Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.108261
Revised: May 26, 2025
Accepted: June 27, 2025
Published online: September 26, 2025
Processing time: 115 Days and 18.3 Hours
Immunoglobulin G4-related disease (IgG4-RD) is a persistent and progressive autoimmune condition marked by inflammation and fibrotic changes in the affected tissues. Cases of IgG4-RD causing pulmonary lesions are relatively rare, and some may be misdiagnosed as pulmonary tuberculosis.
In this report, we present an uncommon instance of IgG4-related lung disease, which was diagnosed through lung tissue biopsy conducted via puncture. A 67-year-old male was hospitalized with a two-month history of cough and sputum production. Chest computed tomography (CT) revealed infiltrative pulmonary tuberculosis in both upper lungs. However, the initial diagnosis was unclear, and the patient received HZRE quadruple therapy for tuberculosis at a local hospital. After 45 days of anti-tuberculosis treatment, the patient's cough and sputum worsened, and he began coughing up blood, prompting transfer to our hospital. Serum tests revealed elevated IgG4 levels. A biopsy of a right lung showed localized fibrous and extensive plasma cell infiltration, with 30-40 IgG4-positive cells per high-power field, and an IgG4/IgG ratio of 40%. These findings led to a diagnosis of IgG4-related lung disease. Following treatment with prednisone and mycophenolate mofetil, follow-up lung CT scans showed significant lesion improvement.
The chest CT findings of IgG4-RD are diverse and nonspecific, often leading to misdiagnosis as pulmonary tuberculosis, especially in primary care settings with limited diagnostic resources. We confirmed the diagnosis of IgG4-related lung disease through histological examination.
Core Tip: Immunoglobulin G4-related disease is a fibrotic inflammatory disease that affects almost all organs and primarily occurs in middle-aged to elderly male patients. It can occur in the mediastinum, airways, lungs, and pleura and is most commonly associated with manifestations outside the chest, which are usually nonspecific. The prognostic characteristics have not yet been determined. Diagnosis relies on a thorough assessment of clinical, biological, and histological findings, as there are no specific biomarkers. Differential diagnoses must always be considered, especially when the disease affects a single organ, is incidentally found on imaging studies, or is unexpectedly diagnosed on pathological specimens.
- Citation: Zhou JL, Zhou XY, Li WJ, Feng S. Immunoglobulin G4-related lung disease mistaken for pulmonary tuberculosis: A case report. World J Clin Cases 2025; 13(27): 108261
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/108261.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.108261
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) represents a chronic and progressive autoimmune disorder that is distinguished by the presence of inflammation and fibrosis in the tissues that are involved[1]. A hallmark of IgG4-RD is the infiltration of IgG4-positive plasma cells and lymphocytes into various tissues. This condition has the potential to impact multiple organ systems, encompassing the biliary tract, renal system, retroperitoneum, prostate, aorta, peri
A 67-year-old Chinese man was hospitalized with a two-month history of cough and sputum production.
The patient had been coughing and producing sputum for 2 months. Chest computed tomography (CT) revealed infiltrative pulmonary tuberculosis in both upper lungs. However, the initial diagnosis was still unclear, and the patient received quadruple HZRE therapy for tuberculosis at a local hospital. After 45 days of anti-tuberculosis treatment, the patient's cough and sputum worsened, and he started coughing up blood, leading to his transfer to our hospital for further evaluation and treatment.
No history of chronic conditions such as hypertension, diabetes, or heart disease.
No family history of tuberculosis.
Upon admission, the patient’s temperature was 36.0 °C. The breath sounds on the right side were found to be reduced, while no abnormal heart sounds were detected. There was no observed dyspnea or respiratory distress. No rales or crackles were detected on auscultation. His oxygen saturation level was 97% on room air.
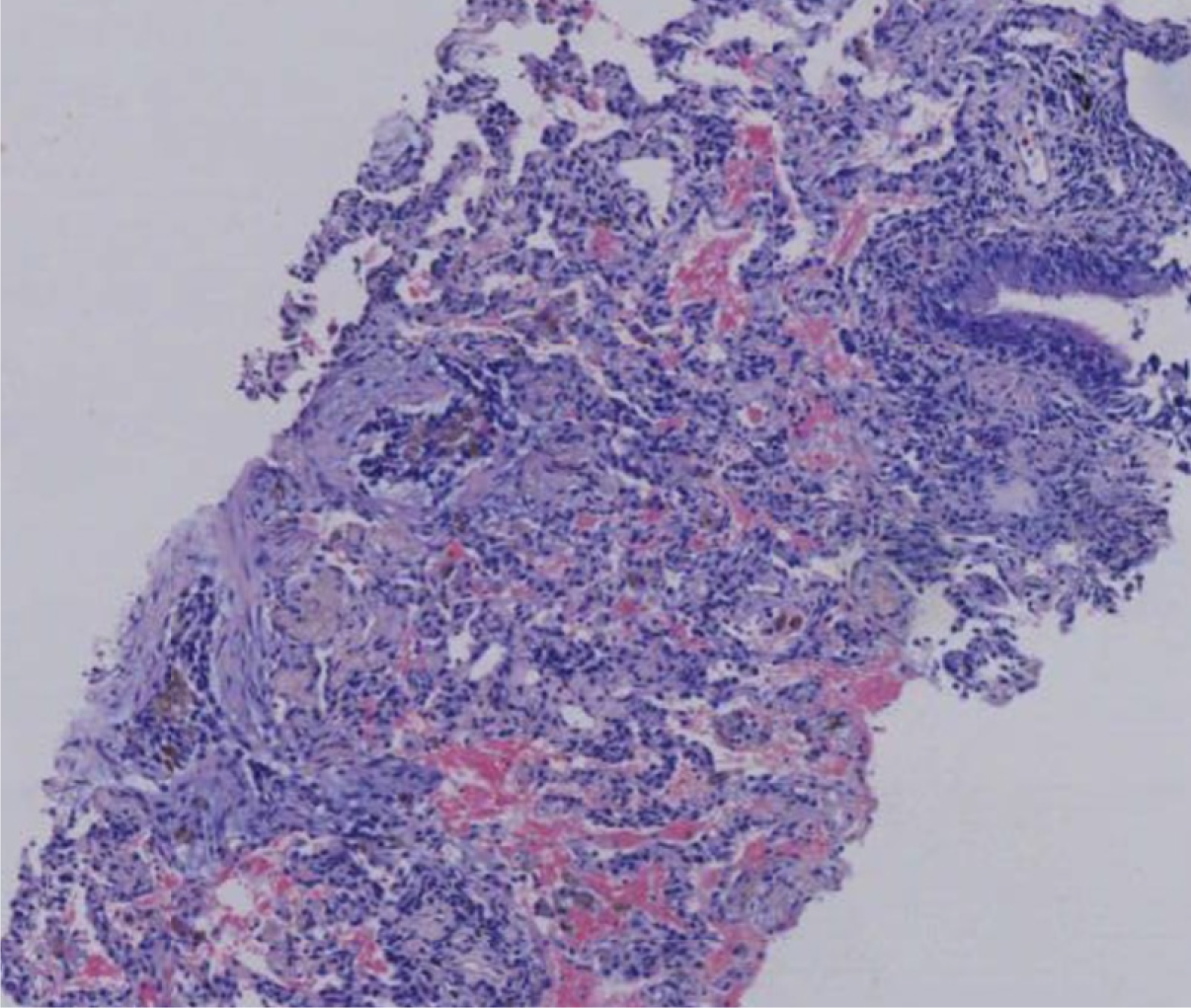
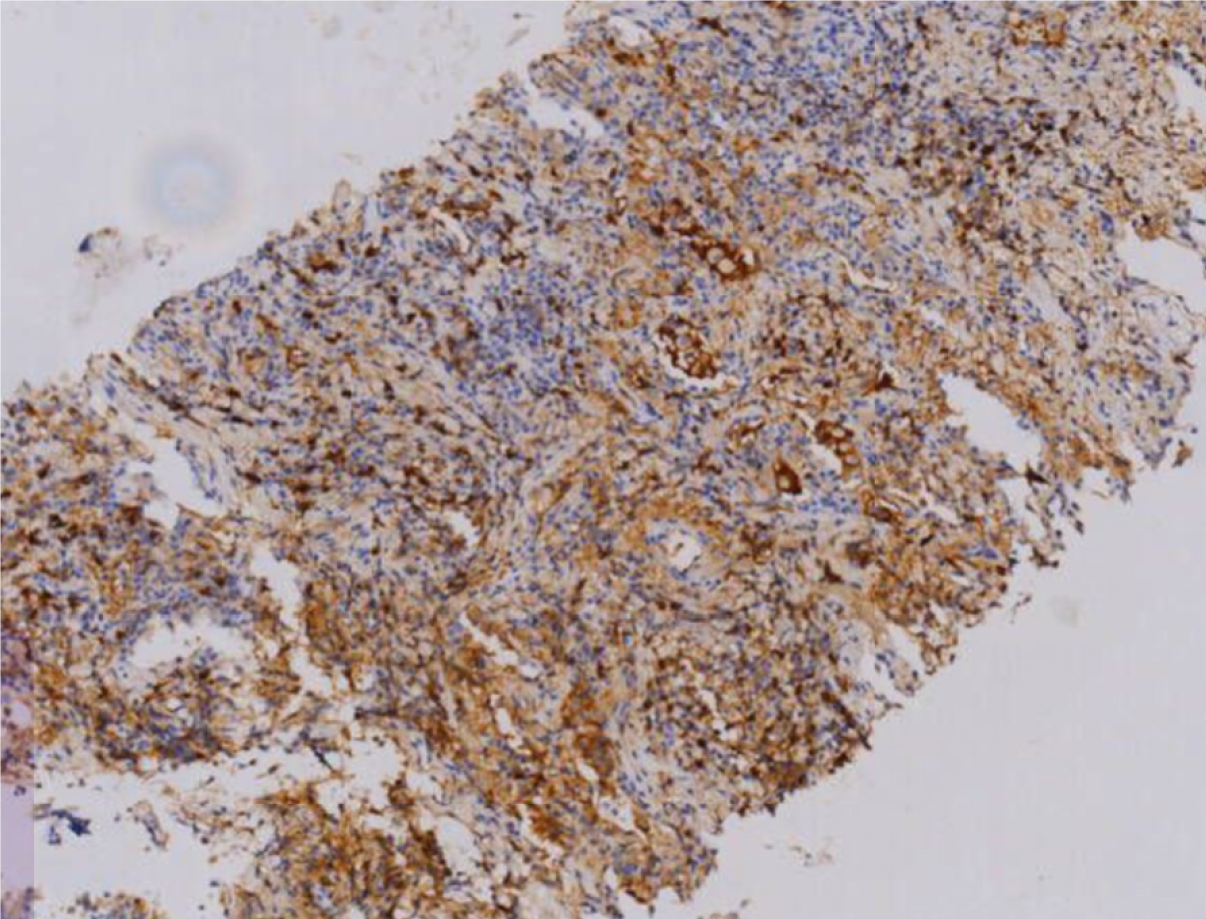
The patient’s hemoglobin level was 107 g/L, uric acid level was 616 μmol/L, and erythrocyte sedimentation rate was 81 mm/hour. Vasculitis-related antibodies and immunoglobulin levels were within normal ranges. Acid-fast staining of sputum and bronchoalveolar lavage fluid, TBNDA, T-SPOT tuberculosis, and tuberculosis antibody tests were all negative. Tumor markers were also within the normal ranges. Serum IgG4 levels were elevated (2.80 g/L). Pathological examination of the right lung biopsy revealed localized fibrous tissue hyperplasia and extensive plasma cell infiltration (Figure 1), with 30-40 IgG4-positive cells per high-power field, and an IgG4/IgG ratio of 40% (Figure 2).
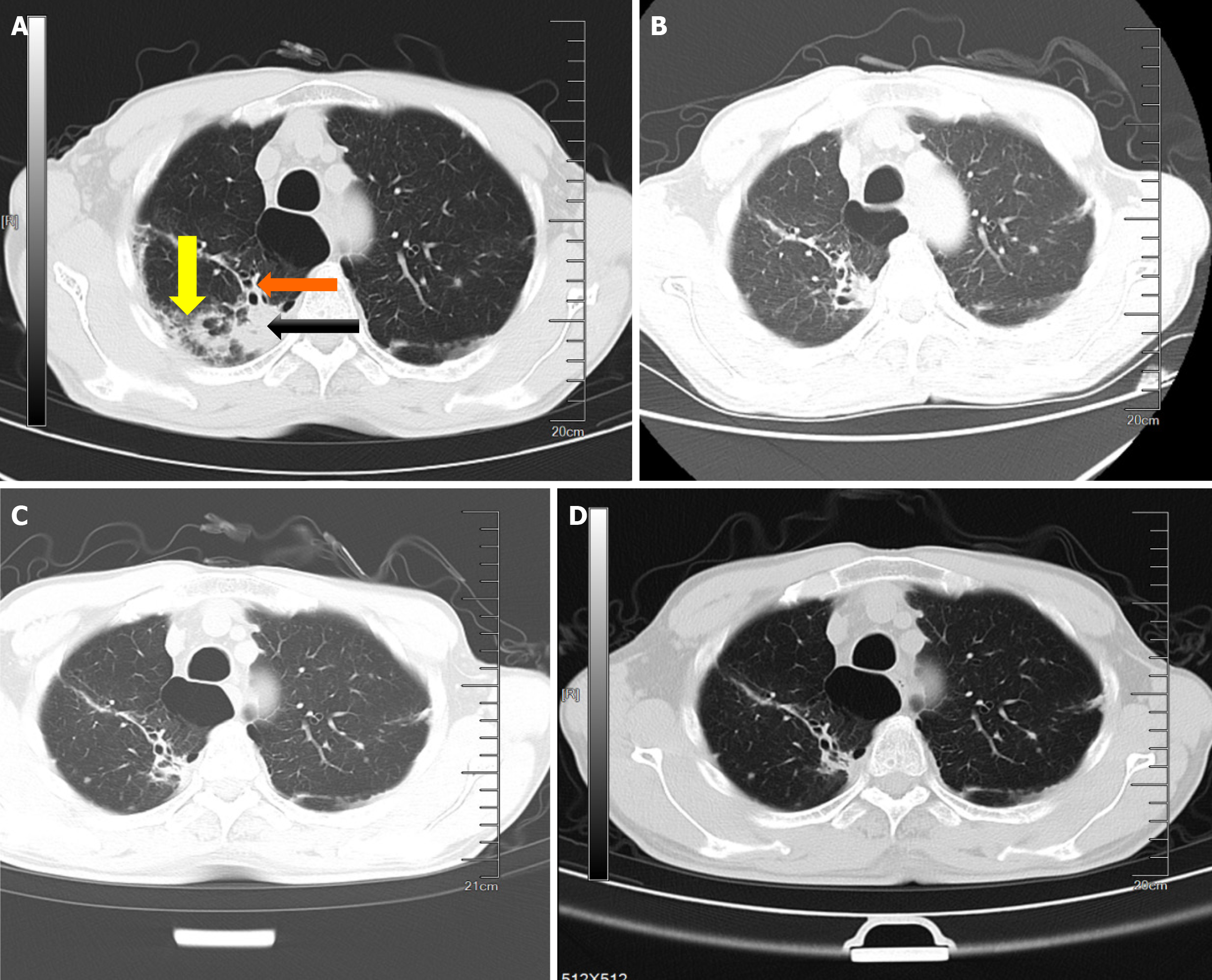
Cardiac ultrasonography revealed mild tricuspid and pulmonary valve insufficiencies. Abdominal ultrasonography revealed multiple cysts in the left kidney and mild prostatic hyperplasia with stone formation. Chest CT indicated bilateral lung infection with a high likelihood of tuberculosis, chronic bronchitis, pulmonary emphysema, and bilateral upper-lobe pulmonary bullae (Figure 3A).
The patient was diagnosed with IgG4-related lung disease.
Following treatment with corticosteroids [oral prednisone (PSL) at 35 mg/day] and immunosuppressants (mycophenolate mofetil at 0.5 g bid) for 1 year, the dosage was reduced to PSL 5 mg/day as maintenance therapy.
Follow-up chest CT scans at 2 and 6 months of treatment showed significant improvement in lung lesions (Figure 3B-D).
IgG4 is the smallest subclass of immunoglobulin G, accounting for approximately 5% of total IgG. It is generally considered non-inflammatory because of its inability to activate the complement system, form immune complexes[5], or effectively bind Fc gamma receptors, thereby limiting antibody-dependent cell-mediated cytotoxicity[6]. Therefore, the role of IgG4 in the pathogenesis of IgG4-RD remains incompletely understood[7]. Innate immunity plays a significant role in the development of gG4-RD. M2 macrophages, which are anti-inflammatory in nature are closely associated with the fibrotic processes characteristic of the disease. These cells are frequently observed in fibrotic regions, and their numbers have been positively correlated with the extent of tissue fibrosis[8]. Recent studies suggests that pulmonary IgG4-RD is linked to Th2-dominant immune dysregulation[9].
Herein, we present a case of IgG4-related lung disease initially misdiagnosed as tuberculosis. We confirmed the diagnosis of IgG4-related lung disease by histological assessment. The patient met the diagnostic criteria for IgG4-related disease. Clinical signs of thoracic involvement are observed in over 10% of cases. These signs are typically nonspecific and vary depending on the location of the lesion, and may include cough, dyspnea, hemoptysis, asthma-like symptoms, or chest pain[10].
Diagnosing IgG4-RD can be challenging for several reasons. First, chest CT scans are the most commonly used imaging method for assessing IgG4-RD thoracic lesions. The chest manifestations of IgG4-RD are diverse and nonspecific[11,12], often leading to misdiagnose as pulmonary tuberculosis, particularly in primary care settings with limited diagnostic resources. Second, although elevated serum IgG4 levels are part of the diagnostic criteria, they are elevated in only 50% of active IgG4-RD cases. Moreover, approximately 5% of healthy individuals may have elevated serum IgG4 levels. Therefore, serum IgG4 levels alone are insufficient for a definitive diagnosis. IgG4-positive plasma cells are also observed in lung tissue across a range of different conditions, such as inflammatory myofibroblastic tumors, non-specific interstitial pneumonia, usual interstitial pneumonia, and necrotizing granulomatous inflammation[13]. These findings suggest that both the quantification of IgG4-positive plasma cells and the ratio of IgG4 to IgG-positive plasma cells may help differentiate true IgG4-related lung disease from its mimics[14]. Third, IgG4-RD often presents as a systemic disease affecting multiple organs. In such cases, the presence of extrapulmonary lesions may provide diagnostic indications. However, the CT imaging characteristics of IgG4-related pulmonary lesions are not specific, which further complicates the diagnostic process. On CT, IgG4-RD resembles lung tumors and nonspecific interstitial pneumonia. However, the main pathological characteristics of IgG4-RD, such as bundled fibrosis and soft lymphoplasmacytic venulitis, are not present in lung tumors or nonspecific interstitial pneumonia[15]. Certain tumor markers may help distinguish between IgG4-related lung disease and lung cancers.
An international consensus statement on IgG4-RD was published in 2015[16] based on expert opinions and a review of existing evidence. It recommends the use of glucocorticoids as a first-line treatment for IgG4-RD, typically at a dose of 0.6 mg/kg. Clinical improvement is usually observed within the first 2 weeks of therapy. After 2-4 weeks at the initial dose, glucocorticoids are generally tapered gradually over a period of 3-6 months. In the present case, mycophenolate mofetil was added to prednisone, which proved to be significantly effective. However, disease-modifying anti-rheumatic drugs (such as azathioprine, mycophenolate mofetil, cyclosporine, and methotrexate) are less effective at inducing remission, they can be useful as steroid-sparing agents[17]. Due to lack of prospective clinical trials[18], experts consensus is divided on whether disease-modifying anti-rheumatic drugs should be introduced early in the course of treatment[17].
Rituximab is utilized more frequently among patients whose conditions have either relapsed or display resistance to glucocorticoid monotherapy or where the administration of glucocorticoids is likely to result in significant adverse effects[19,20]. Inebilizumab reduces the incidence of flares associated with IgG4-RDs and enhances the probability of achieving flare-free complete remission within 1 year. These findings underscore the significance of CD19-targeted B cell depletion as a viable therapeutic strategy for managing IgG4-RD[21,22]. Currently, an international randomized controlled clinical trial is being conducted utilizing anti-CD19 monoclonal antibodies, specifically obexelimab[23], as a B-cell-targeted therapy, with promising results emerging. Furthermore, preliminary evidence suggests the potential effectiveness of interleukin-4-targeted therapies, such as dupilumab[24].
IgG4-RD is a fibrotic inflammatory disease that affects almost all organs and primarily occurs in middle-aged to elderly male patients. It can occur in the mediastinum, airways, lungs, and pleura and is most commonly associated with manifestations outside the chest, which are usually nonspecific. The prognostic characteristics have not yet been determined. Diagnosis relies on a thorough assessment of clinical, biological, and histological findings, as there are no specific biomarkers. Differential diagnoses must always be considered, especially when the disease affects a single organ, is incidentally found on imaging studies, or is unexpectedly diagnosed on pathological specimens. Monitoring IgG4 concentrations revealed an early relapse in some patients. Although IgG4 concentrations decreased with glucocorticoid treatment in most patients in whom they were elevated at baseline, they remained above the normal values[25]. Therefore, the long-term management plan for patients involves monitoring IgG4 levels and obtaining regular lung CT scans. Patients may have to use corticosteroids or immunosuppressants for long periods. This can lead to both bacterial and fungal infections.
We would like to express our sincere gratitude to all authors for their invaluable contributions to this study. Additionally, we extend our heartfelt appreciation to the patient who participated in this case.
| 1. | Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 2. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1867] [Article Influence: 143.6] [Reference Citation Analysis (83)] |
| 3. | Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, Yoshikawa J, Miyayama S, Yasui M, Nakanuma Y, Matsui O. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009;251:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Campbell SN, Rubio E, Loschner AL. Clinical review of pulmonary manifestations of IgG4-related disease. Ann Am Thorac Soc. 2014;11:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Peng L, Lu H, Zhou J, Zhang P, Li J, Liu Z, Wu D, Zhang S, Yang Y, Bai W, Wang L, Fei Y, Zhang W, Zhao Y, Zeng X, Zhang F. Clinical characteristics and outcome of IgG4-related disease with hypocomplementemia: a prospective cohort study. Arthritis Res Ther. 2021;23:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Bateman AC, Culver EL. Challenges and pitfalls in the diagnosis of IgG4-related disease. Semin Diagn Pathol. 2024;41:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Crescioli S, Correa I, Karagiannis P, Davies AM, Sutton BJ, Nestle FO, Karagiannis SN. IgG4 Characteristics and Functions in Cancer Immunity. Curr Allergy Asthma Rep. 2016;16:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Furukawa S, Moriyama M, Tanaka A, Maehara T, Tsuboi H, Iizuka M, Hayashida JN, Ohta M, Saeki T, Notohara K, Sumida T, Nakamura S. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Clin Immunol. 2015;156:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | D'Astous-Gauthier K, Ebbo M, Chanez P, Schleinitz N. Implication of allergy and atopy in IgG4-related disease. World Allergy Organ J. 2023;16:100765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Morales AT, Cignarella AG, Jabeen IS, Barkin JS, Mirsaeidi M. An update on IgG4-related lung disease. Eur J Intern Med. 2019;66:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Fei Y, Shi J, Lin W, Chen Y, Feng R, Wu Q, Gao X, Xu W, Zhang W, Zhang X, Zhao Y, Zeng X, Zhang F. Intrathoracic Involvements of Immunoglobulin G4-Related Sclerosing Disease. Medicine (Baltimore). 2015;94:e2150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Muller R, Ebbo M, Habert P, Torrents J, Gaubert JY, Schleinitz N. Pulmonary IgG4-related disease with favourable response to rituximab: A case report. Respirol Case Rep. 2022;10:e01061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mukhopadhyay S. Differential diagnosis of IgG4-positive plasma cells in the lung. Semin Diagn Pathol. 2024;41:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Yamashita K, Haga H, Kobashi Y, Miyagawa-Hayashino A, Yoshizawa A, Manabe T. Lung involvement in IgG4-related lymphoplasmacytic vasculitis and interstitial fibrosis: report of 3 cases and review of the literature. Am J Surg Pathol. 2008;32:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Ikeda S, Sekine A, Baba T, Okudela K, Iwasawa T, Sakai F, Notohara K, Ohashi K, Takemura T, Ogura T. Abundant immunoglobulin (Ig)G4-positive plasma cells in interstitial pneumonia without extrathoracic lesions of IgG4-related disease: is this finding specific to IgG4-related lung disease? Histopathology. 2017;70:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Iaccarino L, Talarico R, Scirè CA, Amoura Z, Burmester G, Doria A, Faiz K, Frank C, Hachulla E, Hie M, Launay D, Montecucco C, Monti S, Mouthon L, Tincani A, Toniati P, Van Hagen PM, Van Vollenhoven RF, Bombardieri S, Mueller-Ladner U, Schneider M, Smith V, Cutolo M, Mosca M, Alexander T. IgG4-related diseases: state of the art on clinical practice guidelines. RMD Open. 2018;4:e000787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E, Frulloni L, Goto H, Hart PA, Kamisawa T, Kawa S, Kawano M, Kim MH, Kodama Y, Kubota K, Lerch MM, Löhr M, Masaki Y, Matsui S, Mimori T, Nakamura S, Nakazawa T, Ohara H, Okazaki K, Ryu JH, Saeki T, Schleinitz N, Shimatsu A, Shimosegawa T, Takahashi H, Takahira M, Tanaka A, Topazian M, Umehara H, Webster GJ, Witzig TE, Yamamoto M, Zhang W, Chiba T, Stone JH; Second International Symposium on IgG4-Related Disease. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 676] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 18. | Yunyun F, Yu P, Panpan Z, Xia Z, Linyi P, Jiaxin Z, Li Z, Shangzhu Z, Jinjing L, Di W, Yamin L, Xiaowei L, Huadan X, Xuan Z, Xiaofeng Z, Fengchun Z, Yan Z, Wen Z. Efficacy and safety of low dose Mycophenolate mofetil treatment for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxford). 2019;58:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Ebbo M, Grados A, Samson M, Groh M, Loundou A, Rigolet A, Terrier B, Guillaud C, Carra-Dallière C, Renou F, Pozdzik A, Labauge P, Palat S, Berthelot JM, Pennaforte JL, Wynckel A, Lebas C, Le Gouellec N, Quémeneur T, Dahan K, Carbonnel F, Leroux G, Perlat A, Mathian A, Cacoub P, Hachulla E, Costedoat-Chalumeau N, Harlé JR, Schleinitz N. Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients. PLoS One. 2017;12:e0183844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, Deshpande V, Smyrk TC, Chari S, Stone JH. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 21. | Stone JH, Khosroshahi A, Zhang W, Della Torre E, Okazaki K, Tanaka Y, Löhr JM, Schleinitz N, Dong L, Umehara H, Lanzillotta M, Wallace ZS, Ebbo M, Webster GJ, Martinez Valle F, Nayar MK, Perugino CA, Rebours V, Dong X, Wu Y, Li Q, Rampal N, Cimbora D, Culver EL; MITIGATE Trial Investigators. Inebilizumab for Treatment of IgG4-Related Disease. N Engl J Med. 2025;392:1168-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 22. | Perugino C, Culver EL, Khosroshahi A, Zhang W, Della-Torre E, Okazaki K, Tanaka Y, Löhr M, Schleinitz N, Falloon J, She D, Cimbora D, Stone JH. Efficacy and Safety of Inebilizumab in IgG4-Related Disease: Protocol for a Randomized Controlled Trial. Rheumatol Ther. 2023;10:1795-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Perugino CA, Wallace ZS, Zack DJ, Quinn SM, Poma A, Fernandes AD, Foster P, DeMattos S, Burington B, Liu H, Allard-Chamard H, Smith N, Kai X, Xing K, Pillai S, Stone JH. Evaluation of the safety, efficacy, and mechanism of action of obexelimab for the treatment of patients with IgG4-related disease: an open-label, single-arm, single centre, phase 2 pilot trial. Lancet Rheumatol. 2023;5:e442-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Kanda M, Kamekura R, Sugawara M, Nagahata K, Suzuki C, Takano K, Takahashi H. IgG4-related disease administered dupilumab: case series and review of the literature. RMD Open. 2023;9:e003026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 25. | Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011;23:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |