Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3018
Peer-review started: April 8, 2019
First decision: June 12, 2019
Revised: June 16, 2019
Accepted: July 27, 2019
Article in press: July 27, 2019
Published online: October 6, 2019
Cardiac tumors are rare and complex entities. Surgery represents the cornerstone of therapy, while the role of adjuvant treatment remains unclear and, in case of relapse or metastatic disease, the prognosis is very poor. Lack of prospective, randomized clinical trials hinders the generation of high level evidence for the optimal diagnostic workup and multimodal treatment of cardiac sarcomas. Herein, we describe the multidisciplinary clinical management and molecular characterization of a rare case of cardiac myxofibrosarcoma in an elderly woman.
A 73-year-old woman presented signs and symptoms of acute left-sided heart failure. Imaging examination revealed a large, left atrial mass. With suspicion of a myxoma, she underwent surgery, and symptoms were promptly relieved. Histology showed a cardiac myxofibrosarcoma, a rare histotype of cardiac sarcoma. Eight months later, disease unfortunately relapsed, and after a multidisciplinary discussion, a chemotherapy with doxorubicin and then gemcitabine was started, achieving partial radiologic and complete metabolic response, which was maintained up to 2 years and is still present. This report is focused on the entire clinical path of our patient from diagnosis to follow-up, through surgery and strategies adopted at relapse. Moreover, due to their rarity, very little is known about the molecular landscape of myxofibrosarcomas. Thus, we also performed and described preliminary genome analysis of the tumor tissue to get further insight on mechanisms involved in tumor growth, and to possibly unveil new clinically actionable targets.
We report a case of cardiac myxofibrosarcoma that achieved a very good prognosis due to an integrated surgical, cardiac and oncologic treatment strategy.
Core tip: We present the case of an elderly woman affected by myxofibrosarcoma of the left atrium, a rare histotype of cardiac sarcoma. She underwent surgery, however disease unfortunately recurred. Therefore, she started first and second line chemotherapy with doxorubicin and then gemcitabine, obtaining a good and durable radiologic response and a complete metabolic response. An accurate histologic description is reported, and molecular characterization of the tumor, by whole transcriptome sequencing, is also encompassed. This is done in order to better characterize this rare cancer, and to possibly identify pathognomonic molecular events or gene expression profiles shared with other sarcomas amenable for targeted medical therapy.
- Citation: Saponara M, Indio V, Pizzi C, Serban ED, Urbini M, Astolfi A, Paolisso P, Suarez SM, Nannini M, Pacini D, Agostini V, Leone O, Ambrosini V, Tarantino G, Fanti S, Niro F, Buia F, Attinà D, Pantaleo MA. Successful multidisciplinary clinical approach and molecular characterization by whole transcriptome sequencing of a cardiac myxofibrosarcoma: A case report. World J Clin Cases 2019; 7(19): 3018-3026
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3018.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3018
Primary cardiac tumors are a rare entity, being predominantly benign. Only 25% are malignant, with sarcomas accounting for most of them[1]. The clinical presentation depends on tumor size and anatomical location rather than on histological type. It varies from symptoms of congestive heart failure to thromboembolism and arrhythmias[2].
We report the case of an elderly woman who presented signs and symptoms of acute left-sided heart failure caused by a myxofibrosarcoma of the left atrium, which is a rare histotype of cardiac sarcoma.
The molecular characterization of the tumor, by whole transcriptome sequencing, is also encompassed.
A 73-year-old woman presented dyspnea on exertion, paroxysmal nocturnal dyspnea and cough.
She had a history of arterial hypertension, dyslipidemia and diabetes mellitus.
The family history did not reveal any remarkable information.
Physical examination revealed signs and symptoms of acute left-sided heart failure, particularly fatigue and breathing difficulty, whereas no evident signs of deep vein thrombosis were observed.
A chest X-ray showed signs of pulmonary edema. In order to exclude a pulmonary thromboembolism, angio-computed tomography (CT) was performed and results were negative, instead showing a left atrial mass. Two-dimensional transthoracic echocardiography (TTE) showed an enlarged left atrium and severe mitral valve stenosis due to a mobile, round mass (Video). Transesophageal echocardiography (TEE) confirmed a large (46 mm × 32 mm), ovoid and heterogeneous mass, moving with cardiac cycle, and attached with a broad stalk to the mitral posterior leaflet.
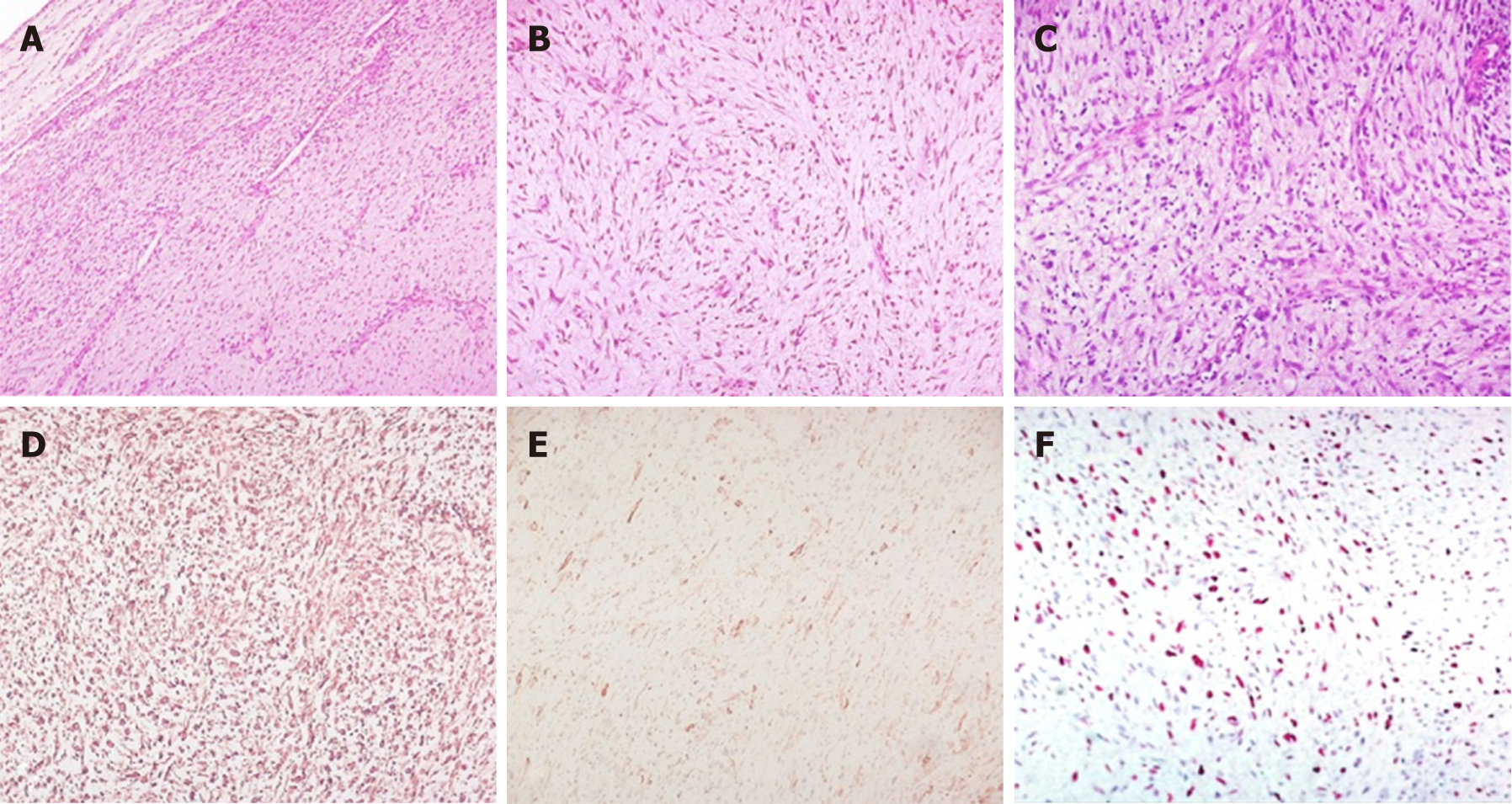
Due to echographic features and location, the mass was thought to be a myxoma, and surgery was planned. Upon macroscopic examination, the mass was made up of solid, greyish tissue. Microscopically, the tumor was composed of a uniform proliferation of spindle-shaped cells with medium cellularity, mild to moderate pleomorphism, and elongated hyperchromatic nuclei. The background was predominantly myxoid and to a lesser extent fibrous, with prominent vascularization (Figure 1). Immuno-histochemistry (IHC) revealed diffuse positive immunoreactivity for vimentin, as well as focal smooth muscle actin. Desmin, caldesmon, CD34, S-100 protein, CDK4, calretinin, wide-spectrum cytokeratin, and epithelial membrane antigen (EMA) were negative. The Ki67 proliferation index was 35%. Therefore, primary cardiac myxofibrosarcoma was diagnosed [Grade 2 according to the NCI grading system, and according to the FNCLCC grading system: Tumor differentiation = 2, mitotic count = 1 (3/10 HPF), tumor necrosis = 1 (< 10%), total score = 4] with microscopically residual disease.
In order to better characterize this rare cancer and to explore molecular events that may be amenable for targeted medical therapy, whole-Transcriptome Paired-End RNA Sequencing analysis was performed.
Total RNA was extracted with the RecoverAll™ Total Nucleic Acid Isolation Kit (Thermo Fisher Scientific, Waltham, MA, United States) from FFPE tumor specimens of the myxofibrosarcoma case, and from five intimal sarcomas and two angiosarcomas. cDNA libraries were synthesized from 100 ng total RNA with TruSeq RNA Exome (Illumina, San Diego, CA, United States). Briefly, cDNA libraries were synthesized from fragmented RNA, ligated to paired-end sequencing adapters and amplified, then coding exon sequences were captured by hybridization to a pool of exonic probes. WTS libraries were quality-checked and sized with Agilent DNA 7500 chips on the Bioanalyzer 2100 (Agilent Technologies, Taiwan), then quantified using a fluorometric assay (QuantITPicogreen assay; Life Technologies, Carlsbad, CA, United States). Paired-end libraries were sequenced at 2 x 80 bp using Illumina Sequencing by synthesis technology on a NextSeq500 instrument (Illumina, San Diego, CA, United States) according to the manufacturer’s recommendations.
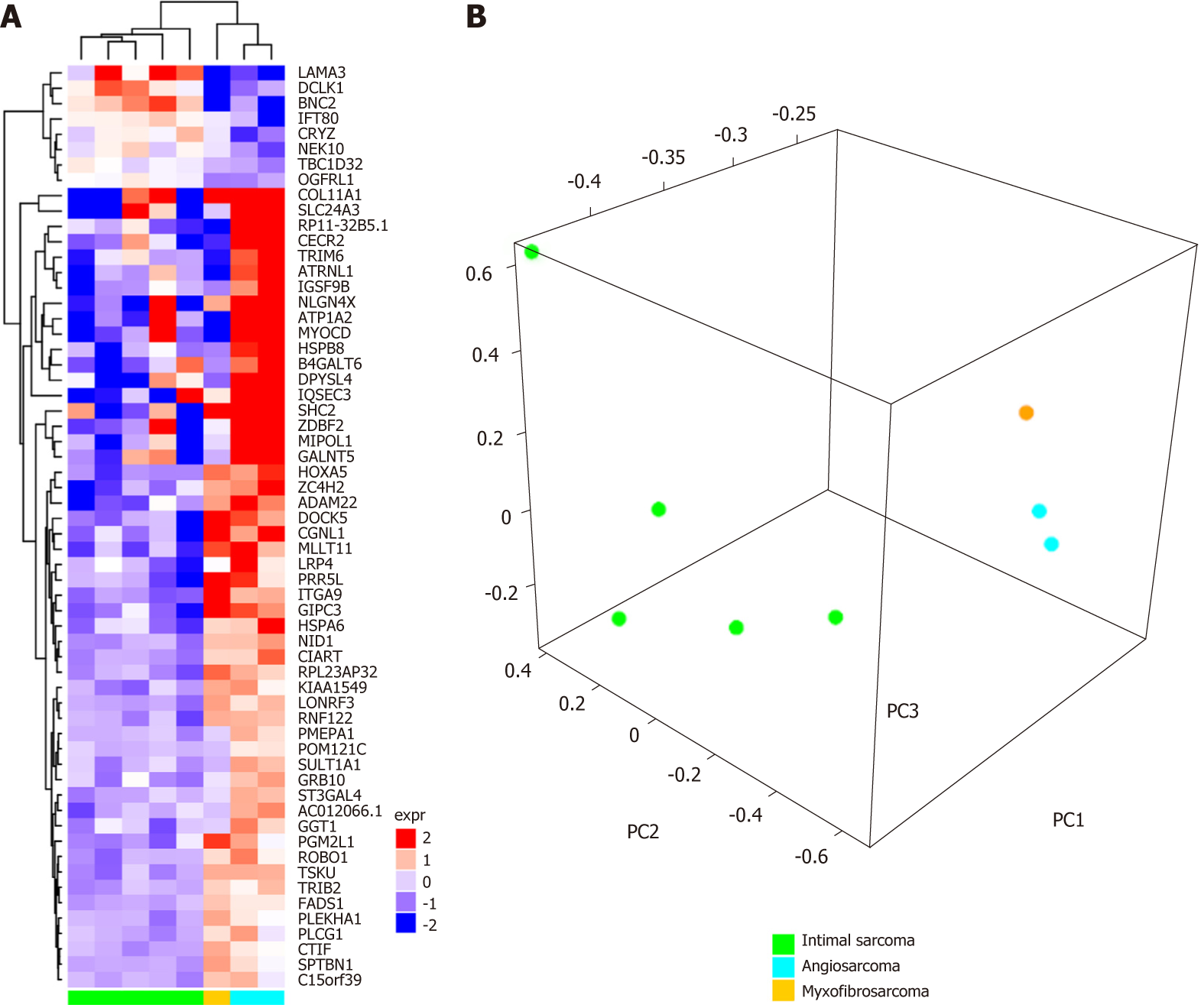
After demultiplexing and adapter trimming, the paired-end reads were mapped with the pipeline TopHat2/Bowtie2 (https://ccb.jhu.edu) on the human reference genome hg38 (http://genome.ucsc.edu). Single nucleotide variants and indels were called with SNVMix2 (https://shahlab.ca) and GATK HaplotypeCaller (https://software.broadinstitute.org), respectively, and chromosomal rearrangements were investigated with several computational tools including ChimeraScan, Defuse, TopHat-Fusion, FusionMap as previously described[3]. No specific mutations or molecular alterations of interest for a targeted therapy were found. We therefore decided to perform gene expression profiling analysis to compare the transcriptional landscape of this rare tumor subtype with other cardiac neoplasms, including two angiosarcomas and five intimal sarcomas, whose sequencing data were already available in the laboratory dataset. First, gene expression was quantified for all samples (myxofibrosarcomas, angiosarcomas and intimal sarcomas) using the python package htseq-count (https://htseq.readthedocs.io) with the Ensembl release 81 annotation features (http://www.ensembl.org) as a reference. Then the R-Bioconductor (https://www.bioconductor.org) package edgeR was used to perform data normalization as count per million (cpm). Secondly, a differential expression analysis of angiosarcoma versus intimal sarcoma was performed with the R-bioconductor package limma, and the most significant up- or downregulated genes were selected (P-value < 0.01, number of genes = 60). This set of 60 genes represented the specific molecular signature capable of distinguishing between angiosarcomas and intimal sarcomas in terms of gene expression profile (Supplementary Table 1). The 60 gene signature was then employed to classify the myxofibrosarcoma with respect to the other cardiac sarcoma by adopting two different methods: Hierarchical clustering (R-bioconductor package ComplexHeatmap, distance “manhattan”, method “complete”) and principal component analysis (R package prcomp). Both analyses highlighted that the myxofibrosarcoma is closer to angiosarcoma in terms of gene expression profile, as opposed to the intimal sarcoma (Figure 2A-B). We found several genes that are co-expressed in myxofibrosarcomas and angiosarcomas and not in intimal sarcomas, suggesting a putative similarity between myxofibrosarcomas and angiosarcomas, which will prove useful in assigning the appropriate chemotherapy regimen. In addition to the global molecular profile analysis, we also evaluated the expression levels of single genes previously identified as up- or down-regulated in pharmacogenomic studies.
In order to accurately stage the disease, a total body CT scan was performed after surgery, which was negative. Considering the advanced age and comorbidities of the patient, recent heart surgery, macroscopically complete resection and absence of disseminated disease, no further therapy was administered, and a follow-up program was started.
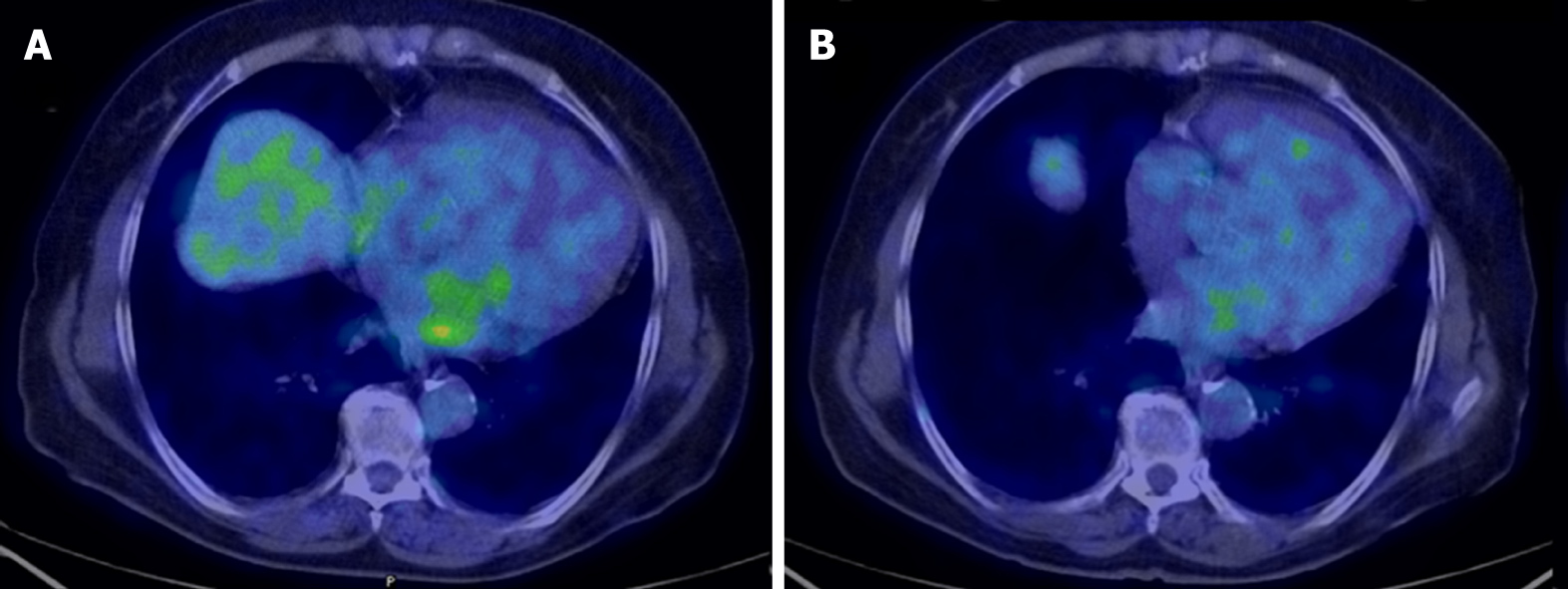
After 8 mo of negative follow-up, the patient presented dyspnea upon exertion. A CT scan showed an ovoid mass of 47 mm x 21 mm attached to the mitral posterior leaflet, which was highly suspicious for a local relapse. No other lesions were described. Cardiac magnetic resonance imaging (MRI) confirmed the mass in the left atrium, showing a heterogeneous fluorodeoxyglucose (FDG) uptake (SUVmax = 4.8) at positron-emission tomography (PET)/CT with fluorodeoxyglucose imaging (18F-FDG PET/CT). In order to downsize the tumor and obtain symptom relief, chemotherapy with doxorubicin was administered. After three cycles of doxorubicin, which is the standard treatment in the first line setting for all sarcoma histotypes, cardiac MRI showed a stable disease with complete metabolic response at 18F-FDG PET/CT (Figure 3), and a close follow-up program was planned. However, imaging also showed a thrombus near the tumor, thus a specific anticoagulant therapy was started.
Unfortunately, 1 year later, the patient presented neurological symptoms of ischemic stroke likely due to a thrombotic event, despite stability of the oncological disease. Thus, appropriate therapy for stroke and subsequent rehabilitation therapy were set up, while close cardiac tumor surveillance was performed.
Two years after surgery, during the surveillance period, the patient had an episode of acute left-sided heart failure. A TTE showed a size increase of the heart mass, confirmed at cardiac MRI. 18F-FDG PET/CT showed metabolic reactivation of disease with heterogeneous FDG uptake (SUVmax = 3.5). After a multidisciplinary evaluation of the case, surgery was considered too risky, but in order to achieve local and systemic disease control, a second line chemotherapy was planned.
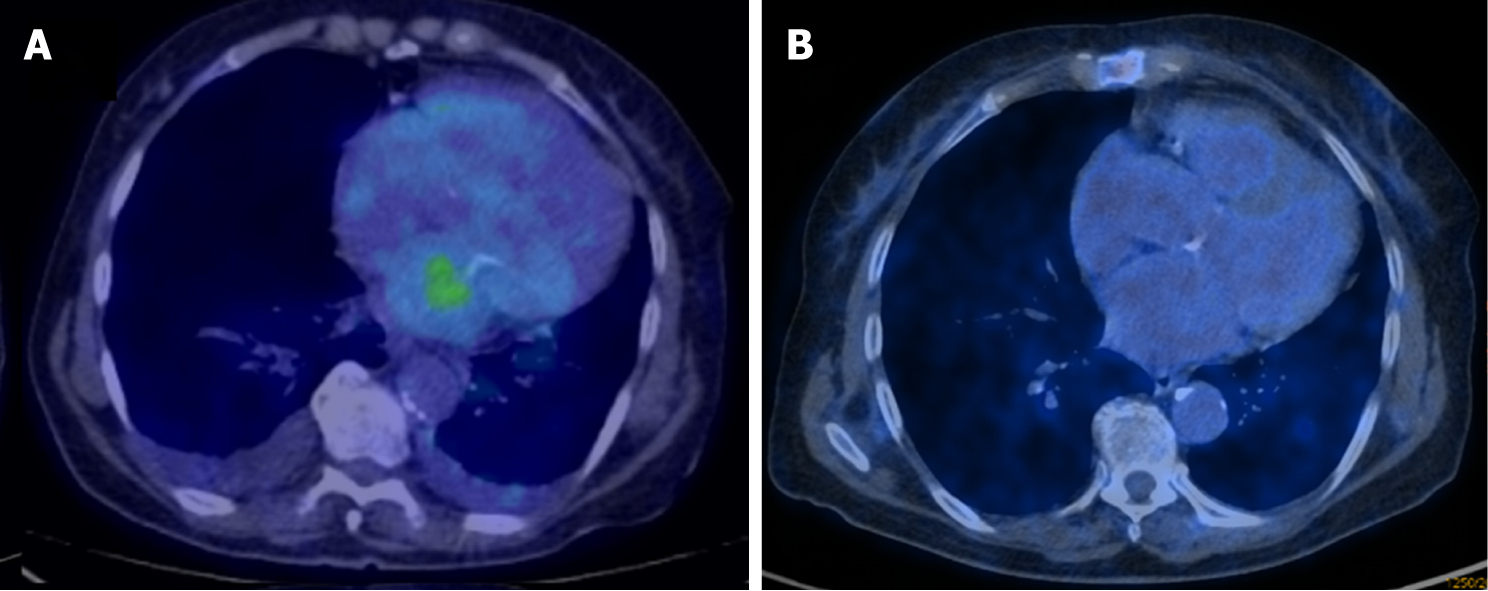
Considering that gene expression profiling findings clustered the disease close to angiosarcoma, which is generally responsive to gemcitabine, we focused on the genes defined by Tooker et al[4] as modulated in relation to gemcitabine resistance and sensitivity. We found several genes that are included in the msigd signature “TOOKER_GEMCITABINE_RESISTANCE_UP” (https://broadinstitute.org/ gsea/msigdb/) that were downregulated in both myxofibrosarcomas and angiosarcomas, including IFI6, LGALS3, ANXA1 and ASS1 (Figure 4A). Conversely, the genes CYB5A, SCD, ADD3, HSPB1, SMS, WWTR1 and RHOB, belonging to the msigdb signature “TOOKER_GEMCITABINE_RESISTANCE_DN” (Figure 4B), appeared upregulated in myxofibrosarcomas and angiosarcomas and not in intimal sarcomas. Taken together, these observations suggested a putative similarity between myxofibrosarcomas and angiosarcomas, also in terms of chemotherapy response. So, we opted for a gemcitabine therapy, which is more promising for such histotypes when compared to other potential second line drugs. Treatment with gemcitabine was therefore started and was well-tolerated. After three cycles, a complete metabolic response at 18F-FDG PET/CT had been obtained, which was confirmed after a further 3 mo of treatment (Figure 5), which is currently ongoing.
Cardiac tumors account only for a small fraction of all cardiac masses, mostly represented by pseudo tumors like thrombi, vegetations, abscesses, and aneurysms. Cardiac tumors are mostly represented by benign tumors (75%), with cardiac myxomas being the most common, accounting for nearly half of them. Primary malignant cases account only for 25% of all cardiac tumors, while secondary cardiac tumors, either by metastatic spread or direct invasion, are far more common (40-50 times more frequent). In one series of over 12,000 autopsies, only seven cases of malignant primary cardiac tumors were identified, accounting for an incidence of less than 0.1 percent[5,6]. Among the primary malignant tumors of the heart, the most frequent are sarcomatous in nature[7]. All types of sarcomas may be observed in the heart, with a predominance of rhabdomyosarcomas in childhood and angiosarcomas or undifferentiated sarcomas in adulthood. Although rare, myxofibrosarcoma is one of the most common sarcoma subtypes in the elderly, with most lesions arising in the extremities. Origin in other organs, like the heart, is an extraordinary event, and the majority of publications are limited to case reports. Given their rarity and the lack of prospective and randomized clinical trials, the level of evidence for the optimal diagnostic workup and multimodal treatment of primary cardiac sarcomas is very low and not well established to date.
Primary cardiac tumor manifestations are rather nonspecific. They may be either asymptomatic, being incidentally discovered during workup for unrelated problems, or may cause symptoms by obstructing the circulation, interfering with heart valves, directly invading the myocardium, invading the adjacent lung, or embolization. They can rarely manifest with constitutional symptoms like fatigue, anorexia or general malaise. The clinical presentation depends on the tumor size and anatomical location rather than on histological type. A cardiac sarcoma located in the left atrium, as in the presented case, can cause obstruction of the mitral valve with symptoms of mitral stenosis like dyspnea, cough and hemoptysis.
When suspected, the first imaging procedure is echocardiography, specially transesophageal echocardiography, which provides better visualization of the tumor. Echocardiography could sensitively and conveniently detect the presence of an intracardiac tumor mass, and it could well reflect location, extent, attachment, diameter of tumor and its hemodynamic consequences. Cardiac MRI and CT provide more detailed information about the morphology, location, cardiac and extracardiac extent of the mass[6,8]. Moreover, in case of suspected cardiac malignancies, CT scans are also useful to assess distant metastasis, if any. The role of 18F-FDG PET/CT is also being investigated, showing promising results[9].
The cornerstone of therapy for cardiac tumors is surgery to accomplish complete surgical resection, with negative margins providing the greatest chance for survival. Instead, the role of adjuvant chemotherapy and radiotherapy remains unclear, given the high percentage of cardiac toxicity of these therapies.
Very recently, Sun et al[8] published pooled analyses of primary cardiac myxofibrosarcomas in order to establish their clinical presentations, pathologic features, treatments and outcome patterns, as well as to develop a rationale for the diagnosis and prognostication of this disease. Results revealed that primary cardiac myxofibrosarcoma afflicted relatively young patients, with a mean age of 42 years. The most common cardiopulmonary symptom reported was dyspnea, accounting for 64.3% of cases, and the most common location was the left atrium, which affected 58% of patients. The median survival time was 14 mo, and statistical analyses revealed that primary cardiac myxofibrosarcomas had a high probability of presenting local recurrences and dismal metastases. Major risk factors significantly related to a poor prognosis included tumors ≥ 40 mm in size or with high-grade.
Very little is known about the molecular landscape of myxofibrosarcomas. Unlike other groups of sarcomas with peculiar genetic alterations and simple karyotypes, such as specific KIT mutations in GIST, myxofibrosarcomas seem to be complex and very heterogeneous, lacking specific or recurrent genetic patterns. Some studies evaluated the gene expression profile of myxofibrosarcomas, aiming to identify potentially actionable targets and biomarkers for personalized treatment strategies in a pathology substantially orphan of effective target therapies[10-12]. Mutations, novel amplifications and copy number alterations have been particularly observed in high grade, metastatic or recurrent myxofibrosarcomas compared with low-grade forms, even if the small case series does not allow for conclusive evaluations.
Herein, we reported a case of primary cardiac myxofibrosarcoma manifesting with dyspnea in an elderly patient. Surgery was initially planned upon the suspicion of a myxoma. Despite the different final diagnosis, removal of the voluminous mass allowed complete symptom relief, and likely positively affected the patient's overall survival together with the multimodality treatment strategy adopted when relapse occurred. In fact, both cardiac and oncologic risk related to a local mass and potential distant spread were promptly recognized and treated. We also performed genome analysis of the tumor tissue to get further insights on mechanisms involved in tumor growth, and to possibly unveil new clinically actionable targets. The most important, yet preliminary, observations concern (1) Confirmation of a considerable complexity in the gene expression profile of myxofibrosarcoma, which could justify the responsiveness to “classical” chemotherapy treatments (such as anthracyclines), and (2) A certain similarity of genomic sequencing between myxofibrosarcomas and angiosarcomas, which could explain similar responsiveness to gemcitabine-based schemes.
According to results reported in the literature, local recurrence rates of myxofibrosarcomas was very high, which is likely related to the peculiar local growth pattern of these tumors, highlighting their aggressive biology and the need for more effective post-treatment strategies[13]. Early diagnosis of primary cardiac myxofibrosarcomas is also crucial, as it might improve the overall survival and improve the quality of life of patients. Both local advanced disease and metastatic disease have poor prognosis. In older retrospective case series, regardless of the type of treatment, most patients died within 12-16.5 mo after initial diagnosis[5]. In more recent series, survival reaches 38.8 mo or even 53.5 mo, but only for patients who underwent complete resection in referral centers[14,15]. Patient selection is also crucial to allow for multimodality treatment, including neoadjuvant chemotherapy that seems to enhance resectability, thus translating into improved patient survival.
Currently, 32 mo after initial diagnosis, our patient is in good general clinical condition. She is recovering from the neurological sequelae of the stroke, leading a normal life in relation to her age, and continuing the well-tolerated chemotherapy treatment with gemcitabine with a good disease control, as showed by recent MRI and PET/CT re-evaluations.
Our case report may support clinicians facing diagnosis, treatment and counseling of patients affected by this rare tumor. Imaging examination, especially echo-cardiography, together with histology and immunohistochemistry should be considered during the diagnosis of primary cardiac masses, specifically those that are voluminous in size. In order to confirm clinical suspicion stemming from first level investigations, other exams such as FDG-PET/CT should be considered, and aimed at planning rational surgery strategies to ultimately improve prognosis.
The rarity of disease, with only a few cases reported in the literature, forbids making stronger recommendations. Therefore, the systemic treatment of patients suffering from myxofibrosarcoma has not substantially changed over the years, which is also based on the highly complex genetic changes and the lack of typical genetic fingerprints. As for the most rare diseases, conveying patients affected by primary cardiac myxofibrosarcoma to reference centers may help to aggregate significant preclinical and clinical data in the quest for more sound scientific evidence. A better understanding of biology, features and outcomes will lead to the development of new, targeted therapeutic strategies for this dismal tumor.
Conflict of interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barik R, Kharlamov AN, Petix NR S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Hudzik B, Miszalski-Jamka K, Glowacki J, Lekston A, Gierlotka M, Zembala M, Polonski L, Gasior M. Malignant tumors of the heart. Cancer Epidemiol. 2015;39:665-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Pathak R, Nepal S, Giri S, Ghimire S, Aryal MR. Primary cardiac sarcoma presenting as acute left-sided heart failure. J Community Hosp Intern Med Perspect. 2014;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Indio V, Astolfi A, Tarantino G, Urbini M, Patterson J, Nannini M, Saponara M, Gatto L, Santini D, do Valle IF, Castellani G, Remondini D, Fiorentino M, von Mehren M, Brandi G, Biasco G, Heinrich MC, Pantaleo MA. Integrated Molecular Characterization of Gastrointestinal Stromal Tumors (GIST) Harboring the Rare D842V Mutation in PDGFRA Gene. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Tooker P, Yen WC, Ng SC, Negro-Vilar A, Hermann TW. Bexarotene (LGD1069, Targretin), a selective retinoid X receptor agonist, prevents and reverses gemcitabine resistance in NSCLC cells by modulating gene amplification. Cancer Res. 2007;67:4425-4433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 491] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, Smith MA, Fletcher CD. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20:391-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 334] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Sun D, Wu Y, Liu Y, Yang J. Primary cardiac myxofibrosarcoma: case report, literature review and pooled analysis. BMC Cancer. 2018;18:512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Saponara M, Ambrosini V, Nannini M, Gatto L, Astolfi A, Urbini M, Indio V, Fanti S, Pantaleo MA. 18F-FDG-PET/CT imaging in cardiac tumors: illustrative clinical cases and review of the literature. Ther Adv Med Oncol. 2018;10:1758835918793569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Heitzer E, Sunitsch S, Gilg MM, Lohberger B, Rinner B, Kashofer K, Stündl N, Ulz P, Szkandera J, Leithner A, Liegl-Atzwanger B. Expanded molecular profiling of myxofibrosarcoma reveals potentially actionable targets. Mod Pathol. 2017;30:1698-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Okada T, Lee AY, Qin LX, Agaram N, Mimae T, Shen Y, O'Connor R, López-Lago MA, Craig A, Miller ML, Agius P, Molinelli E, Socci ND, Crago AM, Shima F, Sander C, Singer S. Integrin-α10 Dependency Identifies RAC and RICTOR as Therapeutic Targets in High-Grade Myxofibrosarcoma. Cancer Discov. 2016;6:1148-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Li CF, Fang FM, Kung HJ, Chen LT, Wang JW, Tsai JW, Yu SC, Wang YH, Li SH, Huang HY. Downregulated MTAP expression in myxofibrosarcoma: A characterization of inactivating mechanisms, tumor suppressive function, and therapeutic relevance. Oncotarget. 2014;5:11428-11441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sanfilippo R, Miceli R, Grosso F, Fiore M, Puma E, Pennacchioli E, Barisella M, Sangalli C, Mariani L, Casali PG, Gronchi A. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18:720-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, Gauthier M, Blouet A, Chaigneau L, Duffaud F, Piperno-Neumann S, Kurtz JE, Girard N, Collard O, Bompas E, Penel N, Bay JO, Guillemet C, Collin F, Blay JY, Le Cesne A, Thariat J. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50:128-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Abu Saleh WK, Ramlawi B, Shapira OM, Al Jabbari O, Ravi V, Benjamin R, Durand JB, Leja MJ, Blackmon SH, Bruckner BA, Reardon MJ. Improved Outcomes With the Evolution of a Neoadjuvant Chemotherapy Approach to Right Heart Sarcoma. Ann Thorac Surg. 2017;104:90-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |