Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.429
Peer-review started: August 14, 2020
First decision: November 14, 2020
Revised: November 20, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 16, 2021
Processing time: 139 Days and 21.4 Hours
Inflammatory myofibroblastic tumor (IMT) is a distinct tumor with a low incidence rate, which can be diagnosed at any age with a predilection for children and adolescents. Although IMT is visible in any tissues and organs, it is more commonly found in the lungs. The clinical and radiological manifestations of IMT lack specificity, hence resulting in frequent misdiagnosis. Surgical resection is currently the main therapeutic approach for IMT. Only scarce cases of IMT treated with metformin have been reported. Here we report the case of an IMT patient with partial penile resection treated with metformin.
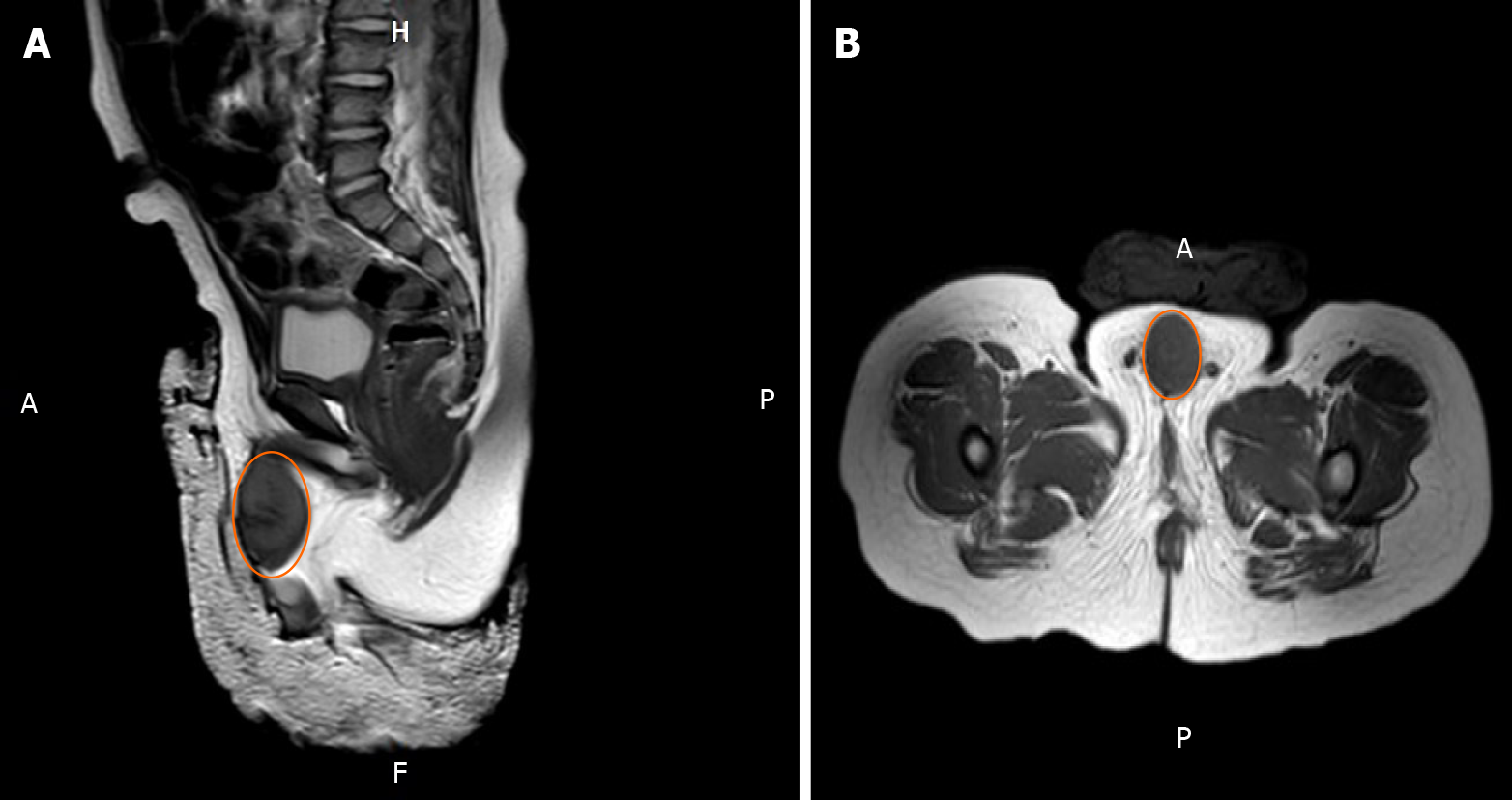
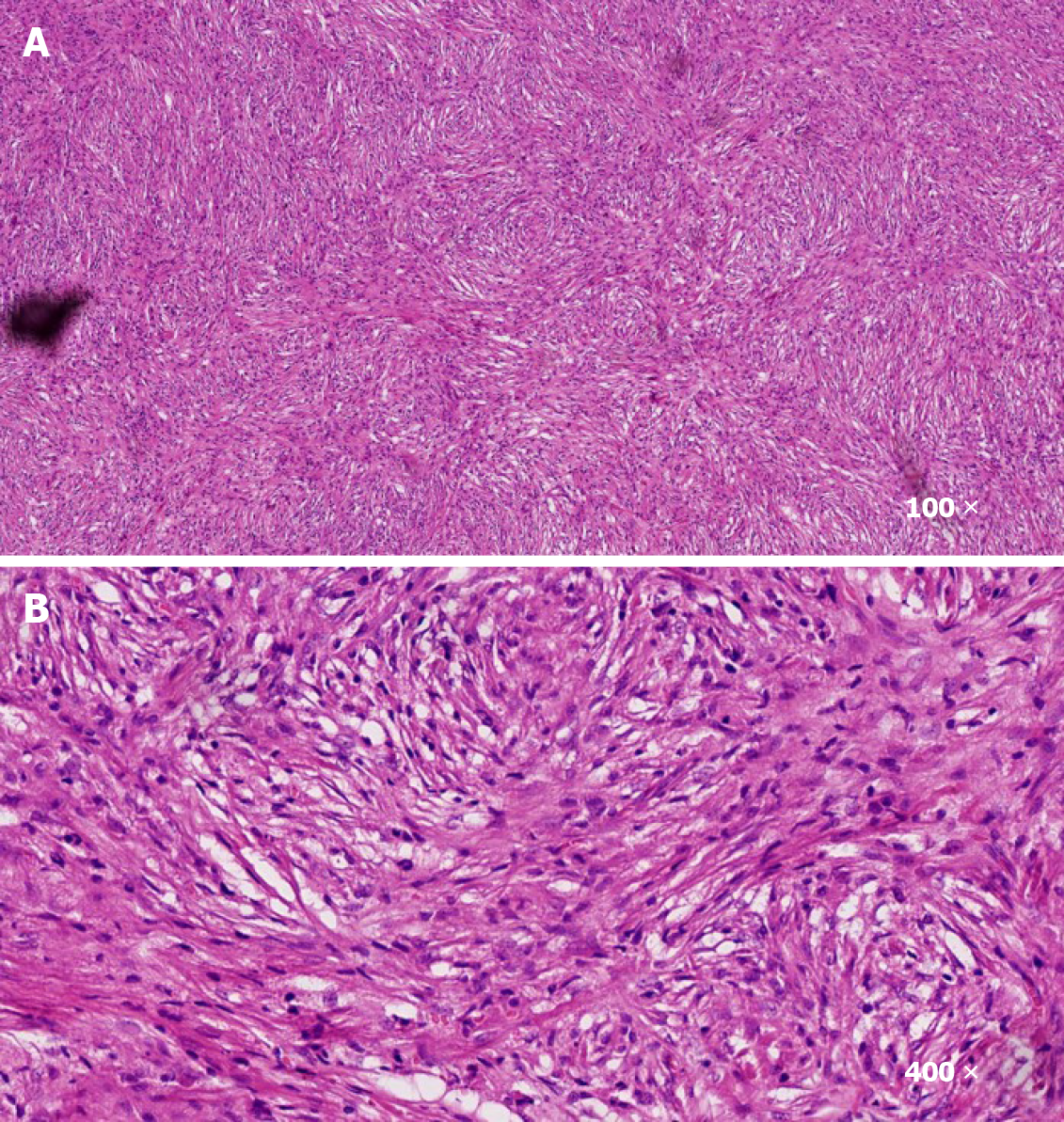
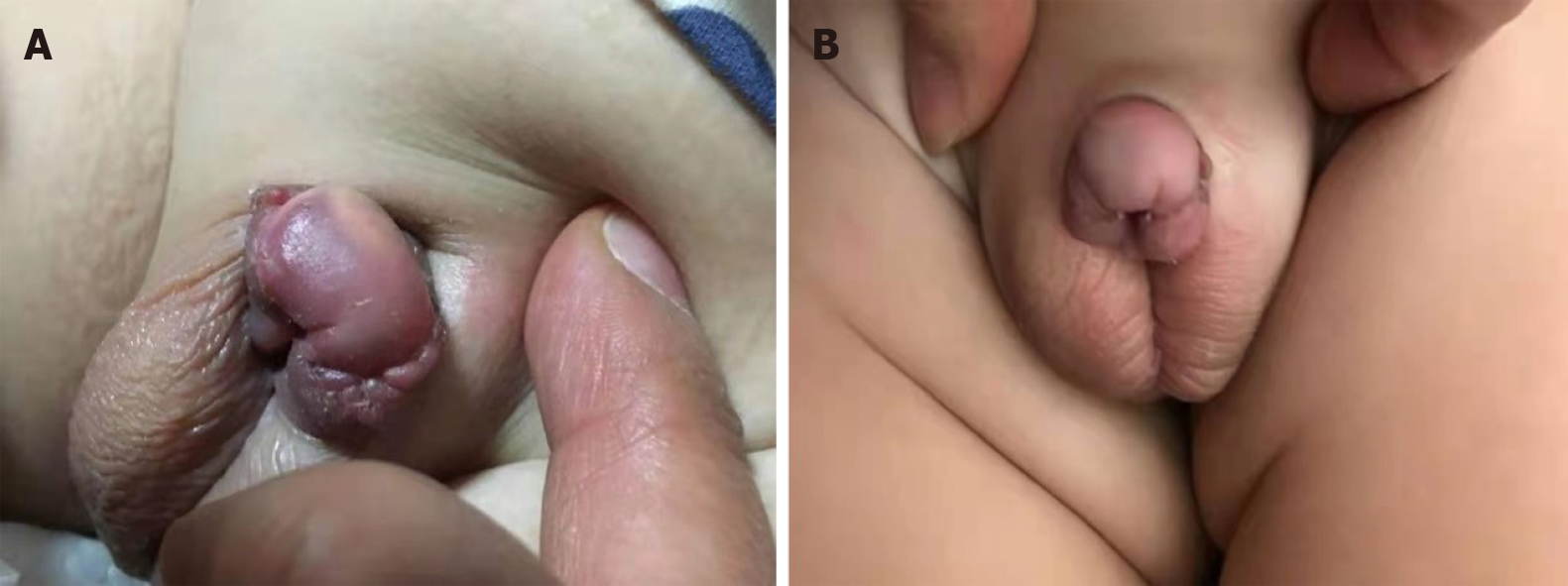
A 1-year-old boy was born with a shorter penis, and his foreskin could not be completely turned over. When he was 6 month old, a well-circumscribed mass on the glans was found, while it did not attract the attention of his parents. The mass gradually increased in size over time before he was admitted to the hospital, where physical examination was performed. It was revealed that the glans hidden behind the foreskin had a mass with a diameter of about 4 cm surrounding the penis. The mass appeared to be hard with a smooth surface and poor mobility. The two testicles examined at the bottom of the scrotum were revealed to have a normal size. Magnetic resonance imaging showed a tumor with rich blood supply encircling the cavernosum with a size of 3.5 cm × 2.1 cm × 2.0 cm. A thick urinary line was found without urine dripping, urgency, and urodynia. Surgical treatment was performed. During the operation, it was observed that the mass had surrounded and invaded the cavernosum without obvious boundaries, and that the tumor occupied about one-half of the penis cross-section as well as infiltrated more than one-half of the glans. In addition, the tumor had caused urethral invasion and anterior urethrostenosis. With the intention of keeping the glans and cavernosum, the tumor at the anterior urethra was partially removed, leaving about 30% of the tumor mass. Pathology analysis demonstrated that the tumor was rich in spindle cells with infiltration of inflammatory cells. Immuno-histochemistry analysis indicated that the cells were positive for CD4, CD99, Ki67, BCL2, and CD68, and negative for ALK, MyoG, S100, SOX10, PR, and EMA. Hence, the tumor was diagnosed as IMT. Metformin was prescribed for the patient after the operation, following which an oral dose of 7 mg/kg was given three times a day after meals. Three months later, it was observed that the remaining tumor had completely disappeared and that the urination process from the urethra opening had resumed normal. In addition, there were no side effects observed. There was also no tumor recurrence. The growth and development of the boy were unaffected as a result of the treatment.
The tumor was observed to have completely disappeared after treatment with metformin. Our finding is of great significance to facilitate future clinical treatment with IMT.
Core Tip: Inflammatory myofibroblastic tumor (IMT) is a rare tumor in children, with surgical resection being the main treatment approach. We report a case of penile IMT with complete surgical resection. The residual lesions were found to have completely disappeared after treatment with metformin, a traditional drug.
- Citation: Liang Y, Gao HX, Tian RC, Wang J, Shan YH, Zhang L, Xie CJ, Li JJ, Xu M, Gu S. Inflammatory myofibroblastic tumor successfully treated with metformin: A case report and review of literature. World J Clin Cases 2021; 9(2): 429-435
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/429.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.429
Inflammatory myofibroblastic tumor (IMT) is a mesenchymal neoplasm of intermediate biological potential[1]. Clinically, IMT occurs at any age and can affect any site of the body, although it is most commonly found in the lungs[2,3]. IMT at early stages exhibits no obvious clinical symptoms. The pathogenesis of IMT is insidious and the etiology remains ambiguous[4]. Currently, surgical resection is the first-line therapeutic approach, unless otherwise prohibited by the site of IMT being at a dedicative anatomic location, or by the occurrence of multiple lesions or com-plications[5]. There have been studies reporting on cases that were prescribed corticosteroids for IMT[6,7]. Here we report a case of penile IMT with incomplete resection treated with metformin. Surprisingly, the tumor was observed to have completely disappeared after metformin was administered for about 3 mo. Our finding may provide an alternative treatment approach for IMT.
A 1-year-old boy was born with a shorter penis, and his foreskin could not be completely turned over.
A round hard mass on the glans was found when he was 6 mo old, with a clear boundary and smooth surface. The mass gradually increased in size over time.
The patient had a free previous medical history.
The patient and his family had a free previous medical history.
The glans hidden behind the foreskin had a mass with a diameter of about 4 cm surrounding the penis. The mass appeared to be hard with a smooth surface and poor mobility. The two testicles were revealed to have a normal size. A thick urinary line was found without urine dripping, urgency, and urodynia.
There were no significant abnormalities in blood analysis.
Magnetic resonance imaging showed a tumor with rich blood supply encircling the cavernosum with a size of 3.5 cm × 2.1 cm × 2.0 cm (Figure 1).
The patient should undergo surgical treatment and obtain histopathological results to confirm the diagnosis.
The tumor should be removed as completely as possible to confirm the diagnosis. The patient could undergo chemotherapy when it is necessary.
It is considered to be a fibroma based on the magnetic resonance image, but it still requires further pathological confirmation.
Surgical treatment was performed. After operation, pathology analysis demonstrated that the tumor was rich in spindle cells with infiltration of inflammatory cells (Figure 2). Immunohistochemistry analysis indicated that the cells were positive for CD4, CD99, Ki67, BCL2, and CD68, and negative for ALK, MyoG, S100, SOX10, PR, and EMA. Hence, the tumor was diagnosed as IMT.
According to the past clinical experience, metformin (Sino-American Shanghai Squibb Pharmaceuticals Ltd., 0.5 g/tablet) was prescribed for the patient after the operation, following which an oral dose of 7 mg/kg was given three times a day after meals.
Three months later, it was observed that the remaining tumor had completely disappeared and that the urination process from the urethra opening had resumed normal (Figure 3). In addition, there were no side effects observed. There was also no tumor recurrence. The growth and development of the boy were unaffected as a result of the treatment.
IMT is a rare tumor commonly found in children and adolescents[1,8]. Tissues of IMT comprise inflammatory cells and mesenchymal spindle cells[9]. To date, the etiology of IMT is unknown, but it may be due to operation, trauma, radiotherapy, infection, the use of steroid hormone, and autoimmune reaction. Whilst about 50% of IMT patients have genetic translocation and rearrangement of the ALK (anaplastic lymphoma kinase) gene on 2p23 that lead to the structural activation of tyrosine kinase and overexpression of ALK protein; the rest of IMT patients lack ALK expression[10]. Although IMT can be found at any anatomical locations, it is commonly diagnosed in the lungs, abdomen, and pelvis. However, IMT is rarely found in the urogenital system. IMT usually presents an intermediate clinical behavior with the potential of recurrence. The rates of recurrence inside or outside of the lungs have been reported to be 2% and 25%, respectively, while the incidence of distant metastasis has been reported to be less than 5%[11]. The radiological features of IMT vary, such as infiltration of inflammatory cells, interstitial fibrosis, and tumor distribution. Histopathological observation has indicated fibrous tissue hyperplasia, fibrous interstitial hyperplasia, and chronic infiltration of inflammatory cells, such as lymphocytes, plasma cells, and eosinophils. Immunohistochemistry analysis has demonstrated that IMT is positive for CD20, CD79a, CD3, CD45RO, SMA, vimentin, CD68, and Action (+), but negative for CD34, CK, and CD35. Although local surgical resection of IMT is recommended, preoperative or intraoperative biopsy is essential for clarifying the catalog of the tumor to avoid non-essential organ resection. It has been previously reported that IMT can be successfully treated using anti-inflammatory medications and glucocorticoids.
Several preclinical, epidemiological, and clinical studies have demonstrated that metformin, a first-line antidiabetic drug, can reduce the overall risk and mortality of neoplasms[12-23]. Metformin is capable of inhibiting protein and lipid synthesis of tumor cells via the activation of adenosine phosphate protein kinase (AMPK) activity, which in turn downregulates the mechanical target of rapamycin complex 1 (mTORC1) signaling pathway, thereby limiting tumor growth and proliferation[24]. Metformin can also suppress the oxidative phosphorylation of mitochondria, resulting in the decrease of ATP level, thus inducing the energy stress of tumor cells and making them vulnerable to energy crisis and cell death in the presence of some mutations[25]. In addition, metformin can also inhibit tumor growth through the downregulation of insulin/IGF-1 levels, and the regulation of cell cycle and immune response. Nevertheless, the mechanism of metformin in the inhibition of IMT is ambiguous. To date, the main molecular targets of metformin are complex I of the mitochondrial electron transport chain, AMPK, and mTORC1. As a member of the conserved serine/threonine protein kinase from the phosphatidylinositol 3 kinase (PI3K) family, mTOR is the target of rapamycin. Regulated by nutrients and growth factors, mTORC1 is the main regulator of cell growth and metabolism via the phosphorylation of target cells. Due to the impacts on downstream oncogenesis, the activity of mTORC1 has been observed to be increased in several tumors[26,27]. Wang et al[28] have indicated that metformin can inhibit the activity of mTORC1 through a number of pathways in the suppression of tumor growth. On one hand, mitochondrial electron chain transport (ECT) produces ATP, which then leads to the downregulation of AMPK. With metformin acting directly on ETC and inhibiting ETC activity to decrease ATP synthesis, the resulting higher ratio of AMP/ATP induces the activation and phosphorylation AMPK, thereby leading to the inhibition of mTORC1[29,30]. On the other hand, metformin can also activate AMPK and inhibit mTORC1 through a mechanism that is independent of the ECT[26]. In addition, the activation of AMPK may repress myofibroblast differentiation through TGF-β1[31]. The activation of AMPK promotes the inactivation/apoptosis of myofibroblasts, which can also be found in other cells, such as alveolar epithelial cells and immune cells. Particularly, the activation of AMPK may also mediate the promotion of decomposition or anti-fibrosis, thus inhibiting the proliferation of IMT[32]. IMT is an extremely rare disease, and the available therapies have been underdeveloped.
Based on this case report, metformin as an easily accessible drug has been found to have the potential to become an ancillary treatment for IMT. However, it is essential to conduct randomized controlled trials with larger sample sizes to confirm our conclusion.
We thank Dr. Gu S for sharing his expertise in treating patients with inflammatory myofibroblastic tumor and his selfless help. Also, we are grateful to Professor Geng HQ, Director of Urology Surgery, Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, for his assistance in the work.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shimada S S-Editor: Huang P L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory Myofibroblastic Tumors: Current Update. Radiol Clin North Am. 2016;54:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Khatri A, Agrawal A, Sikachi RR, Mehta D, Sahni S, Meena N. Inflammatory myofibroblastic tumor of the lung. Adv Respir Med. 2018;86:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Shetty SJ, Pereira T, Desai RS. Inflammatory myofibroblastic tumor of the oral cavity: A case report and literature review. J Cancer Res Ther. 2019;15:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Berhe S, Goldstein S, Thompson E, Hackam D, Rhee DS, Nasr IW. Challenges in Diagnosis and Management of Pancreatic Inflammatory Myofibroblastic Tumors in Children. Pancreas. 2019;48:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Makhlouf HR, Sobin LH. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: how closely are they related to inflammatory fibroid polyps? Hum Pathol. 2002;33:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Fu GX, Xu CC, Yao NF, Gu JZ, Jiang HL, Han XF. Inflammatory myofibroblastic tumor: A demographic, clinical and therapeutic study of 92 cases. Math Biosci Eng. 2019;16:6794-6804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA. Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am J Surg Pathol. 2005;29:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Nistal M, Gonzalez-Peramato P, Serrano A, Reyes-Mugica M, Cajaiba MM. Primary intratesticular spindle cell tumors: interdigitating dendritic cell tumor and inflammatory myofibroblastic tumor. Int J Surg Pathol. 2011;19:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, Klingebiel T, Lehrnbecher T. Crizotinib in ALK+ inflammatory myofibroblastic tumors-Current experience and future perspectives. Pediatr Blood Cancer. 2018;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Mehta B, Mascarenhas L, Zhou S, Wang L, Venkatramani R. Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol. 2013;30:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Park JH, Kim YH, Park EH, Lee SJ, Kim H, Kim A, Lee SB, Shim S, Jang H, Myung JK, Park S, Lee SJ, Kim MJ. Effects of metformin and phenformin on apoptosis and epithelial-mesenchymal transition in chemoresistant rectal cancer. Cancer Sci. 2019;110:2834-2845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:2184-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 14. | Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 15. | Ariaans G, Jalving M, Vries EG, Jong S. Anti-tumor effects of everolimus and metformin are complementary and glucose-dependent in breast cancer cells. BMC Cancer. 2017;17:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Dempsey LA. Anti-tumor role of metformin. Nat Immunol. 2018;19:1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Mu Q, Jiang M, Zhang Y, Wu F, Li H, Zhang W, Wang F, Liu J, Li L, Wang D, Wang W, Li S, Song H, Tang D. Metformin inhibits proliferation and cytotoxicity and induces apoptosis via AMPK pathway in CD19-chimeric antigen receptor-modified T cells. Onco Targets Ther. 2018;11:1767-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Qian W, Li J, Chen K, Jiang Z, Cheng L, Zhou C, Yan B, Cao J, Ma Q, Duan W. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 2018;208:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Rêgo DF, Elias ST, Amato AA, Canto GL, Guerra EN. Anti-tumor effects of metformin on head and neck carcinoma cell lines: A systematic review. Oncol Lett. 2017;13:554-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Samuel SM, Varghese E, Kubatka P, Triggle CR, Büsselberg D. Metformin: The Answer to Cancer in a Flower? Biomolecules. 2019;9:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Suzuki K, Takeuchi O, Suzuki Y, Kitagawa Y. Mechanisms of metformin's anti‑tumor activity against gemcitabine‑resistant pancreatic adenocarcinoma. Int J Oncol. 2019;54:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, Kunisada T, Ozaki T, Udono H. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol. 2019;31:187-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Liang Y, Tian R, Wang J, Shan Y, Gao H, Xie C, Li J, Zhang L, Xu M, Gu S. Melanotic neuroectodermal tumor of infancy successfully treated with metformin: A case report. Medicine (Baltimore). 2020;99:e22303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, Logan PJ, Salt IP, Ahmed SF, Yarwood SJ. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 1001] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 26. | Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017;25:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 933] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, Yan H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res. 2018;37:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 29. | Park J, Joe Y, Ryter SW, Surh YJ, Chung HT. Similarities and Distinctions in the Effects of Metformin and Carbon Monoxide in Immunometabolism. Mol Cells. 2019;42:292-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Li M, Li X, Zhang H, Lu Y. Molecular Mechanisms of Metformin for Diabetes and Cancer Treatment. Front Physiol. 2018;9:1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Li L, Huang W, Li K, Zhang K, Lin C, Han R, Lu C, Wang Y, Chen H, Sun F, He Y. Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway. Oncotarget. 2015;6:43605-43619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, Zmijewski JW. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 410] [Article Influence: 58.6] [Reference Citation Analysis (0)] |