage from Scrotum Secondary to Ventriculoperitoneal Shunt Migration. Asian J Neurosurg. 2023;18:333-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 97. | Tzeng S, Tsai M. Dose Hydrocele in Children Caused by Ventriculoperitoneal Shunt Migration Always Need Surgical Intervention? Watch and Wait - A Rare Cause of Hydrocele. Iran J Pediatr. 2023;33. [DOI] [Full Text] |
| 98. | Chettahi N, Andaloussi AB, Alaoui O, Mahmoudi A, Khattala K, Bouabdallah Y. Rare Instance of Scrotal Migration of Ventriculoperitoneal Shunt: A Case Report. OALib. 2023;10:1-5. [DOI] [Full Text] |
| 99. | Joseph F, Fautin AT, Alfred DD, Christine CC, Didier, S, Paul DVD. Retrograde migration of a ventriculoperitoneal shunt followed by scrotal migration after correction in the same infant with triventricular hydrocephalus: A case report and review of the literature. Asian J Res Rep Neurol. 2023;6:36-42. |
| 100. | Chanchlani R, Sharma PK, Gunasekaran V, Kasundra A. Scrotal migration of peritoneal end of ventriculoperitoneal shunt in an infant - A rare entity. J Neurosci Rural Pract. 2023;14:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Fitra F, Asrizal CW. Downward migration and scrotal extrusion of a peritoneal shunt catheter: A case report and review of literature. Zahra: J Health Med Res. 2023;3:471-476. |
| 102. | Kiyak V, Cansaran S. A Rare Postoperative Complication in a Pediatric Hydrocephalus Case: Migration of the Ventriculoperitoneal Shunt Catheter Into the Scrotal Hernia Sac. Turkiye Klinikleri J Case Rep. 2023;31:101-104. [DOI] [Full Text] |
| 103. | Darwish H, kawtharani S, Housheimy M, Fadlallah H, Moussallem C, Najjar M. Scrotal migration of the distal end of the ventriculoperitoneal shunt: Is there a role for early closure of patent processus vaginalis in the pediatric population. Open J Clin Med Case Rep. 2023;9:2179. |
| 104. | Castrillo A, Rodrigo R, Poca MA, Maluje R, Aguilera-Pujabet M, López M. Migració de la vàlvula de derivació ventriculoperitoneal a l’escrot. Pediatr Catalana. 2023;83:159-161. |
| 105. | Javeed F, Tariq M, Butt H, Rehman L. Scrotal Migration of the Ventriculoperitoneal Shunt: A Case Report and Review of the Literature. Cureus. 2024;16:e63384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 106. | Rehm A, Bannister CM, Victoratos G. Scrotal perforation by a ventriculoperitoneal shunt. Br J Neurosurg. 1997;11:443-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Korfias S, Alexiou GA, Vlachakis E, Sakas DE. Scrotal swelling due to migration of the abdominal catheter of a cyst-peritoneal shunt. Clin Neurol Neurosurg. 2013;115:1918-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 108. | Lee BS, Vadera S, Gonzalez-Martinez JA. Rare complication of ventriculoperitoneal shunt. Early onset of distal catheter migration into scrotum in an adult male: Case report and literature review. Int J Surg Case Rep. 2015;6C:198-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Foster M, Wilson G, Jenkinson MD, Buxton N. A rare case of ventriculoperitoneal shunt malfunction due to scrotal migration of the peritoneal catheter. Br J Neurosurg. 2019;33:566-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Perret C, Bertani R, Pilon B, Koester SW, Schiavini HC. Acute Hydrocephalus Following a Spontaneous Ventriculoperitoneal Shunt Catheter Fracture With Scrotal Migration. Cureus. 2021;13:e14554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 111. | Khoudir M, Harris L, Toescu SM, Vaqas B. Scrotal migration of a ventriculoperitoneal shunt in an adult. A case report and literature review. Brain Spine. 2022;2:100898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Ndongo Sonfack DJ, Tarabay B, Shedid D, Yuh SJ. Unusual presentation of a common neurosurgical shunt procedure in an adult patient. SAGE Open Med Case Rep. 2022;10:2050313X221129770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 113. | Vicaría I, Unzué G. A rare complication of ventriculoperitoneal shunt: Right scrotal abscess due to ventriculoperitoneal shunt catheter migration. Berlin: Springer, 2022. [DOI] [Full Text] |
| 114. | Cooper PR, Levey S, Rubin RC, Jacobs GB, Wille R. Distal shunt herniation simulating testicular torsion. Surg Neurol. 1976;6:269-270. [PubMed] |
| 115. | Villarejo FJ, Blazquez MG. Malfunctioning ventriculoperitoneal shunt due to extrusion of the abdominal catheter into the scrotum. J Neurosurg Sci. 1980;24:187-189. [PubMed] |
| 116. | Kobayashi H, Hayashi M, Kawano Y, Handa Y, Tsuji T, Ishii H. Migration of abdominal catheter of ventriculoperitoneal shunt into the scrotum. Zentralbl Neurochir. 1987;48:232-234. [PubMed] |
| 117. | Albala DM, Danaher JW, Huntsman WT. Ventriculoperitoneal shunt migration into the scrotum. Am Surg. 1989;55:685-688. [PubMed] |
| 118. | Celik A, Ergün O, Arda MS, Yurtseven T, Erşahin Y, Balik E. The incidence of inguinal complications after ventriculoperitoneal shunt for hydrocephalus. Childs Nerv Syst. 2005;21:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 119. | Aly B, Hammad W, Elkhouly H. Uncommon complications of ventriculoperitoneal shunt: Experience in 22 cases. Al-Azhar Assiut Med J. 2015;13:360-373. |
| 120. | Vagholkar K, Pawanarkar A, Vagholkar S, Iyengar M, Pathan K. Unusual encounters in a hernia sac. Int J Res Med Sci. 2016;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 121. | Montasr A. Scrotal migration of the peritoneal catheter of VP shunt. Radiopaedia.org. 2020;. [DOI] [Full Text] |
| 122. | Wu Y, Chen Y, Ng LP, Low SYY. Spontaneous regression of migrated ventriculoperitoneal shunt catheter from scrotum to peritoneum: a case-based review. Childs Nerv Syst. 2024;40:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 123. | Oliveira LB, Hakim F, Semione GDS, Bertani R, Batista S, Palavani LB, Sousa MP, Gómez-Amarillo DF, Mejía-Michelsen I, Pinto FCG, Rabelo NN, Welling LC, Figueiredo EG. Ventriculoatrial Shunt Versus Ventriculoperitoneal Shunt: A Systematic Review and Meta-Analysis. Neurosurgery. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 124. | Campbell D, Sinclair S, Cooke D, Webster D, Reid M. The incidence of VP shunt infection in a middle-income nation: a retrospective analysis of a pediatric population. Front Surg. 2023;10:1304105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 125. | Ferreira Furtado LM, Da Costa Val Filho JA, Moreira Faleiro R, Lima Vieira JA, Dantas Dos Santos AK. Abdominal Complications Related to Ventriculoperitoneal Shunt Placement: A Comprehensive Review of Literature. Cureus. 2021;13:e13230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Atallah O, Badary A, Monib FA, Almealawy YF, Saleh A, Lioi F, Fathallah S, Sapkota A, Kundu M, Sanker V, Das JM. Ventriculoperitoneal shunt extrusion in pediatric patients, clinical patterns and therapeutic strategies: A scoping review. Surg Neurol Int. 2024;15:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Glick PL, Boulanger SC. Inguinal Hernias and Hydroceles. In: Coran AG, editor. Pediatric Surgery 7th Edition. Netherlands: Elsevier, 2012. [DOI] [Full Text] |
| 128. | Chen YC, Wu JC, Liu L, Chen TJ, Huang WC, Cheng H. Correlation between ventriculoperitoneal shunts and inguinal hernias in children: an 8-year follow-up. Pediatrics. 2011;128:e121-e126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Wu JC, Chen YC, Liu L, Huang WC, Cheng H, Chen TJ, Thien PF, Lo SS. Younger boys have a higher risk of inguinal hernia after ventriculo-peritoneal shunt: a 13-year nationwide cohort study. J Am Coll Surg. 2012;214:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 130. | Clarnette TD, Lam SK, Hutson JM. Ventriculo-peritoneal shunts in children reveal the natural history of closure of the processus vaginalis. J Pediatr Surg. 1998;33:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (1)] |