Copyright
©The Author(s) 2021.
World J Clin Cases. Jul 6, 2021; 9(19): 4890-4917
Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.4890
Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.4890
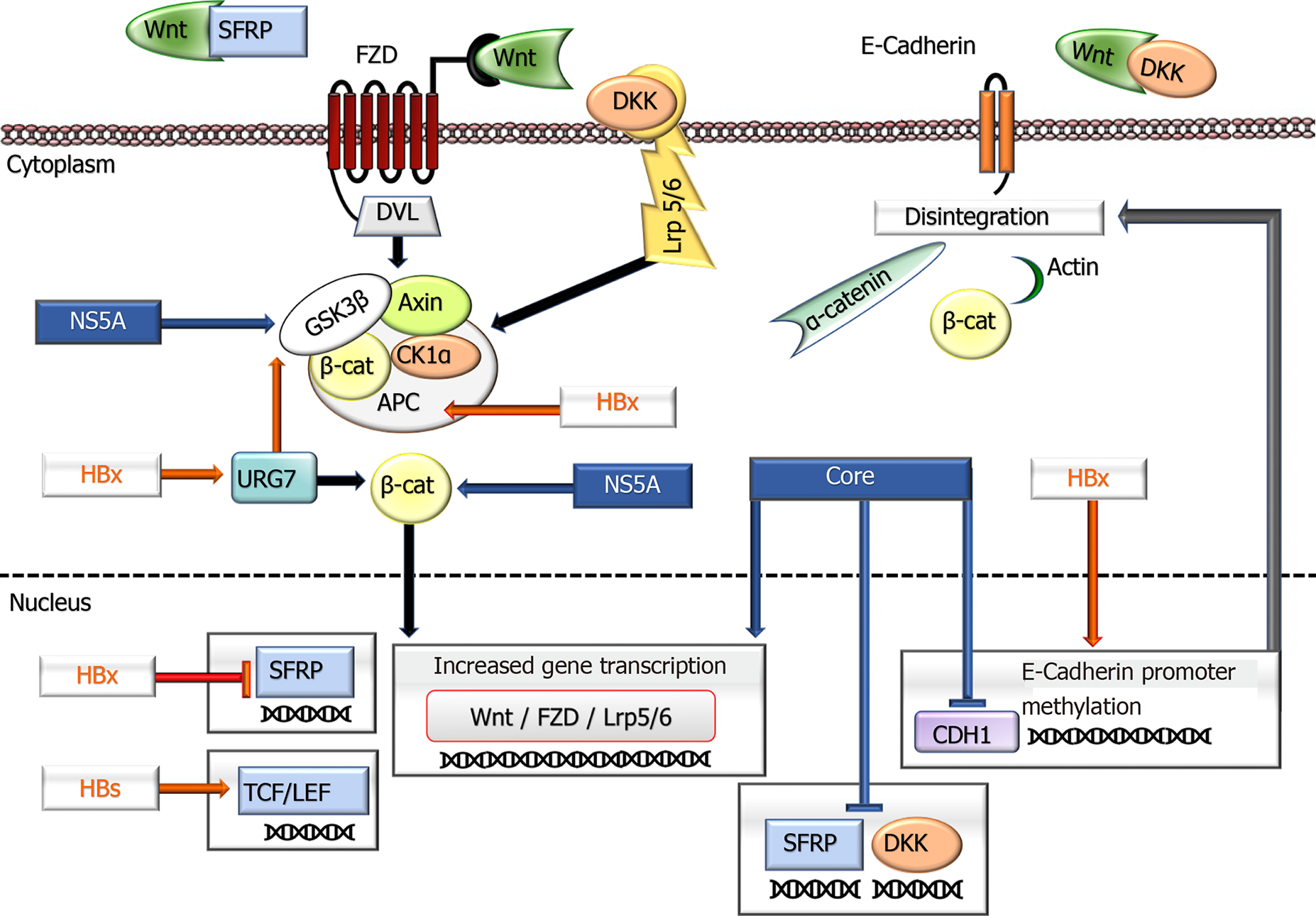
Figure 1 A schematic overview shows the impacts of hepatitis B virus and hepatitis C virus proteins in the Wnt signaling pathway.
In an inactive state, cytoplasmic β-Catenin interacts with a multiprotein degradation complex comprised of CK1a, APC, GSK3β, and Axin, and following phosphorylation, is targeted for proteasome-dependent degradation. On binding Wnt ligands to FZD and LRP5/6 receptors, the scaffolding protein DVL is recruited to the membrane and phosphorylates GSK3β leading to the disassembly of the β-Catenin destruction complex. This event results in the rescue of β-Catenin from proteasomal degradation leading to its accumulation in the cytoplasm and eventually allowing its translocation to the nucleus. Consequently, β-Catenin activates the transcription of target genes through interaction with TCF and LEF family members. Wnt signaling is regulated by secreted proteins, including SFRPs and DKKs, which inhibit Wnt signaling by binding to FZD and LRP5/6 receptors, respectively. Independent of its transcriptional activity, β-Catenin, forming a complex with E-cadherin, also facilitates cellular junctions between cells. The disintegration of E-cadherin production causes the dissociation of the complex and subsequent internalization of β-Catenin, ending with activation of its target genes. Hepatitis B virus and hepatitis C virus proteins deregulate the expression of various components of the Wnt/β-Catenin pathway and contribute to tumor development and behavior. APC: Adenomatous polyposis coli; CK1: Casein kinase 1a; DKK: Dickkopf family of proteins; DVL: Disheveled segment polarity protein; FZD: Frizzled family of the receptor; GSK3b: Glycogen synthase kinase–3b; LEF: Lymphoid enhancing factor; Lrp5/6: LDL receptor-related protein 5/6; SFRPs: Secreted frizzled-related protein; TCF: DNA-bound T-cell factor; ⊥: Inhibition.
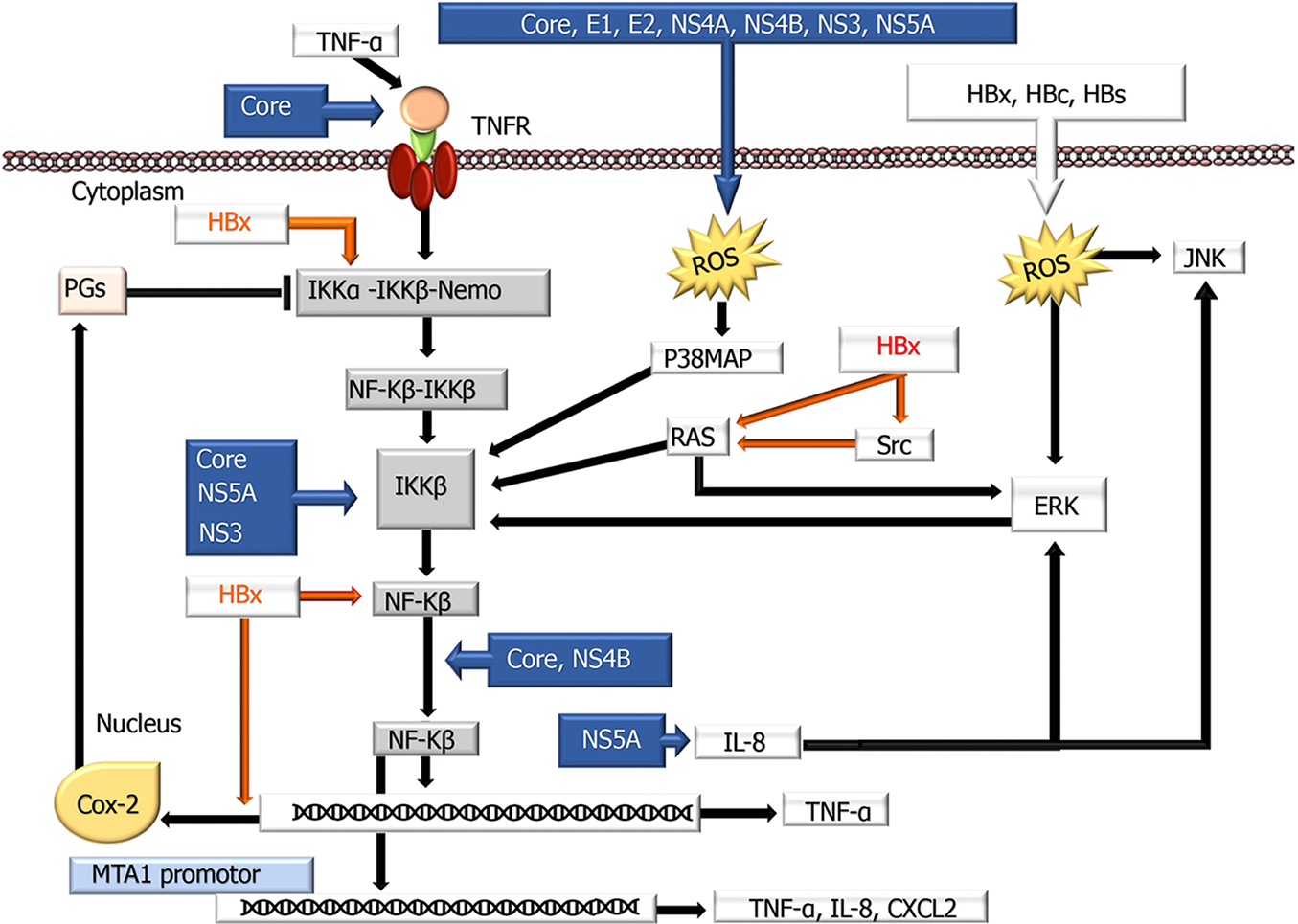
Figure 2 A schematic overview showing the influences of hepatitis B virus and hepatitis C virus proteins on the nuclear factor kappa-Β signaling pathway.
Nuclear factor kappa-Β (NF-κB) normally localizes to the cytoplasm and binds to members of the inhibitory IκB family (IκBα, IκBβ, p105, and p100) of proteins, blocking the nuclear translocation of NF-κB. Therefore, deregulation of the IκB family is required for NF-κB to be translocated into the nucleus. Hepatitis B virus and hepatitis C virus use different mechanisms to modulate these transduction pathways by modulating NF-κB proteins activation, interaction with cellular proteins, interaction with other signaling cascades, and ER stress induction. COX-2: Cyclooxygenase-2; ERK: Extracellular signal-regulated kinase; IKK: IκB kinase; IL: Interleukin; JNK: c-Jun N-terminal kinase; p38 MAPK: p38 Mitogen-activated protein kinase; PG: Prostaglandin; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor-α; TNFR: Tumor necrosis factor receptor; ⊥: Inhibition.
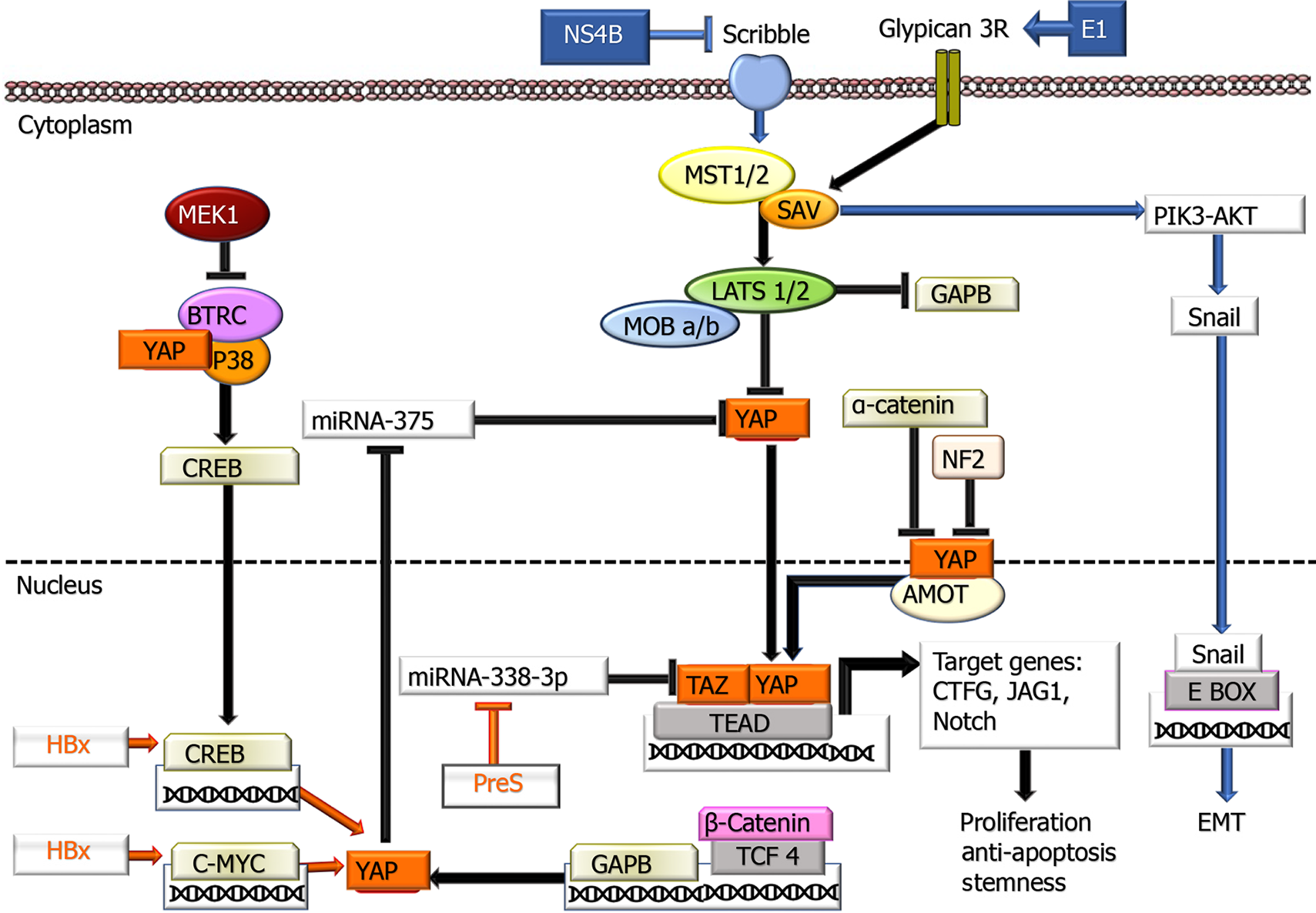
Figure 3 Involvement of hepatitis B virus and hepatitis C virus proteins in the Hippo-Make-TAZ signaling pathway.
In the cytoplasm, YAP/TAZ proteins are inactivated by phosphorylation leading to their cytoplasmic retention. When YAP/TAZ is dephosphorylated, they can translocate into the nucleus and activate the transcription of their target genes through the interaction with the TEA domain transcription factor Scalloped transcription factors. Additionally, YAP stabilizes CREB through interacting with p38MAPK and beta-transducin repeat containing E3 ubiquitin protein ligase. MEK1 also inhibits the latter. On the other hand, GABP is negatively regulated by the Hippo signaling pathway. AMOT: Actin-associated protein angiomotin; BTRC: Beta-transducin repeat containing E3 ubiquitin protein ligase; CREB: cAMP response element-binding protein; LATS1/2: Large tumor suppressor kinase 1 and 2; MST1/2: Mammalian sterile 20-like kinase 1 and 2; P38 MAPK: P38 mitogen-activated protein kinase; NF2: Neurofibromin 2; SAV1: The adaptor proteins Salvador 1; Scribble: A basolateral polarity factor; TEAD: TEA domain transcription factor Scalloped; TCF4: Transcription factor 4; ⊥: Inhibition.
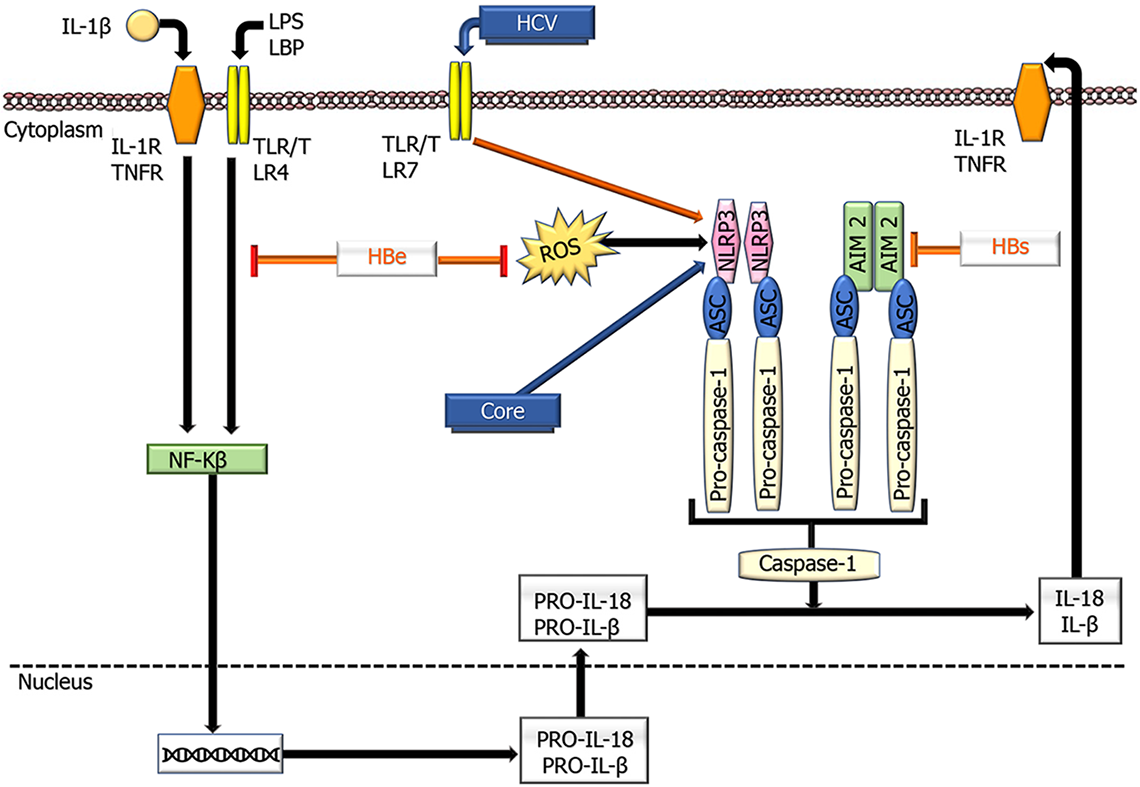
Figure 4 Schematic overview showing the effects of hepatitis B virus and hepatitis C virus proteins on the functioning of NLR family pyrin domain containing 3 and absent in melanoma 2 inflammasomes.
Inflammasome activation is defined by oligomerization of NLR family pyrin domain containing 3 and absent in melanoma 2, which recruits apoptosis-associated speck like proteins and pro-caspase- 1, leading to caspase-1 activation and subsequent conversion of pro-IL- 1β into active IL-1β. HCV: Hepatitis C virus; ASC: Apoptosis-associated speck-like protein containing a CARD; AIM2; Absent in melanoma 2; IL: Interleukin; LPS; Lipopolysaccharides; LBP: Lipopolysaccharide binding protein; NLRP3: NLR family pyrin domain containing 3; TLR: Toll-like receptor; TNFR: TNF receptor; ⊥ : Inhibition.
- Citation: Elpek GO. Molecular pathways in viral hepatitis-associated liver carcinogenesis: An update. World J Clin Cases 2021; 9(19): 4890-4917
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/4890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.4890