Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5999
Peer-review started: January 28, 2021
First decision: April 25, 2021
Revised: May 7, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 26, 2021
Minimal deviation adenocarcinoma is a rare malignancy with a high rate of misdiagnosis and high aggressiveness, and its diagnosis relies on histopathology. Surgical resection is the preferred and most effective treatment, but the outcomes are often unsatisfactory.
A 60-year-old perimenopausal woman was admitted to the hospital and found to have elevated CA19-9 on physical examination without abdominal pain or vaginal bleeding. Clinical examination and positron emission tomography/computed tomography examination were unremarkable, magnetic resonance imaging examination was suggestive of dominant cervical lesions, and methylation examination was suggestive of malignant lesions. Tissue samples were taken from the suspected cervical lesion, and the final pathologic diagnosis was minimal deviation adenocarcinoma. Based on the pathologic diagnosis of suspected minimal deviation adenocarcinoma, radical abdominal total hysterectomy, bilateral oophorectomy, and pelvic and para-aortic lymph node dissection were performed. The final histological report confirmed minimal deviation adenocarcinoma of the cervix, stage IB2, with lymph node metastasis. Minimal deviation adenocarcinoma is a tumor with aggressive clinical behavior.
Patients with minimal deviation adenocarcinoma have a lower survival rate than patients with conventional human papillomavirus-related cervical adenocarcinoma. A precise preoperative pathologic diagnosis may reduce the mortality rate due to missed optimal treatment with multiple surgical interventions. To date, there is no therapeutic consensus; therefore, each case must be treated individually.
Core Tip: Minimal deviation adenocarcinomas of the uterine cervix are mucinous adenocarcinomas not related to human papillomavirus. They are relatively rare, with atypical clinical presentation, low positive rate under cytology and pathological biopsy, high rate of misdiagnosis, high aggressiveness, and tendency to spread easily during the early stage. The methylation test also improves the specificity and sensitivity of early diagnosis of minimal deviation adenocarcinoma of the cervix.
- Citation: Dong Y, Lv Y, Guo J, Sun L. Minimal deviation adenocarcinoma with elevated CA19-9: A case report. World J Clin Cases 2021; 9(21): 5999-6004
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5999.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5999
Gastric-type endocervical adenocarcinoma, also known as malignant adenocarcinoma or minimal deviation adenocarcinoma, is a rare pathologic type of cervical mucinous adenocarcinoma. Its prevalence is about 1%-3% of cervical adenocarcinomas and 0.15%-0.45% of cervical tumors. The average age at diagnosis is 42-57 years. The etiology of minimal deviation adenocarcinoma is unknown and is not associated with human papillomavirus (HPV) infection.
However, it has been reported to be associated with Peutz-Jeghers syndrome (10%) and other ovarian tumors such as mucinous or sex cord tumors. The most common clinical features include serous vaginal discharge (69.4%), spotting, and post-coital bleeding (50%). Pelvic pain was uncommon, but cervical hypertrophy was present in 74.9% of cases[1]. The diagnosis of minimal deviation adenocarcinomas is difficult because of the lack of specific clinical manifestations and benign pathology, and it needs to be differentiated from benign tumors.
A 60-year-old patient presented to the clinic on May 26, 2020 due to elevated CA19-9 found during a health check.
Elevated CA19-9 had lasted more than 3 mo.
The patient had been menopausal for 7 years, with a free previous medical history.
Gynecologic examination suggested normal vulvar development, a smooth vagina, little vaginal discharge, a soft cervix, a uterus of average size, no tenderness, and no abnormalities in the accessories. Triage suggested a smooth rectal wall and rectal pit.
The patient’s CA19-9 level was 3405.89 U/mL on May 13, 2020 and increased to 4972.00 U/mL on June 5, 2020. CEA and CA-125 were normal.
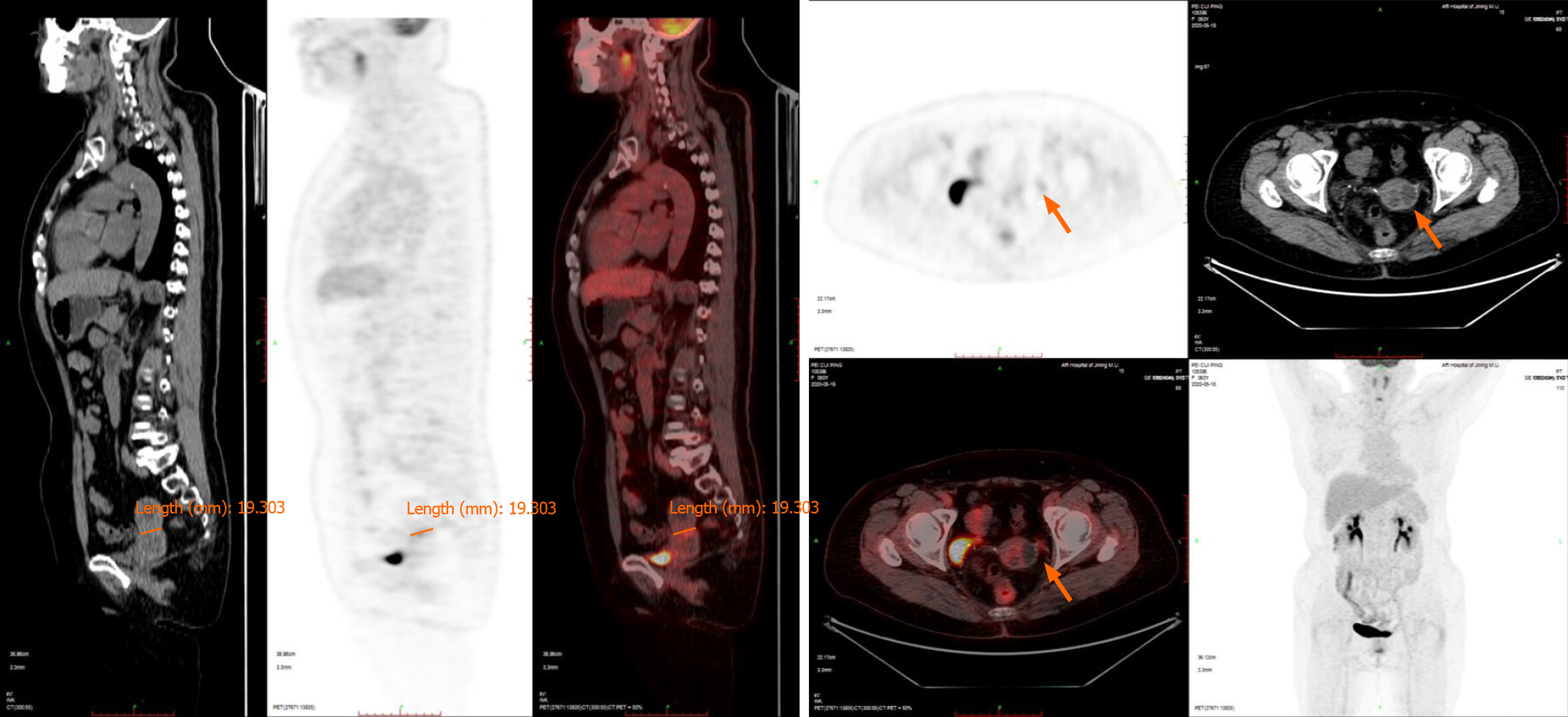
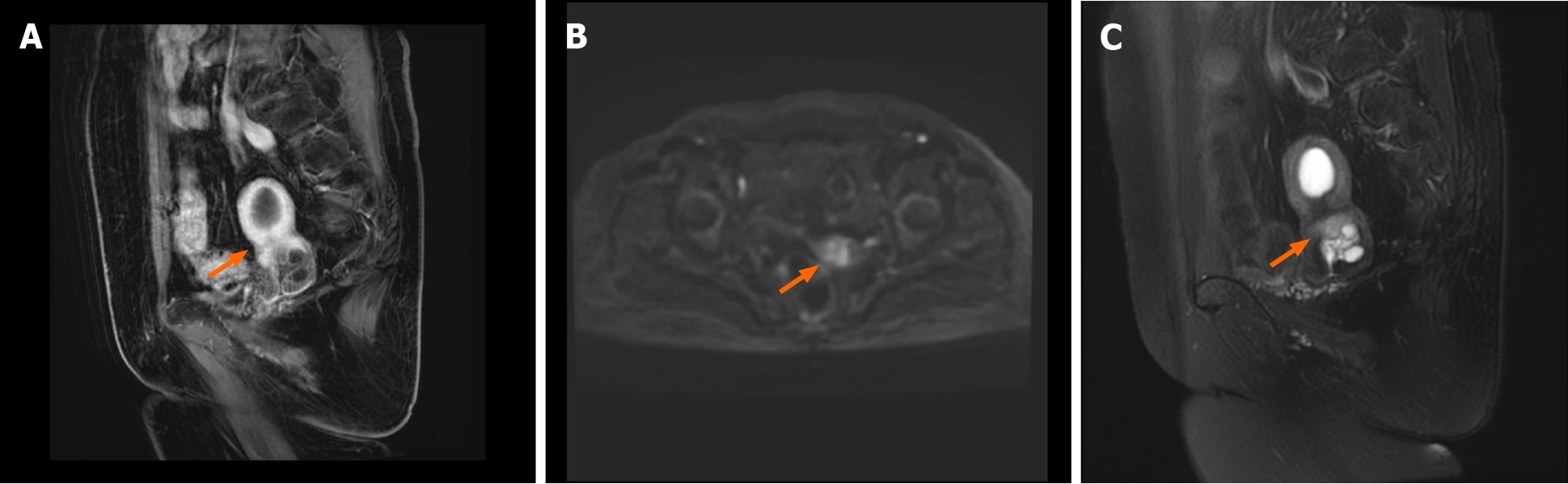
Positron emission tomography/computed tomography (PET-CT) (Figure 1) showed that the uterus was enlarged with cystic, stable occupancy. MRI (Figure 2) suggested fluid in the uterine cavity and a cervical lesion occupying the cervical niche, which was considered a neoplastic lesion, predisposing to cervical cancer.
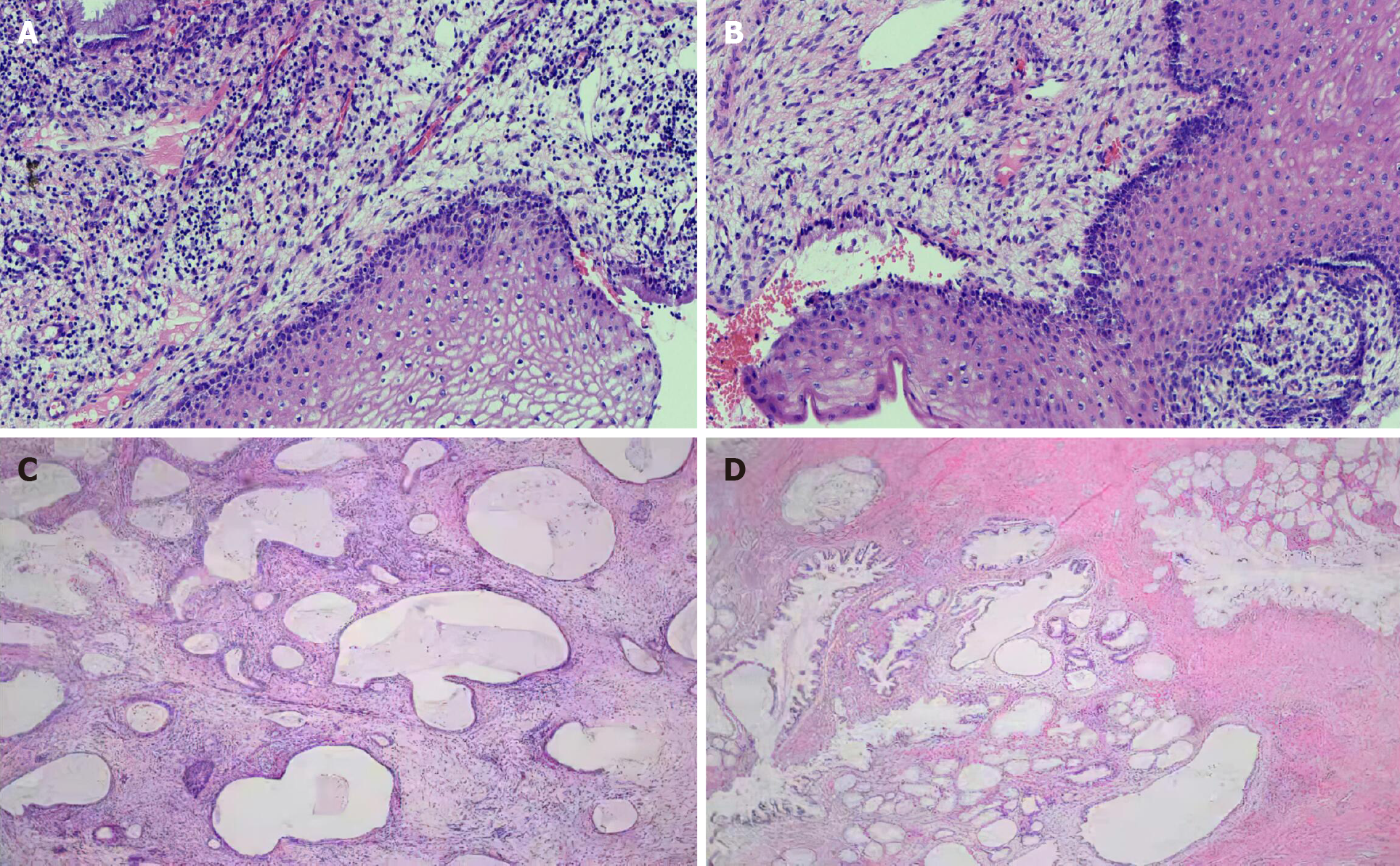
On May 26, 2020, the level of tumor marker CA-199 was 3287.19 U/mL, an E6E7 test was negative, and thinprep cytologic test revealed negative intraepithelial lesion or malignancy. A colposcopy (Figure 3A) on May 28, 2020 suggested cervical inflammation. Further cervical biopsy + pathology (Figure 3B) indicated (cervical 6 o’clock distal) tissue chronic inflammation, bleeding tissue in the uterine cavity with a small amount of mucus epithelium detected in the tissue, chronic inflammation of the tissues of the cervical canal, and significant glandular hyperplasia with hyaline cell mucus papillomatosis. Immunohistochemistry showed the following: CEA (-), P16 (-), NapsinA (-), Ki-67 (+) (< 5%), and HNF-1b (+). On June 2, 2020, the patient underwent loop electrosurgical excision procedure, with an intraoperative resection depth of 3-3.5 cm, and pathological inflammation of the cervix was observed. A PAX1 gene methylation assay performed on cervical exfoliated cells showed the following results: ΔCp = 3.57, indicating that the gene was highly methylated.
The final diagnosis of the presented case was uterine minimal deviation adenocarcinoma.
Since MRI, methylation, and CA19-9 suggested malignant lesions, hysterectomy was performed first, and the next surgical treatment was performed according to the results of intraoperative rapid pathological examination.
Hysterectomy was performed on July 6, 2020, and the uterus was observed anter
The final pathologic diagnosis (Figure 3C and D) was stage IB2 minimal deviation adenocarcinoma. The patient was given treatment with postoperative radiotherapy and chemotherapy. There is no recurrence at present.
Minimal deviation adenocarcinoma of the cervix (MDA), accounting for 1%-3% of all cervical adenocarcinomas and 0.15%-0.45% of all cervical cancers, is a highly differentiated type of mucinous adenocarcinoma[2]. The average age of onset of MDA is 45 years. It is highly aggressive, but the clinical symptoms are atypical and readily missed and misdiagnosed in the early stage. Most of the patients are already in the middle and late stages upon diagnosis, so prognosis is poor[3].
The etiology and pathogenesis of MDA remain unclear. Common cervical adenocarcinoma is usually associated with high-risk HPV infection. However, current studies have found that MDA occurrence is not related to HPV infection. According to the WHO classification, MDA is defined as an HPV-independent cervical adenocarcinoma, which is a rare mucinous adenocarcinoma with gastric gland differentiation[4]. HPV infection was not found in this case. MDA is a highly differentiated type of gastric-type endocervical adenocarcinoma, and it is difficult to distinguish well-differentiated glands from normal glands.
However, MDA shows a typical deep infiltration, random distribution, and structural abnormalities, with some glands showing distinct malignant cellular features with interstitial tissue proliferation. P16 negativity in immunohistochemistry is an essential feature of MDA, suggesting no correlation with HPV infection or with the estrogen receptor[5].
Itoh et al[6] reported that MRI shows the most typical features of the disease among ultrasound, CT, MRI, and other forms of imaging, so MRI is preferred. MDA tissue is highly differentiated, and PET-CT only suggests non-specific signs such as increased cervical volume, cervical redundancy, and abnormal cervical echogenicity, with slightly increased marginal metabolism, which has limited diagnostic value[7]. T2-weighted MRI showed localized polycystic appearance of the MDA, fluid in the uterine cavity, disorderly arrangement of glands, and invasion of the cervical canal wall. In this case, only MRI produced positive results on imaging[8].
The methylation test provides an essential basis for the clinical diagnosis of this case. Recent studies have shown that DNA methylation is closely related to the occurrence of cervical cancer and can be used as a significant molecular marker for cervical cancer screening[9]. At present, many methylation test samples come from cervical cytology, which can avoid the influence of the stromal cells contained in the histological assay on the results. CpG island hypermethylation of tumor suppressor genes was found in cervical cancer patients and did not correlate with the degree of HPV infection[10]. This case was tested for methylation of the PAX1 gene, which is a crucial tumor suppressor gene. Lai et al[11] found PAX1 to be 94.4% highly methylated in cervical cancer tissues. In a subsequent study, they discovered that PAX1 methy
There are few reports about the diagnosis and treatment of MDA. Considering that its molecular biological characteristics (easiness to infiltration, early metastasis and diffusion, and insensitivity to chemoradiotherapy), surgery is the best choice. It is recommended to perform transabdominal hysterectomy + pelvic lymphadenectomy, and adjuvant chemoradiotherapy should be carried out according to whether there are high-risk factors after surgery.
All in all, with the popularity of screening for HPV-related cervical lesions and the wide vaccination of HPV vaccines, the incidence rate of non-HPV-related tumors (such as MDA) may increase. In this patient, PET-CT failed to show its advantage in the diagnosis of malignant tumors, which may be due to less blood perfusion and weak marginal metabolism at the lesion site, but MRI examination could clearly indicate the lesion. Our research showed that for patients with vaginal discharge but negative cytological examination, we should be alert to the possibility of MDA. Deep biopsy or conization should be performed when necessary, combined with auxiliary exam
The methylation test improves the specificity and sensitivity of early diagnosis of MDA, facilitates early treatment, and positively improves the prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amponsah-Dacosta E S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Li G, Jiang W, Gui S, Xu C. Minimal deviation adenocarcinoma of the uterine cervix. Int J Gynaecol Obstet. 2010;110:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Mikami Y, McCluggage WG. Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol. 2013;20:227-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Stolnicu S, Hoang L, Soslow RA. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019;475:537-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M, Clavero O, Klaustermeier J, Jenkins D, Quint WG, Xavier Bosch F, Alemany L, de Sanjosé S; RIS HPV TT study group. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, Manabe T, Akahira J, Ito K, Tase T, Yaegashi N, Sato I, Tateno H, Naganuma H. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and 'adenoma malignum'. Mod Pathol. 2004;17:962-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Itoh K, Toki T, Shiohara S, Oguchi O, Konishi I, Fujii S. A comparative analysis of cross sectional imaging techniques in minimal deviation adenocarcinoma of the uterine cervix. BJOG. 2000;107:1158-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kido A, Mikami Y, Koyama T, Kataoka M, Shitano F, Konishi I, Togashi K. Magnetic resonance appearance of gastric-type adenocarcinoma of the uterine cervix in comparison with that of usual-type endocervical adenocarcinoma: a pitfall of newly described unusual subtype of endocervical adenocarcinoma. Int J Gynecol Cancer. 2014;24:1474-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ronnett BM. Endocervical adenocarcinoma: selected diagnostic challenges. Mod Pathol. 2016;29 Suppl 1:S12-S28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Lima PC, Teixeira J, Aires GN, Andrade LA. Endocervical gastric-type adenocarcinoma, an unrelated HPV tumour: difficulties in screening and diagnosis. BMJ Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kelly H, Benavente Y, Pavon MA, De Sanjose S, Mayaud P, Lorincz AT. Performance of DNA methylation assays for detection of high-grade cervical intraepithelial neoplasia (CIN2+): a systematic review and meta-analysis. Br J Cancer. 2019;121:954-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Lai HC, Lin YW, Huang TH, Yan P, Huang RL, Wang HC, Liu J, Chan MW, Chu TY, Sun CA, Chang CC, Yu MH. Identification of novel DNA methylation markers in cervical cancer. Int J Cancer. 2008;123:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Liou YL, Zhang Y, Liu Y, Cao L, Qin CZ, Zhang TL, Chang CF, Wang HJ, Lin SY, Chu TY, Zhou HH. Comparison of HPV genotyping and methylated ZNF582 as triage for women with equivocal liquid-based cytology results. Clin Epigenetics. 2015;7:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Loureiro J, Oliva E. The spectrum of cervical glandular neoplasia and issues in differential diagnosis. Arch Pathol Lab Med. 2014;138:453-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |