Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4866
Peer-review started: April 11, 2020
First decision: June 13, 2020
Revised: July 27, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: October 26, 2020
Processing time: 194 Days and 4 Hours
Asymptomatic cytomegalovirus (CMV) infection is common in children; in contrast, in children with a weakened immune system, invasive CMV can occur. This is the first case report of a severe manifestation of CMV esophago-enterocolitis in a girl diagnosed with anti-N-methyl-D-aspartate-receptor (anti-NMDAR) encephalitis who received only a moderate dose of corticosteroid therapy.
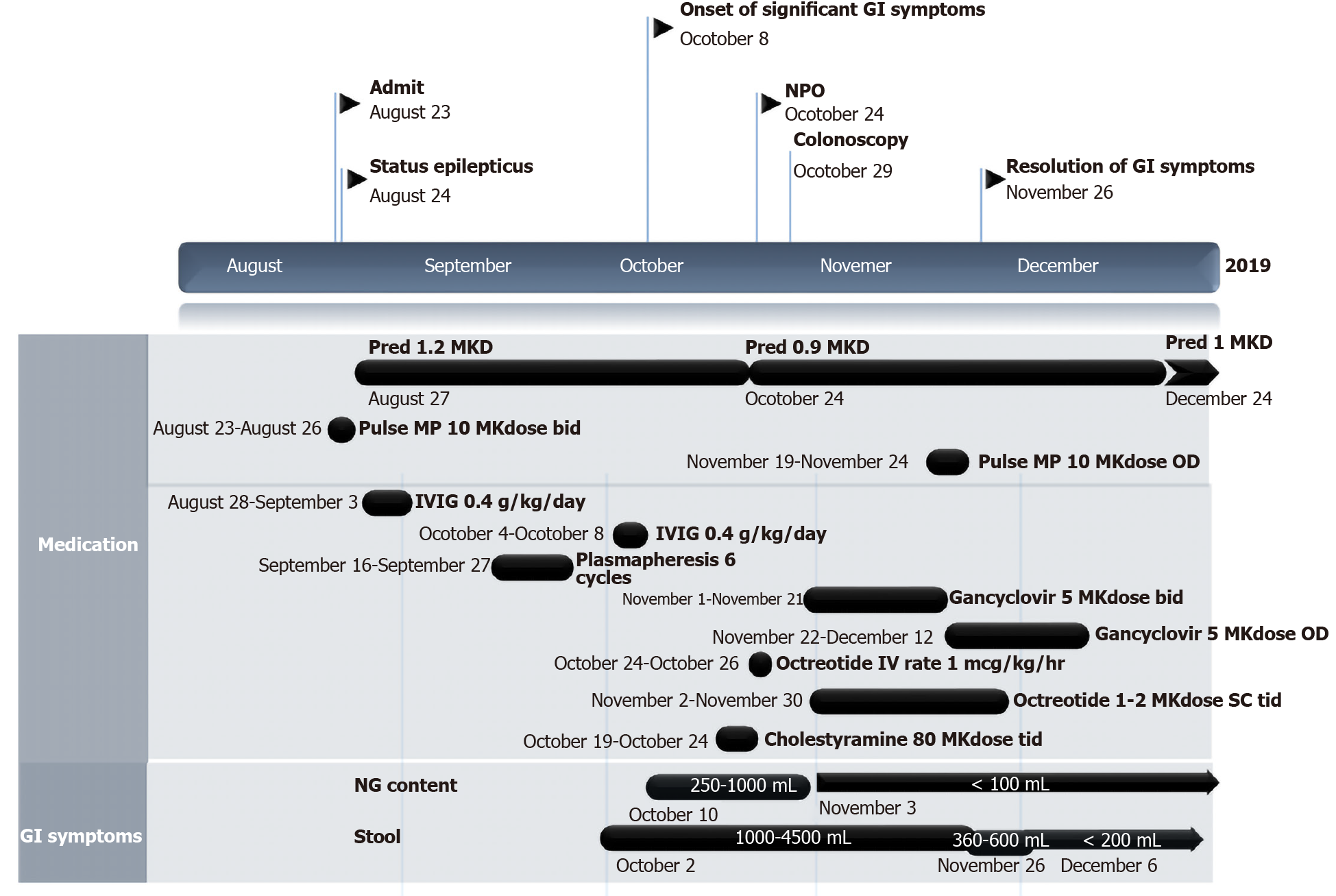
A 12-year-old-Thai girl presented with acute behavioural change and headache for 6 d. Electroencephalogram and positivity for NMDAR autoantibodies were compatible with anti-NMDAR encephalitis. Hence, she received pulse methylprednisolone 10 mg/kg per day for 4 d and continued with prednisolone 1.2 mg/kg per day. On day 42 of corticosteroid therapy, she developed unremitting vomiting and diarrhoea. Endoscopy showed multiple ulcers and erythaematous mucosa along the gastrointestinal tract. Tissue CMV viral load and viral-infected cells confirmed CMV esophago-enterocolitis. Therefore, the patient received ganciclovir 5 mg/kg per dose every 12 h for 3 wk and then 5 mg/kg per dose once daily for 3 wk. Unremitting diarrhoea slowly improved from stool output 1-4 L per day to 1-2 L per day after 3 wk of treatment. Pulse methylprednisolone 20 mg/kg for 5 d was initiated and continued with prednisolone 1 mg/kg per day. After this repeated pulse methylprednisolone treatment, surprisingly, diarrhoea subsided. Immunologic work-up was performed to rule out underlying immune deficiency with unremarkable results.
Unremitting diarrhoea from CMV esophago-enterocolitis subsided with antiviral and methylprednisolone therapy, implying the immune and NMDAR dysregulation in anti-NMDAR encephalitis.
Core Tip: We report a girl with behavioural change who was finally diagnosed with anti-N-methyl-D-aspartate-receptor (anti-NMDAR) encephalitis. She had unremitting vomiting and diarrhoea after 43 d of corticosteroid therapy and was later diagnosed with cytomegalovirus (CMV) esophago-enterocolitis. Surprisingly, these symptoms subsided after antiviral therapy followed by repeated pulse methylprednisolone targeting the anti-NMDAR encephalitis. CMV esophago-enterocolitis in a patient with anti-NMDAR encephalitis has not been reported before and is very rare in patients who receive only moderate-dose corticosteroids. We highlighted that immune dysregulation in autoimmune encephalitis and the linkage of anti-NMDAR receptors and the enteric nervous system might be the cause of severe gastrointestinal involvement.
- Citation: Onpoaree N, Veeravigrom M, Sanpavat A, Suratannon N, Sintusek P. Unremitting diarrhoea in a girl diagnosed anti-N-methyl-D-aspartate-receptor encephalitis: A case report. World J Clin Cases 2020; 8(20): 4866-4875
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4866
Cytomegalovirus (CMV) is a common viral pathogen infecting children. The majority of infected patients are asymptomatic. However, immunocompromised hosts, including individuals who underwent organ transplantation or those who received immunosuppressive drugs, have a high risk of experiencing symptoms of invasive CMV infection[1]. CMV esophago-enterocolitis is a very rare condition in children, and the manifestation of unremitting vomiting and diarrhoea has not been reported in the literature before.
Anti-N-methyl-D-aspartate-receptor (anti-NMDAR) encephalitis has recently been recognized as a type of autoimmune encephalitis in children presenting with behavioural changes, seizures, psychiatric features and oromotor dyskinesia. The treatment outcome depends on prompt first- and second-line immunotherapy[2,3]. Gastrointestinal (GI) manifestations in anti-NMDR encephalitis patients have been reported in few studies as an autonomic dysregulation or complication of autoimmune encephalitis[4,5]. The role of NMDA receptors in the enteric nervous system (ENS) of the GI tract has not yet been elucidated in patients with anti-NMDAR encephalitis.
Here, we present the case of a 12-year-old Thai girl with a diagnosis of anti-NMDAR encephalitis who developed abdominal bloating, feeding intolerance, and bilious vomiting concomitant with watery diarrhoea. Later, CMV esophago-enterocolitis was diagnosed by endoscopy with tissue histopathology and molecular techniques. Interestingly, the complete clinical response of these severe GI symptoms was successful after combination therapy for CMV infection and anti-NMDAR encephalitis.
A 12-year-old Thai girl who was previously healthy was referred to King Chulalongkorn Memorial Hospital due to behavioural change with fever and headache for 6 d.
Six day prior to referral to our hospital, the patient had bizarre behaviour. She had a conversation with herself and cried or laughed without explaining the reason. One day later, she had a fever and progressive headache. She has alteration of consciousness with waxing and waning in her consciousness. She had abnormal movements of her hands and could not write letters. She went to a nearby clinic and received roxithromycin, fexofenadine and paracetamol. She could not sleep at night and slept more during the daytime. Four days prior to admission, she started having aggressive behaviour with incomprehensive language. She did not sleep during the day or night. She had intermittent screaming with visual hallucination. Her visual hallucination was described as a moving snake or the death of relatives. She was admitted to Hua Hin Hospital. At the hospital, she still had a high-grade fever with intermittent episodes of screaming with aggressive behaviours. She underwent computed tomography scan (CT) brain and lumbar puncture. She received high-dose ceftriaxone, acyclovir, azithromycin and oseltamivir before referral to our hospital.
The patient did not have any other medical illness or history of any drug or substance abuse.
There was no history of contagious diseases or neuropsychiatric conditions in the family. There was no history of consanguinity in the family.
On admission, the patient was well-grown and confused and had a low-grade fever with a body temperature of 38.4°C, a pulse rate of 110 beats per minute and a respiratory rate of 24 breaths per minute. The general physical examination was unremarkable. Neurological examination showed confusion with disorientation to time, place and person. Her Glasgow coma scale was E4M5V3. Her cranial nerve examination revealed 3 mm pupils that reacted to light bilaterally. She was incorporated with extraocular movement. Nasolabial folds were symmetric. The uvula was in the midline with a gag reflex present. She did not cooperate for motor examination. When she had aggressive behaviour, she moved all four extremities equally; oromotor dyskinesia and choreoathetosis were noted in bilateral extremities, reflex 3+ was noted in all extremities, Babinski's sign was equivocal and clonus was absent.
Laboratory investigation revealed hemoglobin 123 g/L, white blood cell count (WBC) 4.83 × 109/L (N 65, L28, M7%), platelet count 310 × 109/L, sodium 140 mmol/L, potassium 3.8 mmol/L, chloride 104 mmol/L, bicarbonate 21.4 mmol/L, calcium 2.32 mmol/L, phosphorus 1.12 mmol/L, magnesium 0.97 mmol/L, glucose 5.1 mmol/L, TSH 3.35 µU/mL, FT4 18 ng/L, anti-thyroglobulin < 10, anti-thyroid peroxidase < 9 IU/mL, C3 1.25 and C4 3.8 g/L. Cerebrospinal fluid (CSF) analysis revealed colourless fluid with WBC 13 cells/mm3 (100% mononuclear cells), protein 20.1 g/L and glucose 4.2 mmol/L. Gram staining and culture from CSF did not reveal any evidence of microorganisms. Infectious work-ups were all negative from nasopharyngeal swabs for respiratory syncytial virus and influenza A and B (Polymerase chain reaction, PCR); mycoplasma immunoglobulin (Ig) M and IgG; scrub typhus IgM and IgG; CSF for herpes simplex virus and enterovirus (PCR). Serum and CSF analysis showed that the patient was positive NMDAR autoantibodies. Electroencephalogram (EEG) monitoring showed an absent sleep-wake pattern with generalized slow waves 1-3 Hertz with maximum amplitude at the bilateral frontal area with a delta brush pattern. Ictal EEG demonstrated an abrupt onset of generalized rhythmic delta waves accompanied by clinical screaming and visual hallucination.
The CT brain scan from the local hospital showed small hypodense lesions in the left lentiform nucleus, and follow-up CT scans 8 d later were unremarkable.
This patient presented with fever, behavioural dysfunction, sleep-wake disturbance, oromotor dyskinesia, seizure and visual hallucination. These clinical symptoms suggest infection or inflammation of the central nervous system (CNS), especially in the limbic system, which includes the hypothalamus, hippocampus and amygdala. Based on our patient’s clinical symptoms, the dysfunction also involved the frontal area and basal ganglia. The differential diagnoses were (1) infectious causes from viral, rickettsial or mycoplasma encephalitis or (2) noninfectious causes from autoimmune encephalitis or toxin/metabolic/Hashimoto’s encephalopathy. Oromotor dyskinesis and delta brush EEG patterns are the key features leading to the suspicion of anti-NMDAR encephalitis, and CSF autoantibody results later confirmed our preliminary diagnosis.
The patient received pulse methylprednisolone 10 mg/kg per day for 4 d on the first day after she arrived at our hospital because of EEG delta brush patterns along with antimicrobial agents, phenytoin and risperidone. Her clinical condition did not respond, and she received 2 g/kg intravenous immunoglobulin divided into 5 d followed by plasmapheresis for a total of 10 d. After the first-line regimen, she received a maintenance dose of prednisolone of 1.2 mg/kg per day. During the hospital course, she developed superrefractory status epilepticus and was sent to the paediatric intensive care unit for close monitoring and seizure control. Second-line regimen, such as cyclophosphamide or rituximab, was not given due to septicaemia.
On day 42 of prednisolone 1.2 mg/kg per day, her neurological status did not improve, and she developed upper gastrointestinal bleeding. The vomit was composed of coffee-ground content of 60 mL and bilious content of 30 mL. Her body temperature was 37.8°C. Abdominal examination revealed a distended and soft abdomen with hypoactive bowel sounds. She was consuming nothing orally, so a nasogastric tube was inserted, and intravenous fluid infusion and omeprazole intravenously 80 mg per day were initiated.
On days 43-50, the nasogastric fluid content remained bilious, with a volume of 250-1000 mL per day, and drooling occurred, with saliva content of up to 850 mL per day. There was a large volume of stool with an amount of 1-4 L per day despite no enteral feeding. The content was mostly greenish, watery and minimally mucous. The stool was sometimes mixed with old blood. Regarding the investigations, the haemoculture from the central line and arterial line revealed no microorganism growth. Stool examination revealed no white blood cells, red blood cells or evidence of suspected parasites. Stool culture revealed positive results for Pseudomonas aeruginosa. Imaging modalities were performed. The plain film acute abdomen series revealed decreased bowel gas from the fluid fill loop, and CT of the whole abdomen revealed circumferential wall thickening with mucosal hyperenhancement of the whole intestine, particularly the colon. The drooling from the saliva content could be inhibited with sublingual administration of atropine eye drops. Meropenem 120 mg/kg per day was started for Pseudomonas aeruginosa infection. Regarding intractable diarrhoea, antidiarrhoeal drugs were administered, including cholestyramine 80 mg/kg per dose every 8 h and octreotide 2 mcg/kg per dose subcutaneously every 12 h for many days without any improvement.
On day 50, the patient developed lower GI bleeding with a one-time amount of 400 mL.
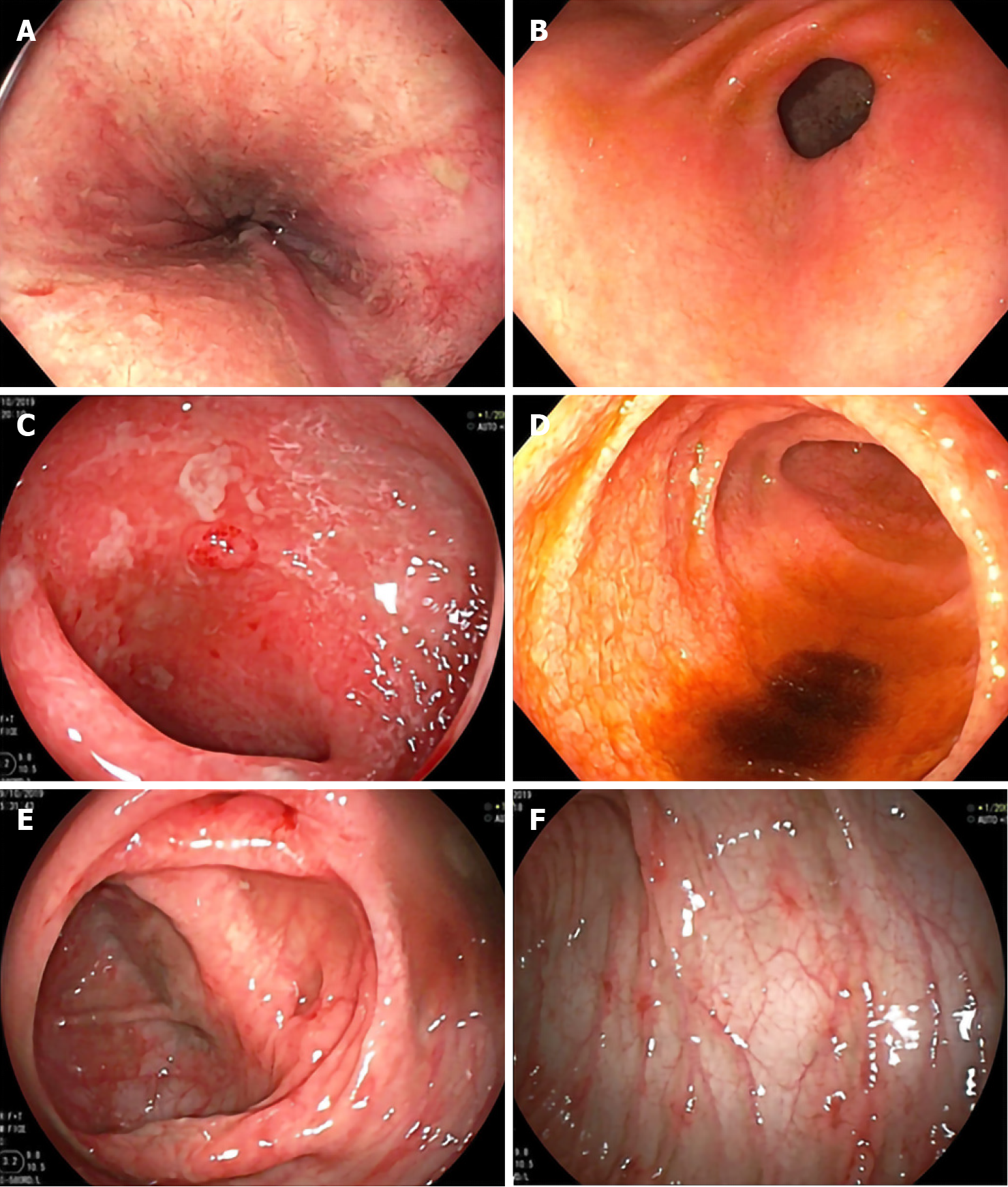
Then, we performed other stool test that revealed negative results for Clostridium difficile toxin, but the serum CMV PCR viral load revealed 5473 copies/mL. An esophagogastroduodenoscopy (EGD) and a colonoscopy showed whitish plaque on erythaematous and breaking mucosa at the mid-distal oesophagus, erythaematous mucosa in the stomach and first part of the duodenum, erythaematous friable mucosa with debris and multiple aphthous ulcers at the terminal ileum, and multiple small shallow ulcers on mild erythaematous mucosa in the caecum, ascending colon, transverse colon, descending colon, sigmoid and rectum (Figure 1). CMV viral load of the colonic tissue was > 500000 copies/mL.
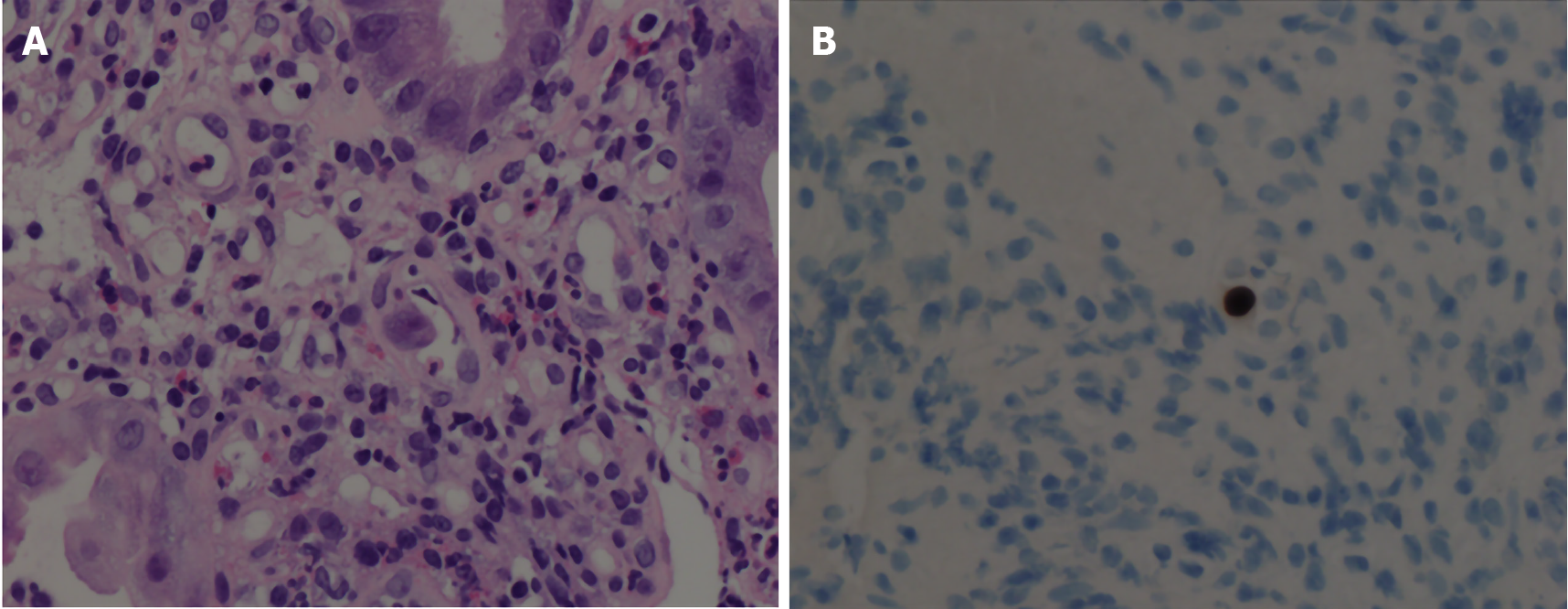
The oesophageal, gastric, ileal and colonic biopsies show mixed inflammatory cells, including neutrophils, eosinophils, lymphocytes, and plasma cells. There were scattered viral-infected cells characterized by enlarged cells with intranuclear and intracytoplasmic inclusions in the oesophagus, ileum and colon. Immunohisto-chemistry for CMV was positive in these cells (Figure 2).
This patient was previously diagnosed with anti-NMDAR encephalitis and received immunosuppressive drugs with pulse methylprednisolone 10 mg/kg per day for 4 d, together with intravenous immunoglobulin 2 g/kg divided into 5 d. Subsequently, she was maintained on prednisolone 1.2 mg/kg per day until day 42 of this medication regimen, her neurological status did not improve, and she developed severe gastrointestinal problems with drooling, unremitted bilious vomiting and diarrhoea. The diarrhoea was a mix of blood, mucous and mainly watery content. The disease itself might cause hypersalivation, vomiting and diarrhoea as a result of autonomic disturbance. However, mucous bloody stool is less likely a GI presentation of anti-NMDAR encephalitis, so the infectious cause was explored. The EGD, colonoscopy and histopathology with immunohistochemistry staining and tissue CMV viral load confirmed the diagnosis of esophago-enterocolitis from invasive CMV infection.
The patient was given ganciclovir 5 mg/kg per dose every 12 h for 3 wk and then shifted to ganciclovir 5 mg/kg per dose once daily for 3 wk. Unremitting diarrhoea slowly improved from stool output 1-4 L per day to 1-2 L per day after 3 wk of treatment and no content from the nasogastric tube. Due to her neurological status, pulse methylprednisolone 20 mg/kg for 5 d was initiated and continued with prednisolone 1 mg/kg per day. After this course of pulse methylprednisolone, surprisingly, diarrhoea subsided with a stool content of only 200-600 mg per day (Figure 3).
Due to the history of autoimmune encephalitis and invasive CMV esophago-enterocolitis, immunologic work-ups were performed to rule out an underlying immune deficiency. Serum IgG and IgM levels were transiently low: IgG 3.6 g/L (normal 6.98-11.94) and IgM 0.52 g/L (0.59-1) with normal IgA levels 0.34 g/L (0.22-2.74). However, the immunoglobulin levels were examined after 18 d of the second dose of pulse methylprednisolone. When the dose of corticosteroids was tapered or 35 d after the repeated dose of pulse methylprednisolone, serum IgG and IgM returned to normal: IgG 8.2 g/L (normal 6.98-11.94) and IgM 0.89 g/L (0.59-1). Flow cytometric analysis of lymphocyte populations was analysed only one time after she received the repeated dose of pulse methylprednisolone for 18 d. The results showed low numbers of total T lymphocytes (1127 cells/µL, 1400-2000), CD4+ T lymphocytes (647 cells/µL, 700-1100), CD8+ T lymphocytes (414 cells/µL, 600-900), NK lymphocytes (129 cells/µL, 27-693), and B lymphocytes (26, 300-500).
After 6 wk of ganciclovir and repeated doses of pulse methylprednisolone, EGD and colonoscopy were performed and showed normal mucosa of the oesophagus, stomach, duodenum, ileum and colon, and no virus-infected cells were seen from the histopathological specimen. Then, cyclophosphamide as the maintenance therapy for anti-NMDAR encephalitis was introduced. No serious infection or diarrhoea occurred after the course of cyclophosphamide. Otherwise, the patient gradually improved her neurological status. She remained stable during 10 mo of follow-up.
We reported a case of a 12-year-old Thai girl with a diagnosis of anti-NMDAR encephalitis who later presented with abdominal bloating, feeding intolerance due to drooling from hypersalivation, unremitting bilious vomiting and diarrhoea. Later, CMV esophago-enterocolitis was diagnosed. Severe gastrointestinal manifestations from invasive CMV are mostly described in heavily immunosuppressed patients, including HIV-infected patients, bone marrow or solid organ transplantation patients or patients with underlying primary immune deficiency. The present study demonstrated that an adolescent girl with anti-NMDAR encephalitis who received a moderate dose of corticosteroids presented with very severe invasive CMV infection of the GI system. Surprisingly, after CMV eradication, diarrhoea slowly improved but completely subsided after administering a higher dose of corticosteroid.
CMV, also known as human herpes virus 5, is one of the herpes viruses. It is highly host-specific to one species[6]. Transmission occurs via direct person-to-person contact, such as contact with infected patient secretion including urine and saliva or sexual transmission. Other routes of transmission are via mother to child, antenatal, perinatal or postnatal routes and through blood product transfusion and organ transplantation. After one is exposed to the virus, CMV will remain in a latent form. Under some appropriate conditions, particularly impaired immune status, the virus can reactivate and cause symptoms. Infection occurs approximately 3 to 12 wk after blood transfusions[6] and approximately 1 to 4 mo after organ transplantation[6] The pathogen can infect many targets including kidneys, pancreas, intestine, liver, heart, lung, tissue allograft, CNS or blood cells[7], and, rarely, skin[8]. The incubation period for horizontal transmission remains unknown. Clinical manifestations vary from asymptomatic, particularly in children, to symptomatic including infectious mononucleosis-like syndrome. In hosts with predominantly T-cell deficiency, it is possible to develop invasive CMV infection. GI involvement of CMV typically presents as mucous bloody diarrhoea, as the colon is mainly involved in up to 94% of cases[9-11]. Several studies have reported the symptom of intractable diarrhoea that most likely occurs in the extremely aged population, infants[12,13] and elderly individuals[10,14]. To the best of our knowledge, this is a first case report of an adolescent girl diagnosed with autoimmune encephalitis who had both intractable diarrhoea and unremitting vomiting from CMV esophago-enterocolitis. The mechanism of secretory diarrhoea in this patient might be from the excessive stimulation of chloride-secretion by the release of tumor necrosis factor (TNF) and interleukin-6 (IL-6) from the CMV-infected monocytes and macrophages during the innate inflammatory response. According to one study, CMV was mainly detected by CD4 and toll-like receptor 2[15], which are mainly expressed in monocytes and macrophages. The CMV-infected cells then undergo reprogramming of gene expression involved in the innate immune response, lead to macrophage overactivation and massive inflammatory cytokine production, including TNF-alpha and IL-6[16]. Moreover, the macrophages mostly reside in the GI mucosa[16].
The potential risk factors for CMV esophago-enterocolitis based on our speculation include a moderate level of corticosteroid exposure. Interestingly, apart from reports on very severely immunocompromised hosts who underwent solid organ transplantation and on primary or secondary T-cell immunocompromised patients, there have been few reports of severe invasive CMV infection in patients who received pulse methylprednisolone or moderate dosage of prednisolone. Ozaki et al[17] retrospectively studied 3733 patients with rheumatic disease and found only 9 patients who had CMV of the upper GI tract. Two patients received pulse methylprednisolone at doses of 0.5 and 1 g per day for 3 consecutive days followed by prednisolone at doses of 40 and 45 mg per day, respectively. Eight patients received more than 1 immunosuppressant (prednisolone, cyclophosphamide or methotrexate). Moreover, there have been 4 other case reports of rheumatic diseases[18-21] in which 2 patients received pulse methylprednisolone followed by prednisolone, and 2 patients received only prednisolone therapy and developed CMV of upper GI tract. The significant risk factors for inactive CMV infection in a previous study of adults with rheumatic diseases were advanced age, lymphopenia and corticosteroid used[22]. Regarding our patient, the girl received a moderate dose of corticosteroids and had secondary lymphopenia after that, which might reflect T-cell suppression leading to the invasive CMV infection. As there is no evidence of recurrent infections before this episode or family history of primary immunodeficiency and because the hypogammag-lobulinemia was improved after the dose of corticosteroids was reduced, underlying immune deficiency in this patient was not likely. Evidently, serum immunoglobulins and numbers of lymphocyte subpopulations can decrease with corticosteroid administration[23,24]. Consequently, our hypothesis is that anti-NMDAR encephalitis itself concomitant with temporary lymphopenia might have been another major factors in this severe CMV infection.
Previous studies in rats[3,25,26] and humans[27] have demonstrated the presence of NMDA receptors in the ENS, which are responsible for visceral hypersensitivity and the motor function of the ENS, leading to abdominal pain, diarrhoea or accelerated gastrointestinal transit time in the setting of colonic inflammation[28]. In the clinical setting of patients with anti-NMDAR encephalitis, only 3 separate case reports have mentioned GI symptoms as the initial presentation or during disease treatment. Li-Ying Liu et al[29] reported a 3-year-old boy who initially presented with severe vomiting and abdominal pain, developed a progressive alteration of mental status to delirium in 2 mo, developed progressive weakness and mutism and was later diagnosed with anti-NMDAR encephalitis. Another case reported by McCoy et al[30] was a 6-year-old boy who presented with diarrhoea and vomiting followed by subacute progression of neuropsychiatric symptoms who was finally diagnosed with anti-NMDAR encephalitis. The last case was reported by Li et al[4]: An 11-year-old girl who presented with acute alteration of consciousness, intermittent irrelevant speech and agitation at night. Diagnosis of anti-NMDAR encephalitis was made from detection of anti-NMDAR antibodies in both the serum and CSF. Two months after her admission, she experienced vomiting, abdominal pain and bleeding following intractable watery diarrhoea. Colonoscopy showed pancolitis, and unfortunately, she died from multiorgan failure 68 d after the symptom of unremitting diarrhoea. These case reports and our present case underscored GI involvement in patients with anti-NMDAR encephalitis. In our case report, we found invasive CMV infection along the GI tract, and severe GI symptoms exhibited a partial response after antiviral therapy and a complete response after the higher dose of immunosuppressant was used to treat anti-NMDAR encephalitis. The pathogenesis of invasive CMV infection in this patient is not exactly well understood. Immune dysfunction in this autoimmune encephalitis patient is unlikely, while complex immune dysregulation is continuing to be studied. The anti-NMDA receptors in the ENS might be stimulated by the inflammatory process following CMV infection, affecting motor function of the ENS and later causing severe GI motility disorders of intractable vomiting and diarrhoea. In general practice, once severe invasive CMV disease is confirmed, immunosuppressive agents should be decreased as much as possible to restore immune function, and initiation of antiviral therapy is merited. Most patients had a good clinical response to antiviral therapy within 1-3 wk of initiation[31-34], which is not consistent with our case in which diarrhoea did not subside after 3 wk of therapy. Hence, we combined the treatment for CMV and anti-NMDAR encephalitis later because the clinical response was not satisfactory, as the immunosuppressant might restore the severe GI symptoms through the attenuation of the NMDA receptors in the ENS. Further studies on GI manifestations or complications in patients with anti-NMDAR encephalitis are needed.
This case report highlighted GI involvement in a patient with anti-NMDAR encephalitis from invasive CMV, with partial response after antiviral therapy but complete response after a high dose of pulse methylprednisolone was provided as the first-line therapy for anti-NMDAR encephalitis. Immune dysregulation in autoimmune encephalitis and the relationship of NMDA receptors with ENS and GI symptoms should be further studied.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 174508.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Fujino Y, Ueda H S-Editor: Wang JL L-Editor: A P-Editor: Ma YJ
| 1. | Vora SB, Englund JA. Cytomegalovirus in immunocompromised children. Curr Opin Infect Dis. 2015;28:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Goldberg EM, Titulaer M, de Blank PM, Sievert A, Ryan N. Anti-N-methyl-D-aspartate receptor-mediated encephalitis in infants and toddlers: case report and review of the literature. Pediatr Neurol. 2014;50:181-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Pruetarat N, Netbaramee W, Pattharathitikul S, Veeravigrom M. Clinical manifestations, treatment outcomes, and prognostic factors of pediatric anti-NMDAR encephalitis in tertiary care hospitals: A multicenter retrospective/prospective cohort study. Brain Dev. 2019;41:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Li R, Jiang L, Li XJ, Hong SQ, Zhong M, Hu Y. Analysis and discussion of the rare complication of autoimmune encephalitis: Two case reports. Medicine (Baltimore). 2018;97:e11202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Pang LY, Ding CH, Wang YY, Liu LY, Li QJ, Zou LP. Acute autonomic neuropathy with severe gastrointestinal symptoms in children: a case series. BMC Neurol. 2017;17:164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | American Academy of Pediatrics, Pickering LK. Red book: 2009 report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village: American Academy of Pediatrics, 2009: 275-280. [Cited in This Article: ] |
| 7. | Razonable RR, Humar A; AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:93-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 365] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 8. | Trimarchi H, Casas G, Jordan R, Martínez J, Schropp J, Freixas EA, Efrón E. Cytomegalovirus maculopapular eruption in a kidney transplant patient. Transpl Infect Dis. 2001;3:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Patra S, Samal SC, Chacko A, Mathan VI, Mathan MM. Cytomegalovirus infection of the human gastrointestinal tract. J Gastroenterol Hepatol. 1999;14:973-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lee CY, Chen YH, Lu PL. Reactivated cytomegalovirus proctitis in an immunocompetent patient presenting as nosocomial diarrhea: a case report and literature review. BMC Infect Dis. 2017;17:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Fakhreddine AY, Frenette CT, Konijeti GG. A Practical Review of Cytomegalovirus in Gastroenterology and Hepatology. Gastroenterol Res Pract. 2019;2019:6156581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Huang Z, Ye Z, Zheng C, Jiang Z, Huang Y. Cytomegalovirus enteritis with intractable diarrhea in infants from a tertiary care center in China. Scand J Gastroenterol. 2020;55:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Sue PK, Salazar-Austin NM, McDonald OG, Rishi A, Cornish TC, Arav-Boger R. Cytomegalovirus Enterocolitis in Immunocompetent Young Children: A Report of Two Cases and Review of the Literature. Pediatr Infect Dis J. 2016;35:573-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | John SG, Dominguez C, Chandiramani V, Vemulappalli T. A rare case intractable diarrhea secondary to Clostridium difficile and cytomegalovirus coinfection. Am J Case Rep. 2013;14:498-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588-4596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 499] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Redman TK, Britt WJ, Wilcox CM, Graham MF, Smith PD. Human cytomegalovirus enhances chemokine production by lipopolysaccharide-stimulated lamina propria macrophages. J Infect Dis. 2002;185:584-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ozaki T, Yamashita H, Kaneko S, Yorifuji H, Takahashi H, Ueda Y, Takahashi Y, Kaneko H, Kano T, Mimori A. Cytomegalovirus disease of the upper gastrointestinal tract in patients with rheumatic diseases: a case series and literature review. Clin Rheumatol. 2013;32:1683-1690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Henson D. Cytomegalovirus inclusion bodies in the gastrointestinal tract. Arch Pathol. 1972;93:477-482. [PubMed] [Cited in This Article: ] |
| 19. | Iwasaki T. Alimentary tract lesions in cytomegalovirus infection. Acta Pathol Jpn. 1987;37:549-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Tokunaga N, Sadahiro S, Kise Y, Suzuki T, Mukai M, Yasuda S, Ogoshi K, Tajima T, Makuuchi H. Gastrointestinal cytomegalovirus infection in collagen diseases. Tokai J Exp Clin Med. 2003;28:35-38. [PubMed] [Cited in This Article: ] |
| 21. | Panteris V, Karakosta A, Merikas E, Peros G, Triantafillidis JK. Gastric Outlet Obstruction due to Cytomegalovirus Infection in an Immunocompromised Patient. Case Rep Gastroenterol. 2009;3:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, Kameda H, Hirohata S, Kondo H, Kumagai S, Tanaka Y. Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford). 2008;47:1373-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Settipane GA, Pudupakkam RK, McGowan JH. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol. 1978;62:162-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 129] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, Zhou H, Wang E, Tsang JS, Nussenblatt R; CHI Consortium. Effects of Systemically Administered Hydrocortisone on the Human Immunome. Sci Rep. 2016;6:23002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Park JH, Moon HS. Cytomegalovirus colitis in immunocompetent patients. Intest Res. 2018;16:504-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Ko JH, Peck KR, Lee WJ, Lee JY, Cho SY, Ha YE, Kang CI, Chung DR, Kim YH, Lee NY, Kim KM, Song JH. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60:e20-e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Qi Q, Chen F, Zhang W, Wang P, Li Y, Zuo X. Colonic N-methyl-d-aspartate receptor contributes to visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2017;32:828-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Zhou Q, Nicholas Verne G. NMDA Receptors and Colitis: Basic Science and Clinical Implications. Rev Analg. 2008;10:33-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Liu LY, Wang YY, Pang LY, Zhang GX, Zou LP. Anti-N-Methyl-D-Aspartate Receptor Encephalitis in a 3-Year-Old Toddler with the Involvement of Severe Autonomic Dysfunctions in Gastrointestinal System: A Case Report. J Pediatr Neurol. 2019;17:41-44. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | McCoy B, Akiyama T, Widjaja E, Go C. Autoimmune limbic encephalitis as an emerging pediatric condition: case report and review of the literature. J Child Neurol. 2011;26:218-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Seo TH, Kim JH, Ko SY, Hong SN, Lee SY, Sung IK, Park HS, Shim CS, Han HS. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology. 2012;59:2137-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 18] [Reference Citation Analysis (0)] |
| 32. | Yerushalmy-Feler A, Padlipsky J, Cohen S. Diagnosis and Management of CMV Colitis. Curr Infect Dis Rep. 2019;21:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Jones A, McCurdy JD, Loftus EV Jr, Bruining DH, Enders FT, Killian JM, Smyrk TC. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin Gastroenterol Hepatol. 2015;13:949-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Jain R, Trehan A, Mishra B, Singh R, Saud B, Bansal D. Cytomegalovirus disease in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2016;33:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |