Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.616
Peer-review started: December 20, 2018
First decision: January 5, 2019
Revised: January 12, 2019
Accepted: January 29, 2019
Article in press: January 29, 2019
Published online: March 6, 2019
Squamous cell carcinoma of head and neck (SCCHN) is the fifth most common cancer worldwide. Inhibition of epidermal growth factor receptor signaling has been shown to be a critical component of therapeutic option. Herein, we report a case of durable complete response to erlotinib.
An 81-year-old Caucasian male who presented with metastatic poorly differentiated squamous cell carcinoma of right cervical lymph nodes (levels 2 and 3). Imaging studies including (18)F-fluorodeoxyglucose positron emission tomography/computed tomography (CT) and contrast-enhanced CT scan of neck and chest did not reveal any other disease elsewhere. Panendoscopic examination with random biopsy did not reveal malignant lesion in nasopharynx, oropharynx, and larynx. He underwent modified neck dissection and postoperative radiation. Within 2 mo after completion of radiation, he developed local recurrence at right neck, which was surgically removed. Two mo after the salvage surgery, he developed a second recurrence at right neck. Due to suboptimal performance status and his preference, he started erlotinib treatment. He achieved partial response after first 2 mo of erlotinib treatment, then complete response after total 6 mo of erlotinib treatment. He developed sever skin rash and diarrhea including Clostridium difficile infection during the course of erlotinib treatment requiring dose reduction and eventual discontinuation. He remained in complete remission for more than two years after discontinuation of erlotinib.
We report a case of metastatic SCCHN achieving durable complete response from erlotinib. Patient experienced skin rash and diarrhea toxicities which were likely predictors of his treatment response.
Core tip: We present a patient with recurrent/metastatic squamous cell carcinoma of head and neck who had durable complete response after completion of 6-mo erlotinib treatment. Patient experienced severe skin rash and diarrhea toxicities from erlotinib. The severity of these adverse effects has been shown to be predictors of treatment response from inhibitors of epidermal growth factor receptor.
- Citation: Thinn MM, Hsueh CT, Hsueh CT. Sustained complete response to erlotinib in squamous cell carcinoma of the head and neck: A case report. World J Clin Cases 2019; 7(5): 616-622
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/616.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.616
Squamous cell carcinoma of head and neck (SCCHN) is the fifth most common cancer worldwide with a global yearly incidence of more than 600000 new cases and around 300000 annual deaths in 2012[1]. Early-stage disease is managed with either surgery or radiation. Two-third of patients present with locally advanced disease, and are generally treated with multimodality therapy, which commonly includes chemotherapy[2]. Tumor control and survival in SCCHN remain unsatisfactory. Even for those who have achieved complete response after initial treatment, the incidence of local recurrence is 50%-60% and the incidence of distant metastases is 20%-30%. Systemic therapy is the mainstay for metastatic disease and unsalvageable recurrence to improve survival and quality of life.
With recent advances in cancer biology, there appear to be common molecular events in SCCHN that are biologically significant in cell survival and invasion, and could be used for therapeutic development such as epidermal growth factor receptor (EGFR). Overexpression of EGFR and its ligand have been reported in 80% to 90% of SCCHN tumors compared with levels in normal mucosa of patients without cancer[3]. Increased EGFR expression has been reported to be a predictor of worse survival in SCCHN patients receiving surgery and chemotherapy[4,5]. The two targeting strategies for inhibition of EGFR are small-molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies directed against the receptor. Cetuximab (a monoclonal antibody against EGFR) has been approved by United States Food and Drug Administration as initial treatment of locally advanced SCCHN in combination with radiation therapy, as first-line treatment of recurrent or metastatic SCCHN in combination with platinum-based therapy plus 5-fluorouracil, and as a single agent for recurrent or metastatic SCCHN progressing from prior platinum-based therapy[2]. Adding cetuximab to platinum-based chemotherapy with 5-fluorouracil as first-line treatment of recurrent or metastatic SCCHN significantly prolonged the median overall survival[6]. Herein, we report a case of sustained complete response to erlotinib, a TKI of EGFR, as first-line treatment for recurrent SCCHN.
An 81-year-old Caucasian male presented with right neck mass.
He had no history of cigarette smoking or alcohol use.
He had an extensive history of multiple skin lesions removed from his scalp, arms, chest and back over 10-15 years prior to presentation. Most of the skin lesions were squamous cell carcinoma except one lesion was in-situ melanoma. Other significant past medical history included diabetes mellitus type II, hypertension, hypothyroidism and remote cerebrovascular accident with mild residual dysarthria and right central vision loss.
Family history was pertinent for mother died of liver cancer at age 86.
Physical examination was unremarkable except right neck lymphadenopathy.
Imaging studies including (18)F-fluorodeoxyglucose positron emission tomo-graphy/computed tomography (CT) and contrast-enhanced CT (CECT) scan of neck and chest revealed right cervical lymphadenopathy. The panendoscopic examination of ENT field did not reveal any abnormality, and the random biopsy of nasopharynx, oropharynx, and larynx showed no evidence of malignancy. Excisional biopsy of right neck mass turned out to be metastatic poorly differentiated squamous cell carcinoma. He underwent right modified neck dissection with seventeen lymph nodes removed from levels 2, 3, 4 and 5. Two of the two lymph nodes from level 2 had evidence of metastatic carcinoma with the largest focus being 2.5 cm and extracapsular extension being present. Three of the five lymph nodes from level 3 were positive for metastatic carcinoma with largest focus being 0.7 cm and extracapsular extension being present. None of the lymph nodes from level 4 or 5 were found to have any metastatic carcinoma. Shortly after postoperative intensity-modulated radiotherapy to right neck with total dose of 66 Gy, he developed local recurrence at right neck and underwent salvage surgery.
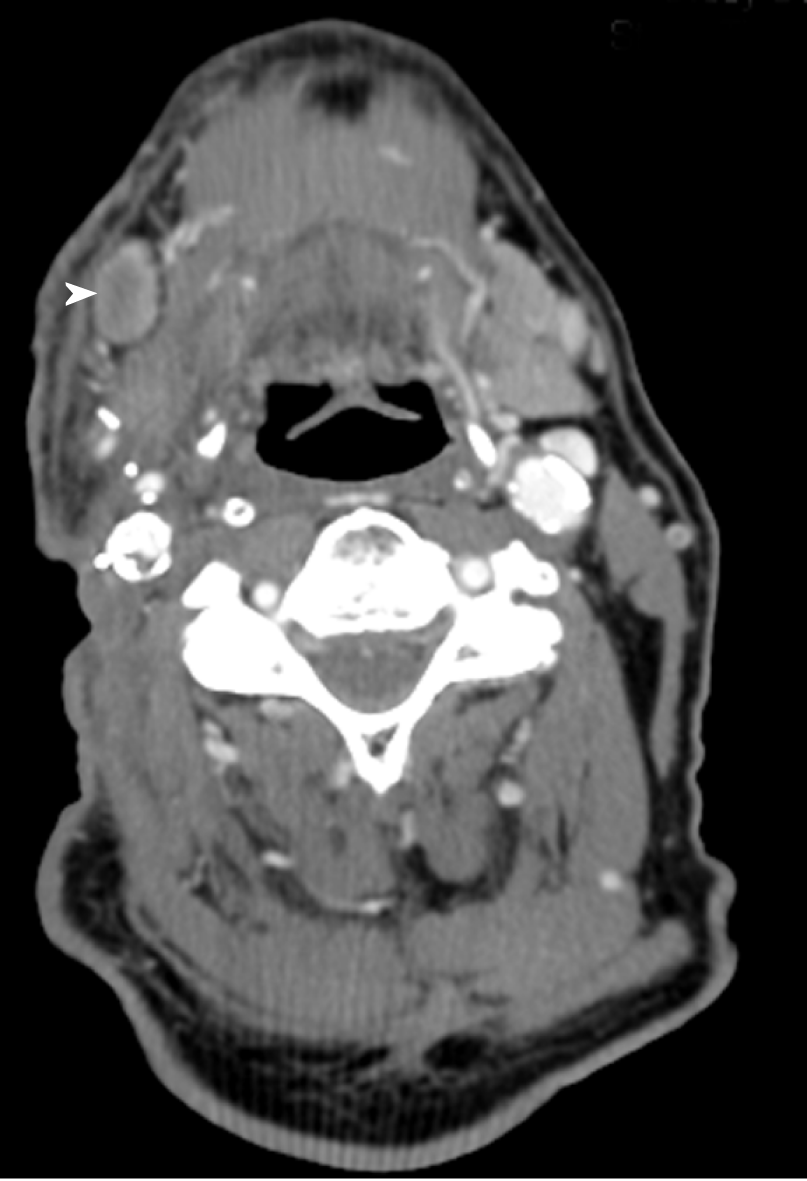
Two mo after salvage surgery, he was found to have palpable skin nodules over the right neck. CECT scan of the neck showed interval increase in the soft tissue thickening and diffuse subcutaneous thickening of the right neck. Focal skin irregularity and interval development of submental, right submandibular adenopathy as well as adenopathy at the right inferior parotid compatible with recur-rent/metastatic SCCHN were noted (Figure 1).
Treatment options were discussed including platinum based chemotherapeutic regimen and targeted therapy. Due to patient’s preference and literature support (see discussion), erlotinib 150 mg orally per day was started. He tolerated the treatment well initially, and achieved partial response after 2 mo of treatment. Subsequently, he developed grade 3 toxicities including skin rash and diarrhea with Clostridium difficile infection requiring dose reduction of erlotinib from 150 mg to 100 mg daily.
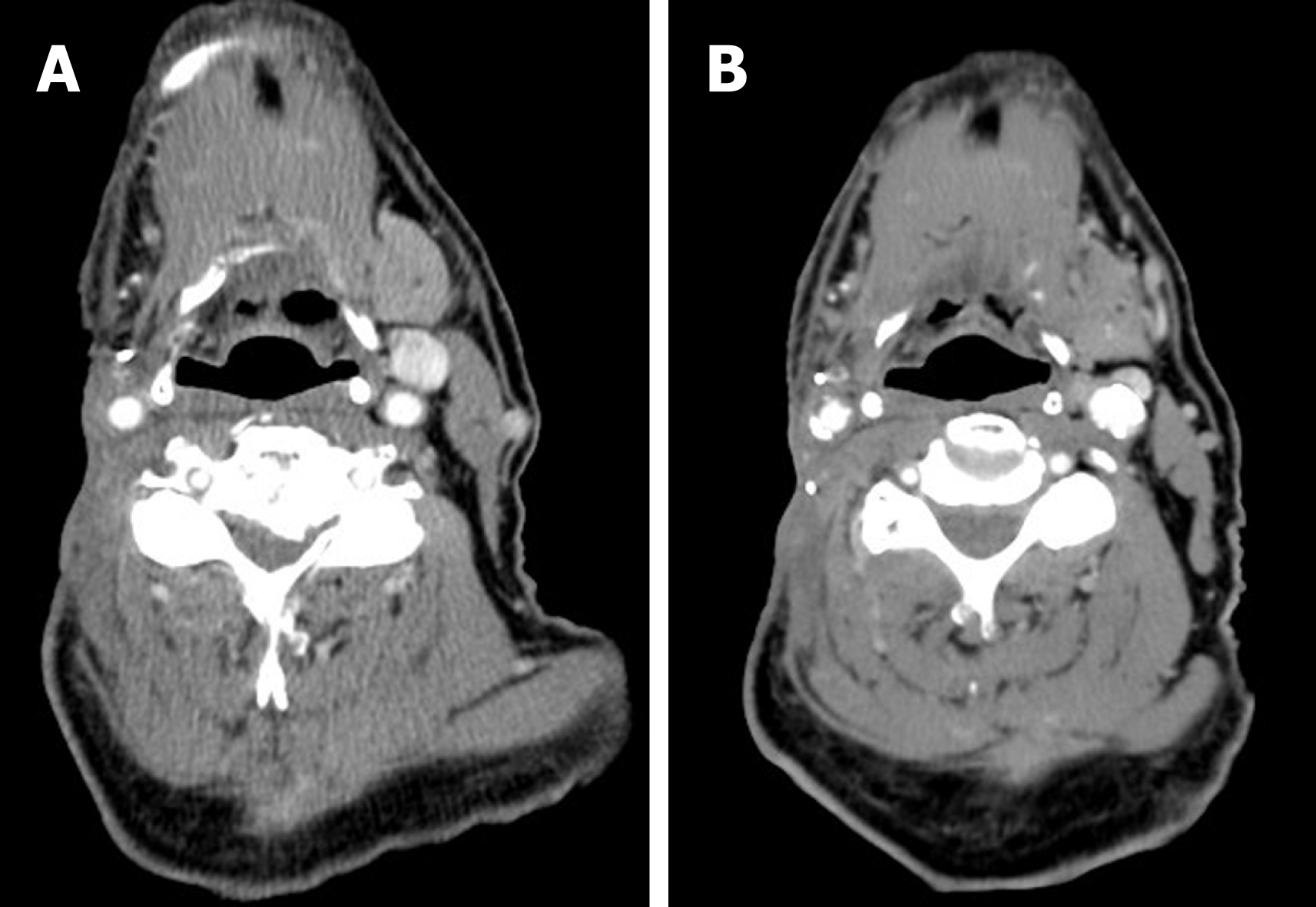
He eventually had complete response after total 6 mo of treatment (Figure 2). Erlotinib was discontinued due to intolerance. He remained free of recurrent disease for more than two years. Subsequently he succumbed to death due to postoperative complication with respiratory failure after resection of an ulcerating skin lesion at right clavicular head.
Our patient presented with SCCHN of unknown primary with cervical lymph node metastasis. His primary site of origin has never been identified over the course of the subsequent workup and follow-up. Retrospective analyses indicate SCCHN of unknown primary represents about 3% of newly diagnosed SCCHN[7]. Most SCCHN of unknown primary may represent clinically occult oropharyngeal cancer. The management of SCCHN of unknown primary is aimed at curative for most patients; cervical lymphadenopathy indicates locally advanced disease and is amenable for multimodality treatment such as surgery and radiotherapy.
Our patient developed regional recurrence shortly after initial surgery with neck dissection and post-operative radiotherapy. Despite of salvage surgery, he developed recurrent disease at prior surgical site two mo later. Due to suboptimal performance status and patient’s preference, he received systemic therapy with erlotinib, which is an EGFR TKI. EGFR TKIs inhibit EGFR and downstream signaling leading to apoptosis. Advantages of TKIs include the ease of oral administration. As shown in Table 1, erlotinib, lapatinib, gefitinib and afatinib have been studied in phase II/III trials[8-11]. Most of them showed promising activities in SCCHN, but failed to demonstrate improved survival compared to chemotherapy. A multicenter phase II study evaluated erlotinib in the treatment of recurrent or metastatic SCCHN showed the overall objective response rate of 4.3% in 115 patients. Forty-seven percent of patients received erlotinib at 150 mg daily throughout the entire study, 6% had dose escalations, and 46% required dose reductions and/or interruptions. Stable disease for a median duration of 16.1 wk was noted in 38% of patient. The median progression-free survival was 9.6 wk, and the median overall survival was 6 mo. Better overall survival was observed in patients who developed grade 2 or higher rash. Rash was the most common adverse event, observed in 79% of patients, followed by diarrhea, which was seen in 37% of patients. Most of the adverse events were mild to moderate[8].
Retrospective analyses of clinical trials investigating EGFR TKIs in SCCHN have shown skin rash and diarrhea are common toxicities and severity of these side effects correlates with therapeutic responses[12]. Skin rash has been shown to a predictor of response to EGFR TKI in patients with non-small cell lung cancer, likely due to skin injury caused by inhibition of EGFR signaling in epidermal cells[13]. Many factors may affect severity of skin rash including genetic variations in EGFR and metabolism of EGFR TKI[14]. Higher drug levels may result from polymorphisms in metabolizing enzymes such as cytochrome P450 family. The severe skin rash and diarrhea toxicities seen in our case are likely predictors of good treatment response from erlotinib.
In non-small cell lung carcinoma, patients with activating mutations in the EGFR tyrosine kinase domain are sensitive to EGFR TKIs. However, the incidence of this type of EGFR mutations is low and fails to predict sensitivity to EGFR TKIs in SCCHN[15]. Van Allen et al[16] reported a patient with locally advanced SCCHN achieving a near-complete pathological response after 13 d of neoadjuvant erlotinib treatment. After surgical resection, histologic evaluation revealed 2 residual foci (approximately 2 mm each) of invasive, moderately differentiated squamous cell carcinoma within the primary site but there was no evidence of lymph node metastasis. The patient did not receive adjuvant therapy and had no evidence of disease recurrence 24 mo postoperatively. Whole exome sequencing of the pretreatment tumor revealed a MAPK1 E322K somatic mutation, which was further implicated in mediating erlotinib sensitivity. This response to erlotinib occurred in the context of an activating somatic MAPK1 E322K mutation, which led to increased EGFR ligand production and EGFR activation in preclinical studies[17].
Recent adoption of next-generation sequencing in genomic testing of tumor has provided opportunity to advance precision medicine. Dumbrava et al[18] reported a case of metastatic SCCHN who progressed on a phase II clinical trial with erlotinib, docetaxel and cisplatin. Patient’s tumor was subsequently biopsied and found to have amplification of fibroblast growth factor genes by next-generation sequencing. Patient was treated with fibroblast growth factor receptor inhibitor on clinical trial and achieved complete response for about 9 mo[18].
We report a case of durable complete response to erlotinib in SCCHN. Patient experienced skin rash and diarrhea toxicities which were likely predictors of his treatment response. The use of next-generation sequencing in genomic profiling of tumor samples may select patients who will likely respond to targeted therapy.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bhalla AS, Dasgupta S S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19780] [Article Influence: 2197.8] [Reference Citation Analysis (17)] |
| 2. | Marur S, Forastiere AA. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc. 2016;91:386-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 703] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 3. | Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579-3584. [PubMed] [Cited in This Article: ] |
| 4. | Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 826] [Cited by in F6Publishing: 860] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Etienne MC, Pivot X, Formento JL, Bensadoun RJ, Formento P, Dassonville O, Francoual M, Poissonnet G, Fontana X, Schneider M, Demard F, Milano G. A multifactorial approach including tumoural epidermal growth factor receptor, p53, thymidylate synthase and dihydropyrimidine dehydrogenase to predict treatment outcome in head and neck cancer patients receiving 5-fluorouracil. Br J Cancer. 1999;79:1864-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2546] [Cited by in F6Publishing: 2367] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 7. | Strojan P, Ferlito A, Medina JE, Woolgar JA, Rinaldo A, Robbins KT, Fagan JJ, Mendenhall WM, Paleri V, Silver CE, Olsen KD, Corry J, Suárez C, Rodrigo JP, Langendijk JA, Devaney KO, Kowalski LP, Hartl DM, Haigentz M, Werner JA, Pellitteri PK, de Bree R, Wolf GT, Takes RP, Genden EM, Hinni ML, Mondin V, Shaha AR, Barnes L. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck. 2013;35:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 9. | Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Hirsch FR, Varella-Garcia M, Ghiorghiu S, Hargreaves L, Armour A, Speake G, Swaisland A, Vokes EE. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27:1864-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, Clement PM, Gauler T, Cupissol D, Grau JJ, Guigay J, Caponigro F, de Castro G, de Souza Viana L, Keilholz U, Del Campo JM, Cong XJ, Ehrnrooth E, Cohen EE; LUX-H&N 1 investigators. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | de Souza JA, Davis DW, Zhang Y, Khattri A, Seiwert TY, Aktolga S, Wong SJ, Kozloff MF, Nattam S, Lingen MW, Kunnavakkam R, Stenson KM, Blair EA, Bozeman J, Dancey JE, Vokes EE, Cohen EE. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:2336-2343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:e155-e160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Ruan Y, Jiang J, Guo L, Li Y, Huang H, Shen L, Luan M, Li M, Du H, Ma C, He L, Zhang X, Qin S. Genetic Association of Curative and Adverse Reactions to Tyrosine Kinase Inhibitors in Chinese advanced Non-Small Cell Lung Cancer patients. Sci Rep. 2016;6:23368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Liu S, Kurzrock R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat Rev. 2014;40:883-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Perisanidis C. Prevalence of EGFR Tyrosine Kinase Domain Mutations in Head and Neck Squamous Cell Carcinoma: Cohort Study and Systematic Review. In Vivo. 2017;31:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Van Allen EM, Lui VW, Egloff AM, Goetz EM, Li H, Johnson JT, Duvvuri U, Bauman JE, Stransky N, Zeng Y, Gilbert BR, Pendleton KP, Wang L, Chiosea S, Sougnez C, Wagle N, Zhang F, Du Y, Close D, Johnston PA, McKenna A, Carter SL, Golub TR, Getz G, Mills GB, Garraway LA, Grandis JR. Genomic Correlate of Exceptional Erlotinib Response in Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2015;1:238-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Wen Y, Li H, Zeng Y, Wen W, Pendleton KP, Lui VW, Egloff AM, Grandis JR. MAPK1E322K mutation increases head and neck squamous cell carcinoma sensitivity to erlotinib through enhanced secretion of amphiregulin. Oncotarget. 2016;7:23300-23311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Dumbrava EI, Alfattal R, Miller VA, Tsimberidou AM. Complete Response to a Fibroblast Growth Factor Receptor Inhibitor in a Patient With Head and Neck Squamous Cell Carcinoma Harboring FGF Amplifications. JCO Precision Oncology. 2018;1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |