Copyright
©The Author(s) 2021.
World J Clin Cases. Nov 16, 2021; 9(32): 9857-9868
Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9857
Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.9857
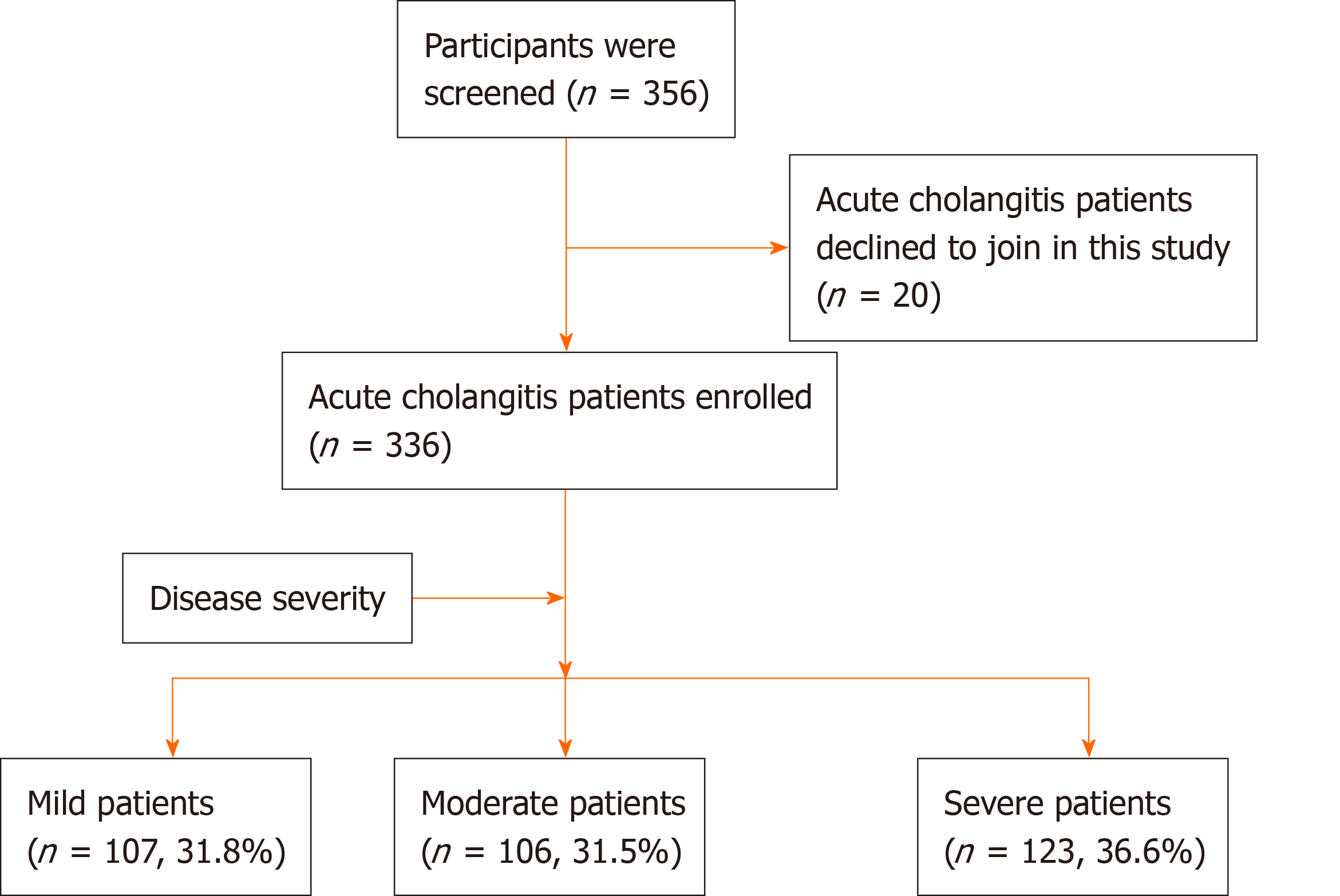
Figure 1 Flowchart of the participants.
Diagnostic criteria and severity grading of acute cholangitis were determined by the Tokyo guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis.
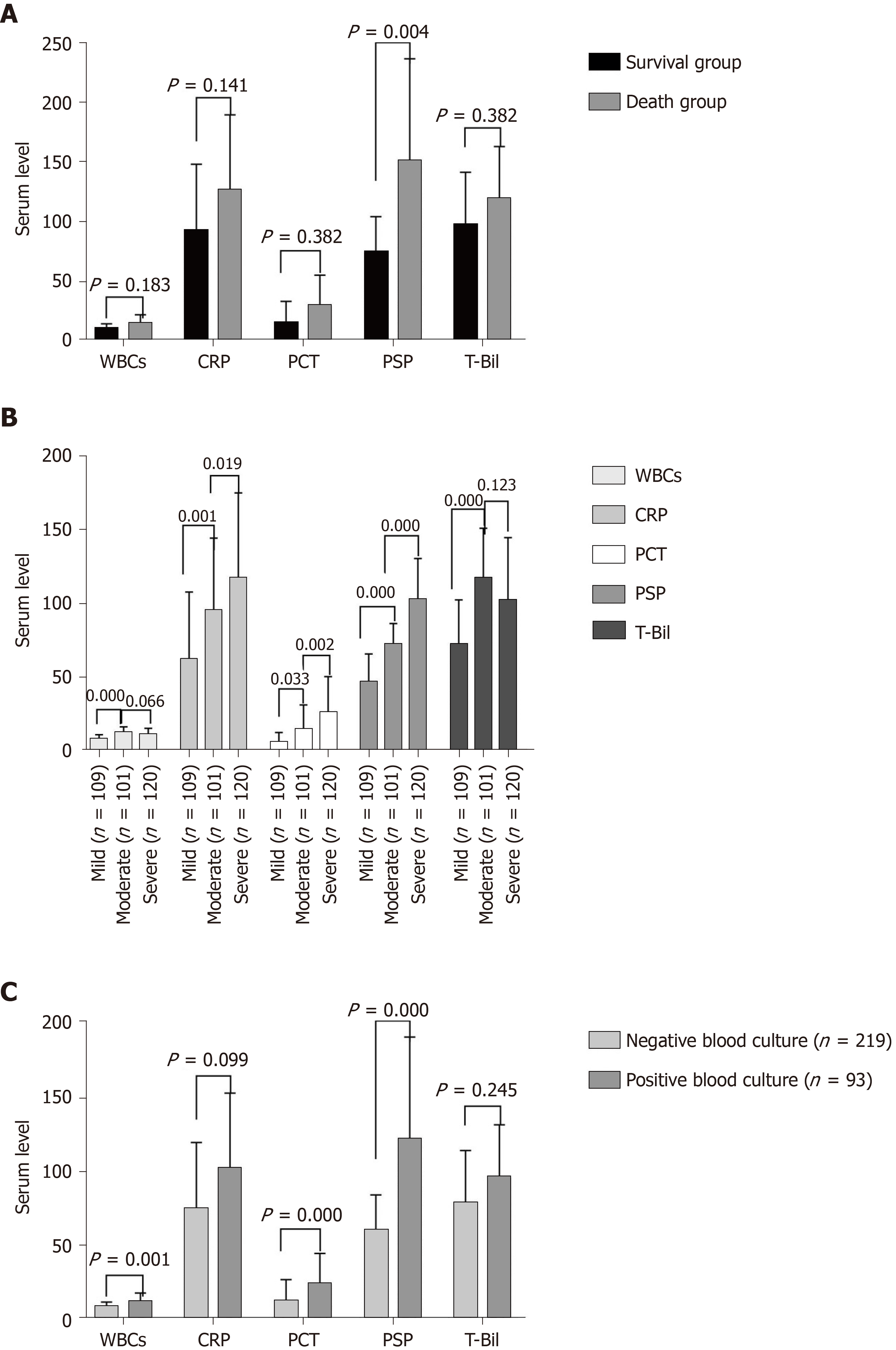
Figure 2 Biomarkers in acute cholangitis.
A: Biomarkers in the survival group and death group of acute cholangitis; B: Biomarkers in grading of acute cholangitis; C: Biomarkers in negative and positive blood culture groups, including white blood cells (× 109/L), C-reactive protein (mg/L), procalcitonin (ng/mL), presepsin (pg/mL), and total bilirubin (µmol/L). P < 0.05 was set as the significance level in A and C; P < 0.017 was set as the significance level in B. The presepsin value in the figure is the result of presepsin concentration divided by 30. WBC: White blood cell; CRP: C-reactive protein; PSP: Presepsin; T-Bil: Total bilirubin.
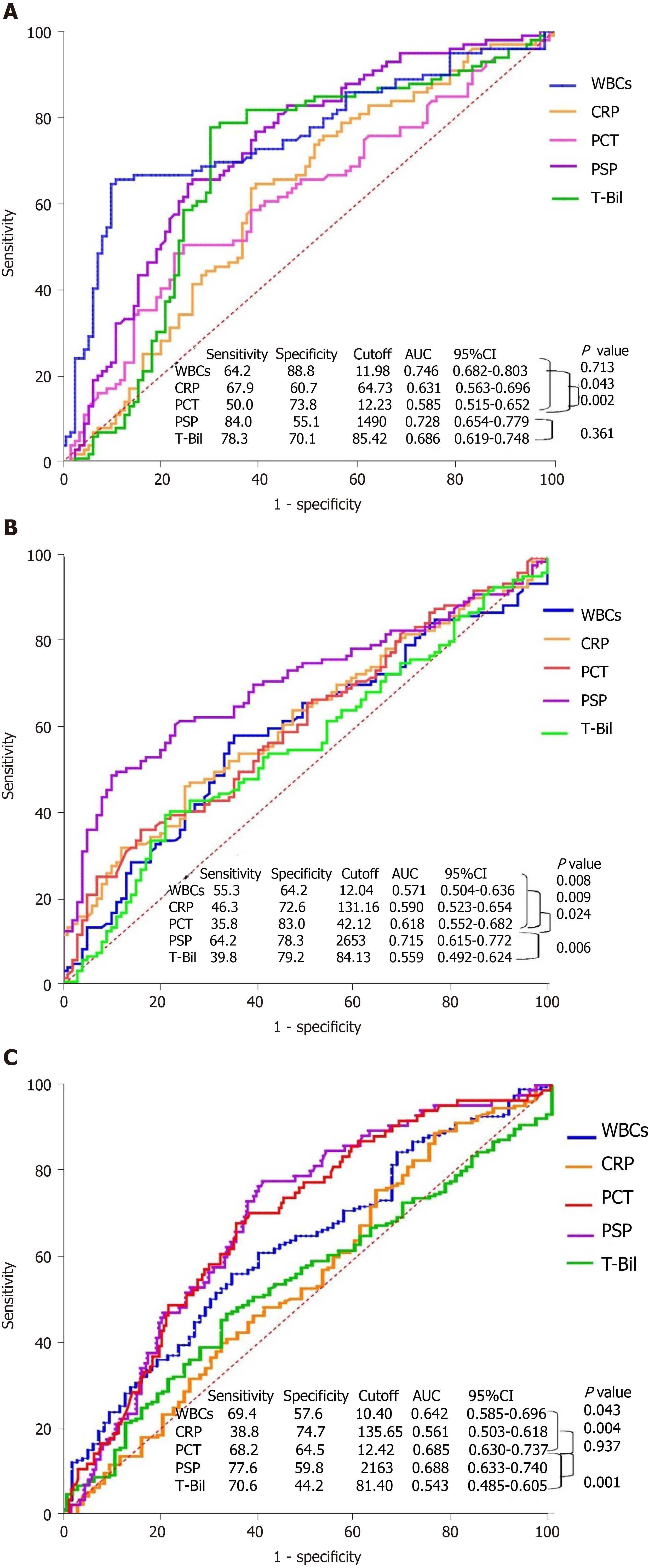
Figure 3 Receiver operating characteristic curves for white blood cells, C-reactive protein, procalcitonin, presepsin, and total bilirubin for classification of severity and prediction of positive blood culture in acute cholangitis.
The Delong test was used to compute the P value for comparison of the area under the receiver operating characteristic curve. A: Mild acute cholangitis vs moderate acute cholangitis; B: Moderate acute cholangitis vs severe acute cholangitis; C: Positive blood culture in acute cholangitis. P < 0.05 was set as the significant level. WBC: White blood cell; CRP: C-reactive protein; PCT: Procalcitonin; PSP: Presepsin; T-Bil: Total bilirubin; AUC: Area under the receiver operating characteristic curve.
- Citation: Zhang HY, Lu ZQ, Wang GX, Xie MR, Li CS. Presepsin as a biomarker for risk stratification for acute cholangitis in emergency department: A single-center study. World J Clin Cases 2021; 9(32): 9857-9868
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/9857.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.9857