Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5724
Peer-review started: March 9, 2021
First decision: April 4, 2021
Revised: April 16, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 16, 2021
Processing time: 119 Days and 17.7 Hours
Splenosis is a rare benign disease that often disguises itself as a malignant tumor. There are few articles providing a comprehensive description of splenosis, especially cases located in the stomach being treated by laparoscopic surgery.
A 44-year-old man presented with recurrent upper abdominal pain for more than half a year. The patient had splenic rupture caused by trauma more than 10 years ago and underwent splenectomy. An abdominal contrast-enhanced computed tomography scan revealed an irregular soft tissue density. Gastroscopy revealed an approximately 3.0 cm × 3.0 cm mucosal eminence at the posterior wall of the upper segment of the gastric body. Biopsy was not performed since the lesion was found under the mucosa and the gastric mucosa appeared normal. According to these findings, a diagnosis of gastric stromal tumor was made, although a definitive differential diagnosis was not known before surgery. When laparoscopic resection of the gastric stromal tumor was performed, an astonishing finding was made when postoperative pathology showed that the lesion comprised typical spleen tissue.
This case highlights the strong similarities between splenosis and malignant tumors. A detailed medical history combined with various effective auxiliary examinations can help improve differential diagnosis.
Core Tip: Here we report a patient whose preoperative examinations revealed a gastric stromal tumor located in the body of the stomach. Nonetheless, the postoperative pathology showed that the tumor consisted of splenic tissue. The patient’s prior splenectomy was a predisposing factor, indicating that patient history together with other auxiliary examinations should be used to assist diagnosis. We summarize the diagnostic references and surgical indications of splenosis, providing an important tool for clinical practice.
- Citation: Zheng HD, Xu JH, Sun YF. Splenosis masquerading as gastric stromal tumor: A case report. World J Clin Cases 2021; 9(20): 5724-5729
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5724.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5724
Splenosis is autologous ectopic spleen implantation caused by traumatic splenic rupture or iatrogenic splenectomy[1]. Von Kutter first put forward the concept of splenosis in 1910. It has been reported that the incidence of splenosis after traumatic splenic rupture can be as high as 67%. Most of cases of splenosis involving the stomach reported in the previous literature involve lesions located in the fundus of the stomach, while splenosis located in the posterior wall of the gastric body is rare. Here, we describe such a case showing laparoscopic ectopic splenectomy of the posterior wall of the gastric body.
A 44-year-old man presented with recurrent upper abdominal pain for more than half a year.
More than half a year before admission, the patient complained of recurrent upper abdominal pain. No symptoms of bloating, diarrhea, or black stool were described. In this period of time, the patient did not receive any treatment.
The patient had splenic rupture caused by trauma more than 10 years ago and under
The patient had no relevant family history.
There were no positive signs in the physical examination.
Laboratory examinations including routine blood, liver and kidney function, and tumor marker tests were all unremarkable.
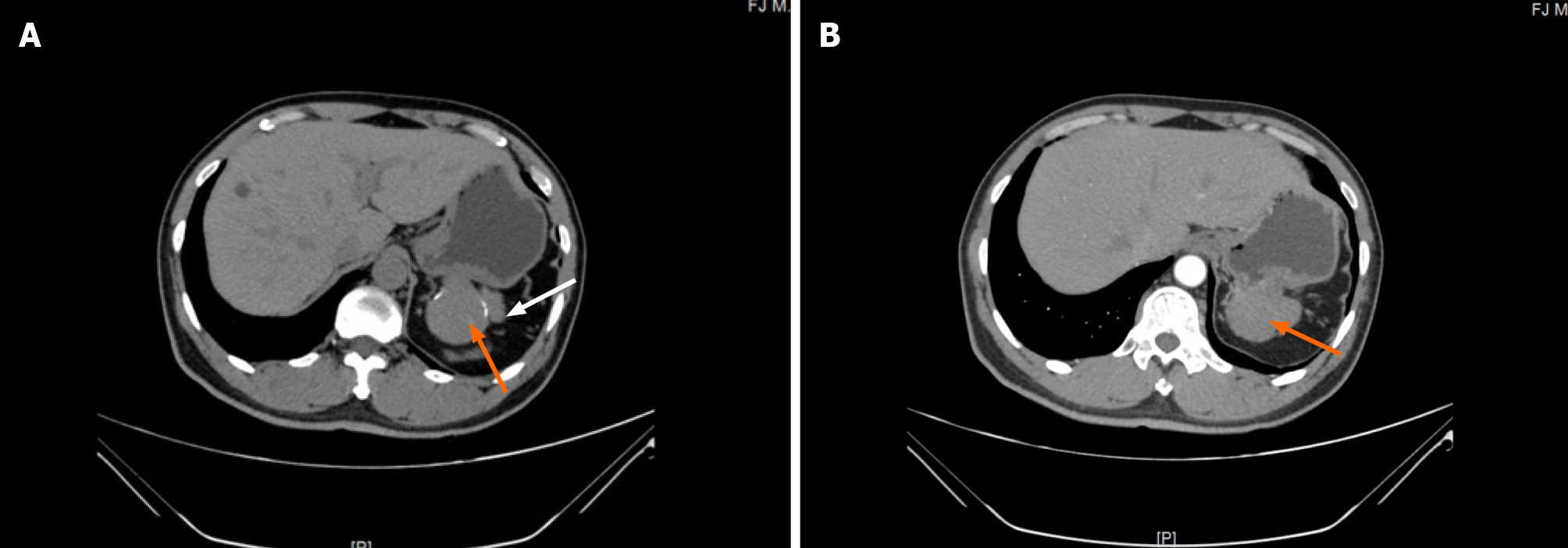
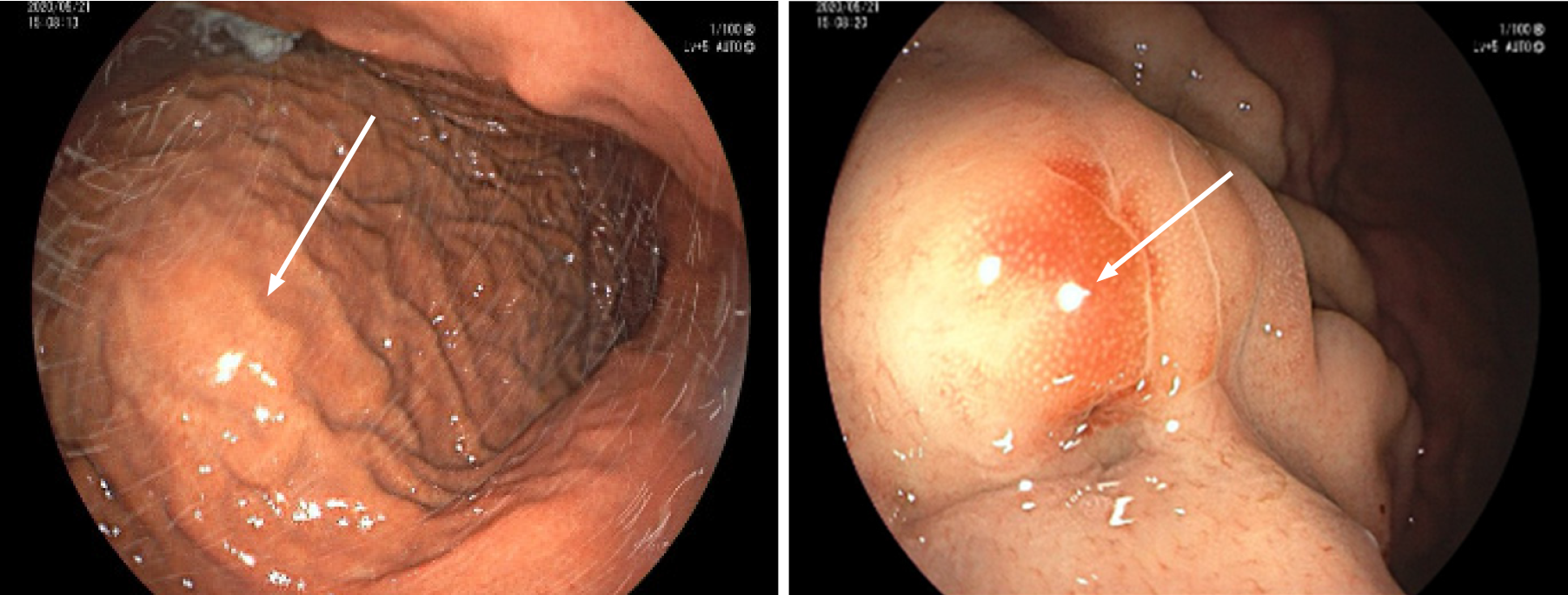
An abdominal contrast-enhanced computed tomography (CT) scan revealed an approximately 5.64 cm × 4.00 cm irregular soft tissue density that was not clearly defined from the stomach and pancreas, like an extra gastric tumor and similar to a stromal tumor, and there was slight inhomogeneous enhancement (Figure 1). Gastroscopy showed an approximately 3.0 cm × 3.0 cm mucosal eminence at the posterior wall of the upper segment of the gastric body resembling a gastric stromal tumor (Figure 2).
The final diagnostic suspect was a gastric stromal tumor.
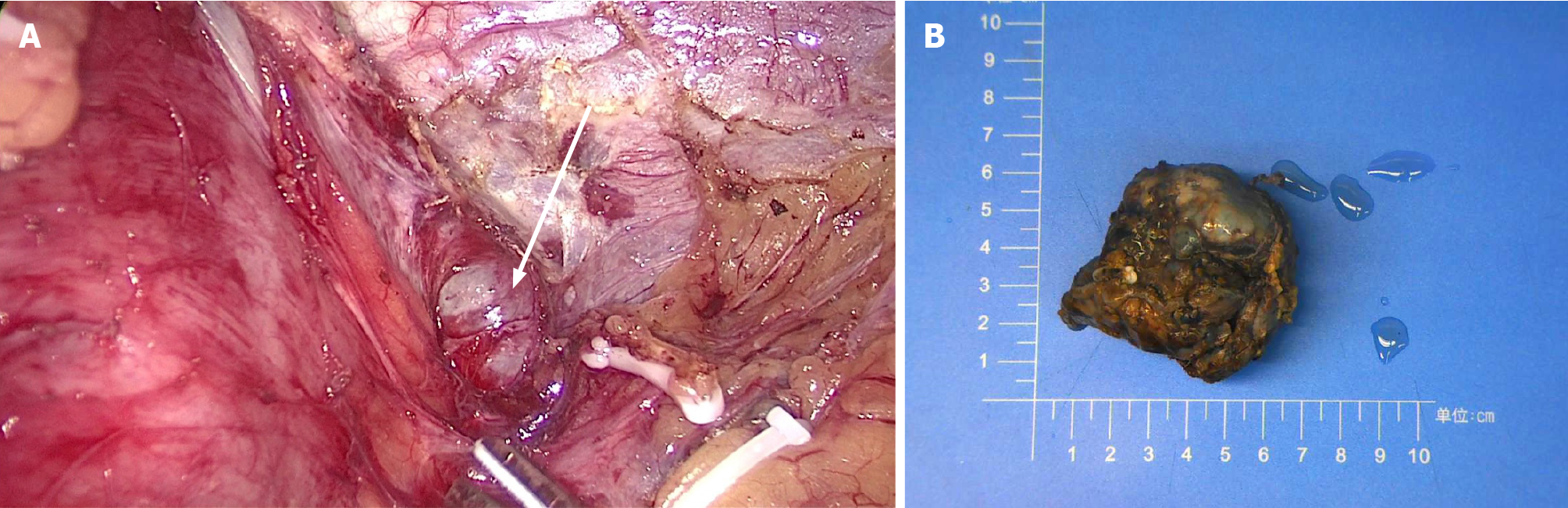
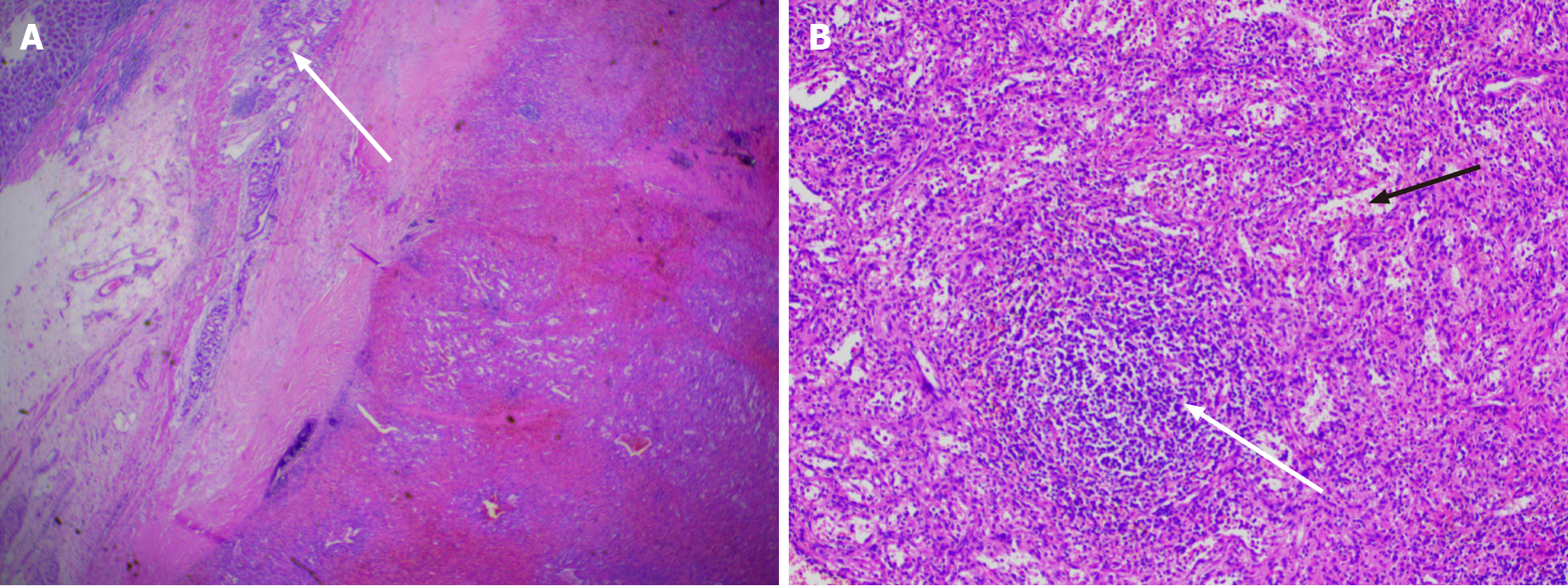
Laparoscopic resection was performed according to the diagnosis of a gastric stromal tumor. After disinfecting the abdominal skin with 5 g/L povidone-iodine solution, inhalational-intravenous anesthesia was maintained using cisatracurium besilate, propofol, dexmedetomidine, remifentanil, and sevoflurane. During the operation, a mass of about 5.0 cm × 3.0 cm was observed between the left upper stomach and the pancreas, and a 6.5 cm × 5.0 cm × 3.8 cm piece of specimen was obtained and sent for pathological examination (Figure 3). To everyone’s surprise, postoperative pathology showed that the mass consisted of typical spleen tissue with obvious splenic sinus, splenic cord, and splenic corpuscle. In addition, gastric mucosal epithelium can be seen around the spleen tissue (Figure 4). Intraoperative blood loss was 25 mL.
After the operation, we used “sulbenicillin sodium” to prevent infection, replenished fluids, and gave nutritional support, etc. The patient recovered well after the operation and was discharged on postoperative day 5. No recurrence was found during regular follow-up.
Splenosis refers to a heterotopic spleen that shows spreading of the white and red pulp tissue to the surrounding area or involves translocation to other tissues through blood circulation after rupture of the spleen[2]. The most common sites of implantation include the omentum, ileum, serous membrane of the colon, parietal peritoneum, mesentery, and diaphragm. Less common are the liver, pancreas, stomach, bladder, gall bladder, kidney, ureter, and uterus. The pathogenesis of this disease includes: (1) Splenic pulp spreading to adjacent tissues, forming a planting metastasis[3]; (2) Entry of splenic pulp into other organs via circulation, including, rarely, the brain and liver[4]; and (3) A unique liver pathway where red blood cell progenitors are transferred from the spleen to the liver via the portal vein (Chinzei and Lenox LE[5,6] and others). Here the local hypoxic environment of the liver could stimulate the secretion of erythropoietin, activating BMAP/Madh5 signaling pathways, inducing the proliferation and differentiation of the transplanted splenic red blood cell progenitors, and leading to intra-hepatic splenosis. The splenosis in this case is likely to be formed via pathway 1.
Splenosis is usually asymptomatic and can provide some degree of normal splenic function, and such cases can be treated conservatively. However, it can also cause hemorrhage, abdominal pain, bowel obstruction, bowel infarction, or torsion of splenic implants[7]. Reinglas et al[8] reported a case of massive hemorrhage of the upper digestive tract involving splenosis in the gastric fundus, resulting from the dilatation of the gastric fundus artery. Agha et al[9] made a retrospective analysis of misdiagnosed gastric splenosis, finding that all cases involved the gastric fundus with three of eight cases showing acute upper gastrointestinal bleeding. Therefore, surgical intervention should be actively considered when splenosis involves critical organs or where serious complications are possible such as gastrointestinal bleeding and intestinal obstruction. After literature review, our summary of surgical indications for splenosis are: (1) Repeated bleeding, abdominal pain, hematochezia, and other discomfort seriously affecting lifestyle; (2) Invasion of key organs or the digestive tract, resulting in uncontrollable bleeding; (3) Recurrent adhesive intestinal obstruction; (4) Splenosis obstructing the digestive tract through mechanical pressure; and (5) Lesions that are difficult to distinguish from malignant tumors. In this case, the patient’s recurrent abdominal pain seriously affects the quality of life, and it was difficult to distinguish splenosis from malignant tumors, so there was an indication for surgery.
Splenosis is usually found accidentally during imaging or abdominal exploration. However, because of the non-specificity of conventional imaging (ultrasound, CT, and MRI) in diagnostic procedures, it is often misdiagnosed as abdominal or metastatic tumors, lymphoma, endometriosis, or ectopic testis[10]. As in our case, it was misdiagnosed as gastric stromal tumor through CT and gastroscopy. Current studies have shown that superparamagnetic iron oxide enhanced magnetic resonance imaging (SPIO-MRI) is an effective method. 99mTc-labeled thermal denatured red blood cell (99mTc-DRBC) scintigraphy remains the preferred method of choice for the non-interventional evaluation of splenosis. Crivellaro et al[11] diagnosed a case of ectopic spleen by this technique successfully. If these methods cannot make a definitive diagnosis, histological examination should be performed. At present, the commonly used methods are EUS-FNA[12] and laparoscopic exploration. When splenosis is located in the gastric tissue, the characteristics of gastric stromal tumor are often present on imaging and endoscopy, leading to misdiagnosis. In our case, such auxiliary approaches would have been useful to differentiate benign diseases (such as leiomyoma, lymphangioma, and neurogenic tumor) from malignant diseases (such as leiomyosarcoma and metastatic tumor), gastrointestinal stromal tumor, and non-neoplastic lesions (ectopic pancreatic tissue and intramural pseudocyst)[13]. It nonetheless remains important to enquire about detailed medical histories[14].
To help better diagnose splenosis, we propose the following physicians diagnostic reference: (1) Patient history of splenic trauma or splenectomy; (2) Lesions revealed by CT scan that cannot be clearly distinguished from tumors; (3) Lesions showing significantly low signals on T2W1 of SPIO-MRI or significant concentrated signals using 99mTc-DRBC; and (4) Histopathological diagnosis (EUS-FNA or postoperative pathology) suggesting splenic tissue. Excluding cases of accessory spleen, clinicians should consider splenosis for patients meeting criteria 1, 2, and 3, with cases confirmed by pathological diagnosis (criteria 4).
Laparoscopic splenectomy has become the preferred modality for selective splenectomy[15]. In 1995, Higgins and Crain[16] reported the first successful laparoscopic treatment of pelvic splenosis. The minimal invasiveness and enhanced recovery after surgery of this technique have seen laparoscopy become the first choice option. However, despite advantages over open surgery, the rarity of splenosis has limited a more thorough evaluation of its safety and effectiveness. Our evaluation of previous cases in PubMed, Ovid, and other databases found only 15 reports of laparoscopic ectopic splenectomy, while laparoscopic gastric ectopic splenectomy was rarely reported[17,18].
Misdiagnosis of splenosis often occurs since clinicians do not have a comprehensive understanding of this condition. Therefore, this report of laparoscopic gastric body ectopic splenectomy has clinical value and significance to the literature.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cazorla E, Sperti C, Wan L S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Carrara S, Rahal D, Repici A. A Case of Gastric Splenosis Mimicking a Stromal Tumor. Clin Gastroenterol Hepatol. 2016;14:e50-e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Xuan Z, Chen J, Song P, Du Y, Wang L, Wan D, Zheng S. Management of intrahepatic splenosis:a case report and review of the literature. World J Surg Oncol. 2018;16:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Brewster DC. Splenosis. Report of two cases and review of the literature. Am J Surg. 1973;126:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Luo X, Zeng J, Wang Y, Min Y, Shen A, Zhang Y, Deng H, Gong N. Hepatic splenosis: Rare yet important - A case report and literature review. J Int Med Res. 2019;47:1793-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kwok CM, Chen YT, Lin HT, Su CH, Liu YS, Chiu YC. Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses. 2006;67:1330-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Lin YC, Liao CC, Lai HC. Intraperitoneal splenosis mimics peritoneal carcinomatosis of leiomyosarcoma and ovarian cancer. Taiwan J Obstet Gynecol. 2020;59:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Reinglas J, Perdrizet K, Ryan SE, Patel RV. Splenosis involving the gastric fundus, a rare cause of massive upper gastrointestinal bleeding: a case report and review of the literature. Clin Exp Gastroenterol. 2016;9:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Agha FP. Regenerated splenosis masquerading as gastric fundic mass. Am J Gastroenterol. 1984;79:576-578. [PubMed] [Cited in This Article: ] |
| 10. | Deng Y, Jin Y, Li F, Zhou Y. Splenosis mimicking an extramural duodenal mass: A case report. Oncol Lett. 2014;8:2811-2813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Crivellaro C, Cabrini G, Gay E, Sara R, Rossetti C. Intrathoracic splenosis: evaluation by 99mTc-labelled heat-denatured erythrocyte SPECT/CT. Eur J Nucl Med Mol Imaging. 2011;38:412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Fujita A, Nakahara K, Matsuda K, Ozawa SI, Itoh F. Splenosis diagnosed by EUS-guided FNA. Gastrointest Endosc. 2020;92:1129-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Garlipp B, Zeh M, Scheidbach H, Kuester D, Lippert H. [Peritoneal splenosis 26 years after traumatic splenic rupture--rare differential diagnosis of a subepithelial gastric mass--case report and review of the literature]. Z Gastroenterol. 2011;49:344-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | de Lara Bendahán V, García Gámez EM, Borrega Harinero C, Lara Fernández SM. Abdominal splenosis: the importance of the medical history. Emergencias. 2018;30:133. [PubMed] [Cited in This Article: ] |
| 15. | Kuriyama N, Maeda K, Komatsubara H, Shinkai T, Noguchi D, Gyoten K, Hayasaki A, Fujii T, Iizawa Y, Murata Y, Tanemura A, Kishiwada M, Sakurai H, Mizuno S. The usefulness of modified splenic hilum hanging maneuver in laparoscopic splenectomy, especially for patients with huge spleen: a case-control study with propensity score matching. Surg Endosc. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Higgins RV, Crain JL. Laparoscopic removal of pelvic splenosis. A case report. J Reprod Med. 1995;40:140-142. [PubMed] [Cited in This Article: ] |
| 17. | Bonatti HJR, Sahmel RO, Erlich RB. Laparoscopic Resection of a Left Upper Quadrant Mass Leading to a Surprise Diagnosis. Case Rep Surg. 2020;2020:8365061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Nicolas G, Schoucair R, Shimlati R, Rached L, Khoury G. Laparoscopic gastric band removal complicated by splenosis. Clin Case Rep. 2016;4:807-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |