Peer-review started: June 6, 2018
First decision: June 6, 2018
Revised: August 15, 2018
Accepted: December 24, 2018
Article in press: December 24, 2018
Published online: January 18, 2019
Processing time: 63 Days and 1.6 Hours
Coronary artery disease (CAD) screening and diagnosis are core cardiac specialty services. From symptoms, autopsy correlations supported reductions in coronary blood flow and dynamic epicardial and microcirculatory coronaries artery disease as etiologies. While angina remains a clinical diagnosis, most cases require correlation with a diagnostic modality. At the onset of the evidence building process much research, now factored into guidelines were conducted among population and demographics that were homogenous and often prior to newer technologies being available. Today we see a more diverse multi-ethnic population whose characteristics and risks may not consistently match the populations from which guideline evidence is derived. While it would seem very unlikely that for the majority, scientific arguments against guidelines would differ, however from a translational perspective, there will be populations who differ and importantly there are cost-efficacy questions, e.g., the most suitable first-line tests or what parameters equate to an adequate test. This article reviews non-invasive diagnosis of CAD within the context of multi-ethnic patient populations.
Core tip: Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide. Globalisation has seen an epidemiological shift in demographics and risk for populations. In planning a cost-effective health service it is important to understand demographic risk and variables in interpretating and managing CAD.
- Citation: Iyngkaran P, Chan W, Liew D, Zamani J, Horowitz JD, Jelinek M, Hare DL, Shaw JA. Risk stratification for coronary artery disease in multi-ethnic populations: Are there broader considerations for cost efficiency? World J Methodol 2019; 9(1): 1-19
- URL: https://www.wjgnet.com/2222-0682/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5662/wjm.v9.i1.1
The lay term “Heart Attack”, implying coronary heart disease (CHD) is associated with significant anxiety among all communities. The first descriptions for ischemic heart disease (IHD) can be traced back to Homer and many other ancient civilizations. In Western medicine “Angina Pectoris” as first described by Heberden was derived from Latin “infection of the throat”, Greek “strangling” and Latin “pectus or chest”, took a century to consolidate its nature as a syndrome, by Osler and focus on the coronary arteries[1-5]. As clinical medicine, physiology and pathology, three pillars of health sciences coalesced, clinicopathophysiology of diseases became the norm in clinical discussions. Further collaborations between cardiologists, epidemiologists and biostatistician in the 1940’s paved the way for the Framingham Study, the first to put a numerical face to coronary artery disease (CAD) by objectively quantifying risk factors[5]. Subsequent evolutions were in diagnosis, therapies and now cost efficacy.
Australian cardiology practice today is fundamentally different, with new clinical paradigms in CAD emerging while traditional scientific collaborations have somewhat diminished. Examples include variations in symptoms as with angina equivalent symptoms, clinical impact of risk factors and epidemiology for pathological dynamism of coronary vascular tone. This manifests as presentations and rates of progression outside traditional models. These differences can be seen within racial or socioeconomic groups and more importantly between groups who have never been formally enrolled into studies but are now mainstay in cardiology clinics from migration and other factors.
When CAD is suspected referral for cardiac services is made for risk stratification. From the history, patients acquire a risk score that guides conformation via a physiological and anatomical test of the coronary arteries. Presently there are no conclusive guidelines that address the cost efficacy of risk stratifying multi-ethnic patients from presentation to diagnosing CAD should they not be represented in guideline derived studies. This review is focused on exploring the paradigm that may exist when treating a diverse population for CAD from the constraints of traditional evidence. The critical question is whether a one shoe fits all approach is sufficient to answer the questions in a multiethnic, broad aged and demographically diverse populations . We focus on the Health system in the Western suburbs of Melbourne.
CAD the largest contributor to cardiovascular diseases (CVD) with 46% male and 38% female global CV deaths, is also the most prevalent non-communicable disease and greatest cause for morbidity and mortality worldwide; has common denominators in diet, obesity and physical inactivity which if eliminated can reduce risk by > 80%, including those with diabetes mellitus and stroke. The chronology of CAD from early observational studies has revealed temporal associations in its natural history, associated risk factors and with interventions (“primary”, “secondary” and even “primordial”strategies). Consequences of CAD rest with the burden of IHD and its sequelae, measured with years of life lost from death and years of disability lived with nonfatal acute myocardial infarction (MI), angina pectoris, or ischemic heart failure. Globally total age standardized IHD incidence and mortality rates have reduced, increases in the global burden of IHD has developed predominately from population growth, aging and discrepancies in regional socioeconomic development[6,7].
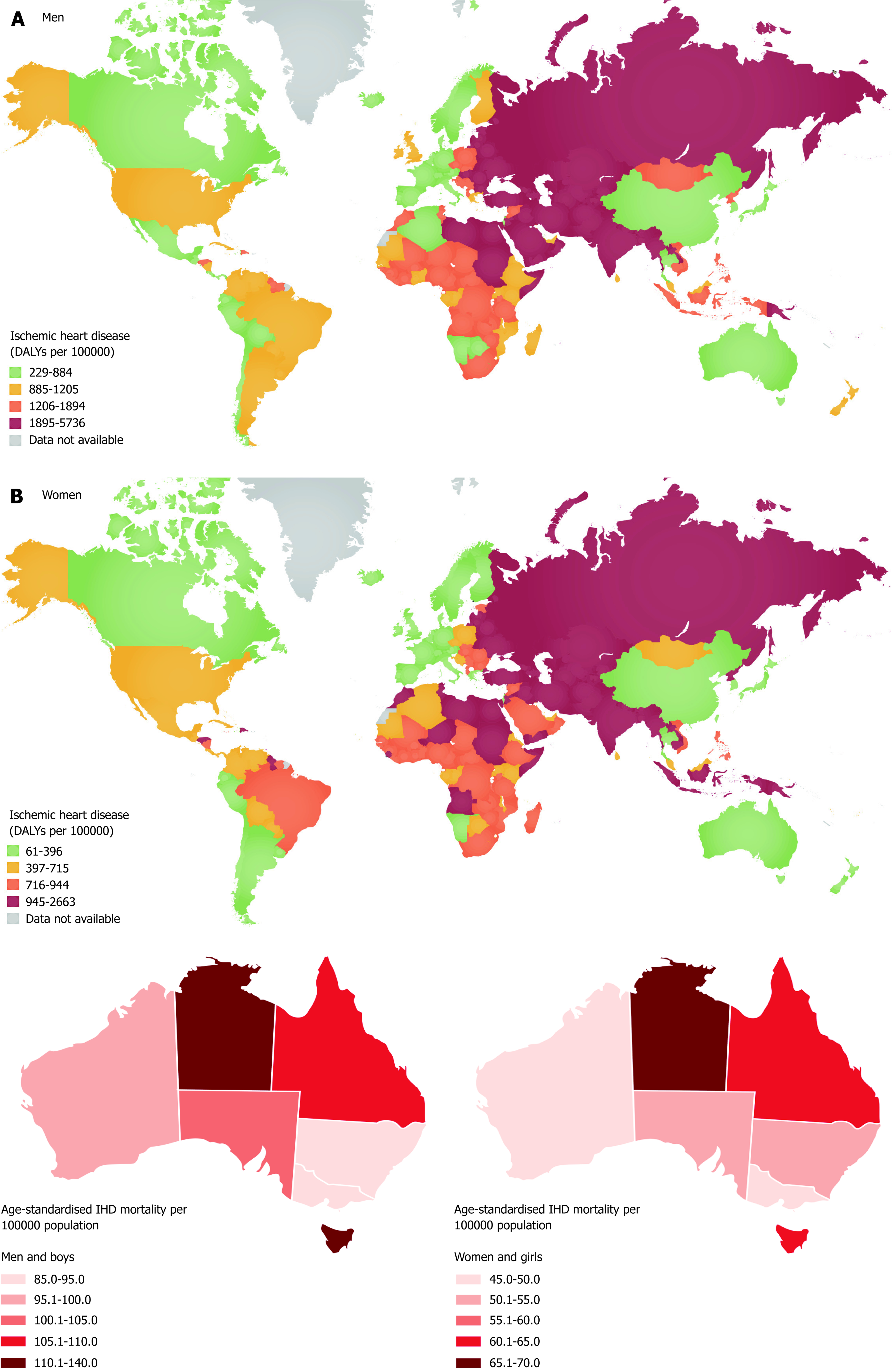
The greatest trend in the West today is regional divergence where prevalence peaked in the last century, but with increases in both prevalence and burden in Asia, Middle East and lower socioeconomic regions. Since 1990 there was 35% increase in CAD related deaths worldwide to 7 million (Figure 1). Geography, ethnicity and gender are factors that influence incidence, prevalence of risk factors and overall risk. Death rates (per 100000) vary 20 times from 35 to > 733 between South Korean and Ukrainian males; and around 30 times from 11 to 313 between French and Ukrainian women[8-15]. Community and global studies have gradually built on the evidence from Framingham Health Study (FHS) showing the “epidemiologic transition” of mortality from middle age (stage 3) to elderly (stage 4) in developed nations. The pattern of epidemiological transition can be trends, a rise and fall, continued rise or plateau[13]. As many developed nations have absorbed a diverse ethnic and sociodemographic population it is unclear which trend could eventuate in future. There is evidence on the one hand the rise and fall pattern is stalling among young adults, but uncertain trends for Aboriginal and new migrants.
Australia has seen a national rise and fall trend for IHD deaths, for example 29637 deaths (23% of all deaths) vs 22983 (17% of all deaths) were reported in 1996 and 2006 respectively[16]. Table 1 and Heart Foundation report summarizes the epidemiology in context[17]. A divergent pattern does emerge when data is broken down into states and regions within states. Remoteness, certain ethnics groups and socioeconomic demographics do not fit into the rise and fall trend when data is looked at in totally or if mortality is reduced, IHD burden remains high, e.g., Aboriginal and Torrens Strait Islanders, lower socioeconomic status, younger and advanced ages[18]. This then raises the question about fixed screening protocols for diagnostics and preventive strategies and its cost-efficacy.
| Study geography | Clinical summary (Population data available on). Demography; epidemiology; morbidity; mortality; regional variations in RF and outcomes; epidemiological transition; gaps |
| *Major international studies[6-8] | Year, study, participants, sex and ethnicity: |
| US studies: (1946) The minnesota businessmen study – C, 281 M, < 55 yr; (1948) Framingham heart study - ; (1984) CARDIA – AA/C, 5115 M/F, 18-30 yr; (1987) ARIC – AA/C, 15792 M/F, 45-64; (1989) Strong Heart – AI, 4549 M/F, 45-75 yr; (1989) Cardiovascular health study – AA/C, 5888 M/F, 65-102 yr; (2000) Jackson heart AA, 5302 M/F, 21-94 yr; (2000) MESA AA/C/Ch/H, 6814 M/F, 45-80; (2006) Hispanic community health study/study of latinos – H, 15079 M/F, 18-72. | |
| Global: 1958 The seven countries study – C, 12763 M, 40-59 yr; (1979) MONICA – ME; 15m M/F, 25-64 yr; (1999) INTERHEART – ME, 15152 M/F, age/sex matched; (2002) PURE – ME, 153996 M/F, 45-69 yr; | |
| Japanese: (1965) Ni–Hon–San study – J, 20k M, 45-69 yr | |
| Europe: UK: (1967, 1985)Whitehall, Whitehall II – C, 18403 M/10314 M-F, 40-64/35-55 yr; Iceland 1968, 2003) Rejkavik, AGES studies – C, 9141/2499 M, 34-79; Germany (1979) PROCAM – C, 4043M/1333F, 50-65 | |
| Summary of epidemiology findings: | |
| Caucasian male population are baseline comparator group for epidemiology data | |
| High income countries: | |
| International trends shown strong ↓ mortality in high-income countries since 1980 | |
| Mortality gaps exist with ethnic differences (probably genetics) either ↑ or ↓ risk or even protection. | |
| Globally: | |
| Age-standardized acute myocardial infarction incidence and angina prevalence have ↓ and ischemic heart failure prevalence has increased since 1990 (6) | |
| High age-standardized IHD mortality in Eastern Europe, Central Asia, and South Asia point to the need to prevent and control established risk factors in those regions and to research the unique behavioral and environmental determinants of higher IHD mortality.(7) | |
| Much of the dramatic CHD mortality increases in Beijing can be explained by rises in total cholesterol, reflecting an increasingly “Western” diet. Without cardiological treatments, increases would have been even greater.(6-4 Critchley J) | |
| Gaps in knowledge: | |
| Paucity of data in older > 75 yr | |
| Ethnic, family and true genetic contributions to CAD with improved modifiable risk factor control | |
| Australia[17,18] | Mortality, morbidity and cost: |
| Death rates >Japan but < other high-income countries e.g. UK, Germany USA | |
| ATSI Deaths 1.5-3 x and IHD burden.0 0Smoking ATSI | |
| Ischaemic heart disease results in more. Australian deaths than any other single cause for both men and women. | |
| Death rates from heart disease are substantially higher among ATSI Australians, ranging from 1.5 to 3 times higher than in non-Indigenous Australians. | |
| Of all Australians aged 2 yr and over, 5% report living with heart, stroke or vascular disease. Among people aged 85 yr and over, this proportion rises to two in every five people (40%). | |
| In 2012–2013 the Pharmaceutical benefits scheme paid approximately $1.8 billion for cardiovascular system medicines, representing 21% of total benefits paid in that year. | |
| Risk factor: | |
| ↓ Smoking M:F18:14%: ATSI > 2x double non-Indigenous (41% daily smokers). | |
| < 10% of all met the NHMRC guidelines for vegetable consumption. In a national secondary school survey, 24% met recommendations for consumption of vegetables and 42% met recommendations for fruit consumption. | |
| Most Australians (58%) were either sedentary or had low levels of activity. Australians spent an average of 38.8 only 30% of children met physical activity recommendations, and only 10% met both physical activity and screen-time recommendations. | |
| 13% of men and 10% of women reported drinking alcohol at levels likely to present a risk to health. Total per capita alcohol consumption fell between the early 1970s and the early 1990s, but has been relatively steady since then. | |
| One-third of Australians had high blood cholesterol (above 5.5 mmol/L). Almost four in every five Australians with abnormal cholesterol or triglyceride levels were not receiving treatment for it. | |
| One in five Australians had high blood pressure and the prevalence was higher in men than women. One in four Aboriginal and Torres Strait Islander Australians had high blood pressure. The prevalence of high blood pressure rose substantially with age, from less than 10% in the 25 to 34-year age group to almost 50% in people aged 75 years and over. | |
| More than two-thirds of men were classified as overweight or obese, as were 55% of women. One-quarter of children aged 2 to 17 years were classified as overweight or obese. | |
| The overall prevalence of diabetes in the Australian public was more than 5%, with a further 5% at increased risk of developing diabetes. | |
| The prevalence of mental disorders in 2007 was 17.6% in men and 22.3% in women; anxiety disorders were the most prevalent mental disorders in both sexes. Cardiovascular disease was responsible for nearly 44000 deaths in Australia in 2012, including more than 20000 deaths from ischaemic heart disease. |
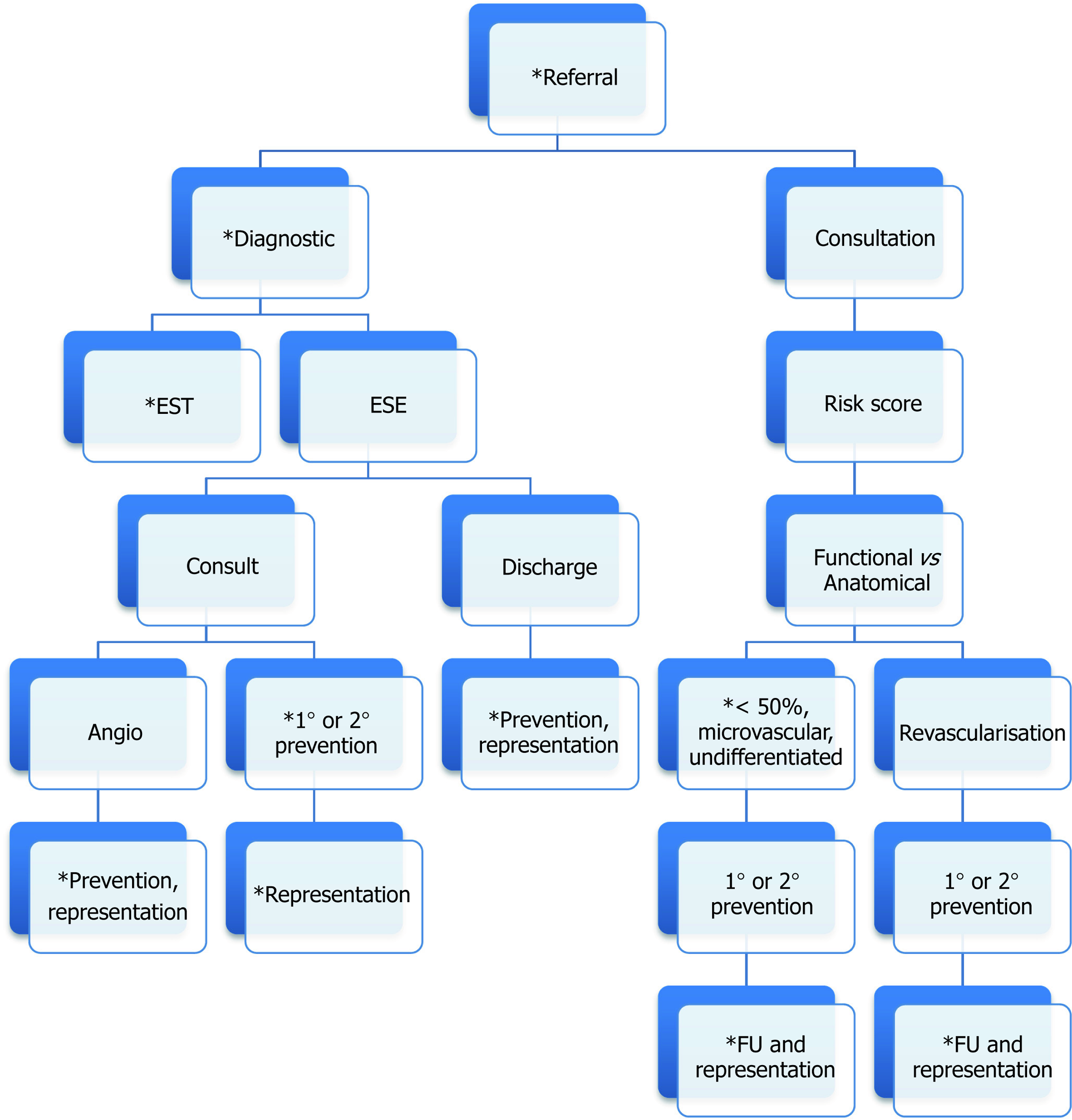
Medicare billing specialist reviews mandates prior primary care work-up. The volume of work leads to early triaging by general practitioners or emergency departments for first specialist encounter, where medicare funding regulations provide some basic framework. Figure 2 highlights the common pathway seen across medicare funded systems. Using a “three phase screening perspective”, this is however not a foolproof system for optimal cost-efficacy or outcomes as many aspects of each phase are not explored through a specialty lens. Let’s explore:
Clinical presentation: Firstly is documenting typical angina symptoms or equivalence, secondly eliciting markers of silent ischemia (discussed in risk scoring) and thirdly clinicopathophysiological correlations e.g., CAD in women. Regards symptoms, chronic stable angina is the first presentation in 50% of cases, sudden cardiac death unstable angina or infarctions in 30%. The typical presentation of anginal chest pain or “anginal equivalents” include variations in pain referred between jaw and umbilicus, shortness of breath and constitutional symptoms such as nausea, fatigue, sweating or dizziness[6,19,20]. Diamond proposed a classification of typical, atypical or non-angina, based on number of descriptors e.g., substernal location, exertional and response to rest or nitroglycerin, found good angiographic correlation between men of all ages and in older women[21]. Secondly, is silent ischemia which remains a controversial topic. On the third point, pathophysiological variations: in women for e.g., plaque burden is more diffuse and less calcific, epicardial disease less likely among < 65 years of age and smaller diameter arteries with more vascular dysfunction. Clinically we see a biphasic presentation with microvascular CAD at younger ages and higher associations of stress and mood changes from the history[22]; similarly a gradient of risk is seen from Asian to African American and Indigenous patients in severity and earlier age of onset[23-31]. Factoring these variations with language barriers can be challenging, as demonstrated by Shafiq et al[32] with patient and physician discordance in symptom reporting. Such factors could even account for variations in observed management and outcomes[33].
Risk scoring and pretest probability (PTP): From the history an assessment is made of a person’s incidence or future risk of CAD. Only patients at the lowest risk of both are discharged from surveillance. Higher scores are also documented after observation of atherosclerosis and treatments such as coronary artery bypass grafting surgery or percutaneous coronary intervention. Scores determine the choice of diagnostics investigations and predict hard CAD endpoints of nonfatal or fatal MI[6]. The FHS importantly demonstrated CVD risks with inverse relationship between various lipoproteins in cholesterol, diabetes, women and hypertension. These findings also highlighted genomic determinants and risk clusters as the foundations for multivariable or global risk assessments[6,34-36]. Additional prospective observational studies added in other risk factors including age, gender, ethnicity, diet, cigarette use, exercise, sedentary lifestyles, excess weight and family or past history of CHD, as among the more important to shape scoring systems. Such systems have been developed for clinics (e.g., Framingham Heart Disease Risk Scores) emergency departments [e.g., ADAPT (Protocol for Cardiac Event Risk), Emergency Department Assessment of Chest Pain Score] or exercise (e.g., Duke Treadmill Score).
Jensen et al[37] explored 5 risk scores (Diamond–Forrester, Updated Diamond–Forrester, Duke, Morise, CORSCORE), all use age, sex and symptoms; Duke and Morise also use tobacco use, diabetes and hypercholesterolemia, while Duke uses MI and electrocardiogram (ECG) changes and Morise uses family history of CAD, body mass index, oestrogen and hypertension. The most efficient risk models in predicting CAD are the Duke, updated Diamond–Forrester, and CORSCORE among patients presenting with angina, while the most user friendly is updated Diamond–Forrester with the lowest number of clinical variables[37]. Integration of this model into clinical care is discussed subsequently.
The premise of physiological testing is the ischemic cascade and the mechanisms for detecting the varying events[38]. Stressors (mode of exercise or pharmacological agent) and diagnostic imaging modalities will contribute to the overall short or long term statistical binary classifications for the screening tests in this case sensitivity and specificity. Within this broad classification it has never actually been identified or tested if there are categories (sub-populations e.g., demography) who require new observations prior to committing to the current stress guideline classification.
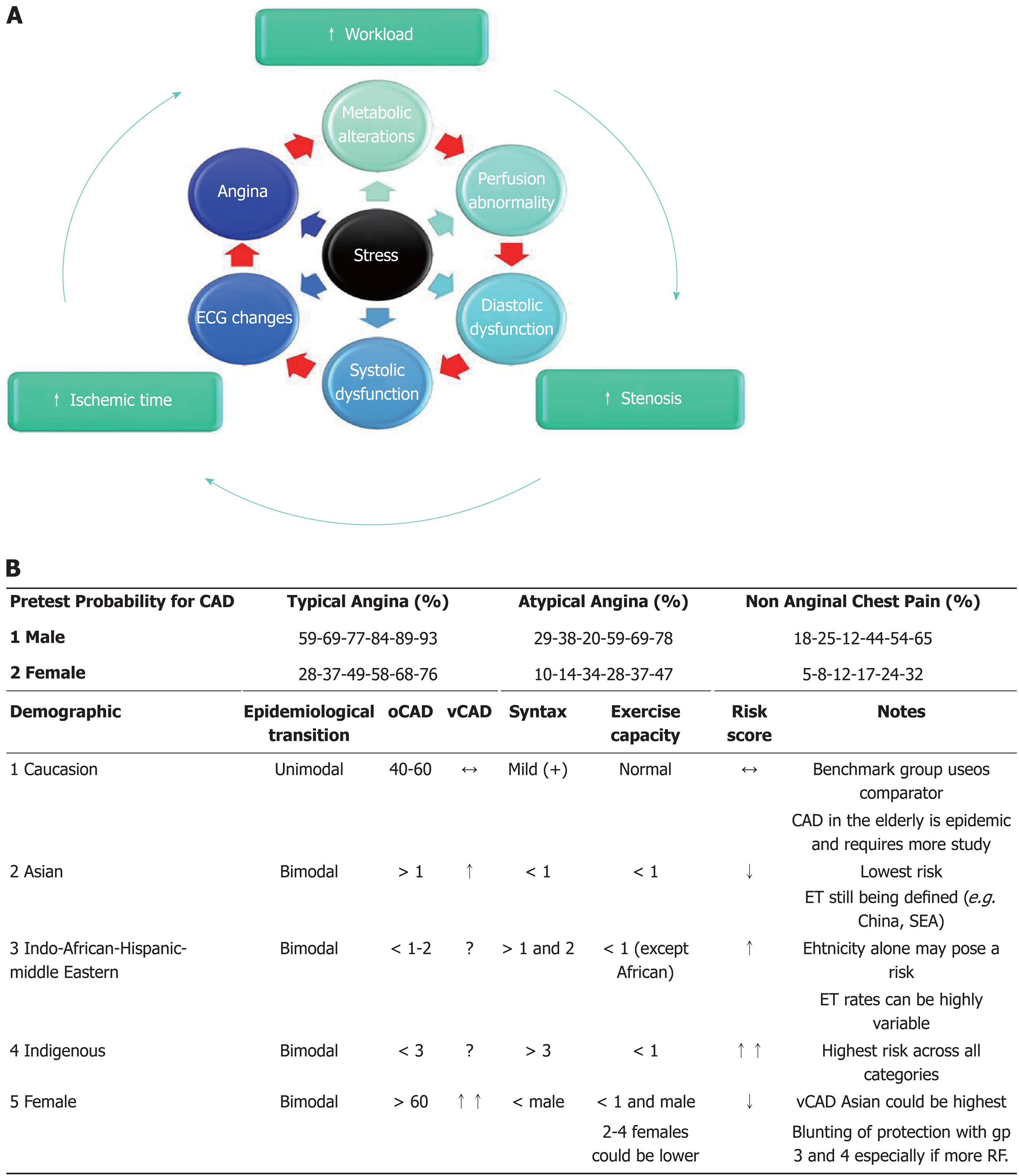
Ischemic cascade in physiological stress tests: The ischemic cascade describes “an assumption of linear” temporal sequence of pathophysiological changes where the preceding change triggers the subsequent in response to increasing myocardial supply-demand imbalance (Figure 3). Maznyczka et al[39] proposed a more individualized concept that places the patient holistically at the center of the sequence, coining the term “ischemic constellation”. This paradigm proposes no test as gold standard, that different cascade events can be abnormal at any temporal stage based on factors that can interrupt cascade linearity. These include stenosis severity or dynamism in the vascular bed, extent of stress or nature of stimulus, prior treatments, comorbidities, ischemic preconditioning and other individual factors[39]. This would suggest that from our traditional model of PTP, symptoms, ECG and diagnostic imaging, where the latter carries greatest discriminatory capacity; in the new model under varying conditions any factor could assume greater importance.
With this in mind important points to consider: (1) PTP (a) adequacy of scoring prior to referrals; and (b) standardizing scores for fixed factors e.g., age, ethnicity, family history, birthplace, etc; (2) stress modality (a) adequacy of stress duration and achieving > 85% mean predicted heart rates across age, gender and ethnicity. Achieving 10 METS is an accepted target, however the comfort and recovery periods are not factored as risks[106]; (b) the effects on the ischemic cascade with pharmacological stressors; and (c) Long term prognosticator of pharmacological stressors; (3) contextualizing ECG changes with risk and normal imaging: (a) strongly positive ECG change (> 2 mm) in low to intermediate risk females; and (b) marginally positive e.g., > 1 mm but < 2 mm in males; (4) marginally positive imaging without symptom or ECG changes; (5) when to combine physiological test and coronary imaging modality to a risk monitoring plan at baseline; (6) lower limit criteria for classifying negative or low future risk (no cardiac follow-up required); and (7) long term planning for undifferentiated chest pains and dynamic coronary ischemia and future health services utilization.
Evidence for using the ischemic constellation in multiethnic communities: Among the six cascade events, baseline characteristics and stressor can determine which factor is more prominent with stress provoked ischemia. The most powerful test finding would be from individuals who have achieved greater that predicted exercise stress with some direct vascular atherosclerotic scoring. We cite from experience that some ethnicities especially when associated with lower/higher body mass and older may not meet the physical challenges of the treadmill to achieve generic targets. Body mass, ethnicity and older adults indexed charts are not available. Language barriers in this group could also alter the interpretation of symptoms. ECG changes could have variable importance among, troponin negative acute chest pain discharges [40-42]. In acute chest pain presentations negative troponins could miss significant CAD[40,43], while high sensitivity troponin assays are plagued with specificity issues[44-51]. In summary, circumstances associated with ethnicities in some health clusters can alter the PTP, for e.g. difficulties with interpreting symptoms, the thresholds to provoke ischemia and constellation factors influencing the measured diagnostic parameters; greater vigilance is needed with post test decisions[40,41,52], including those who have normal coronary angiography[53]. Broadening decision support algorithms, presently inadequately used for more diverse demographic, could be part of future planning[54-59].
Imaging modalities, Ca Score and computer tomography coronary angiography (CTCA): When deciding on the imaging modality several factors have to be considered, including: availability, accessibility, reproducibility, cost and safety (Table 2). ECG based exercise stress testing remains first line for younger males with low risk that are able to exercise. Direct referrals for stress echocardiography is debated presently, but evidence points to superior cost efficacy when using a patient centric approach[60,61]. Much of the exercise and imaging quality deficits can be overcome by pharmacological agents e.g., dobutamine and contrast agents[60]. Appropriate remuneration strategies for this have not been factored outside tertiary centers. The main advantages appear to be access, accuracy, reproducibility and cost. The access may also be the Achilles heel for cost efficacy as guidelines for repeat testing are not well regulated. SPECT has equivalent accuracy but falls short in other parameters. A main advantage could be in those patients with baseline resting wall motion abnormalities, to quantitate location and size of infarct and obtain a gated blood pool scan ejection fraction. Cardiac MRI is safe and accurate but has other access and cost issues of SPECT. With existing dilated cardiomyopathies we suggest a baseline ESE for functional assessment combined with a baseline MRI or GBPS ejection fraction. Should an MRI be available, combination with cardiac CTCA without additional functional assessment, could be considered[61].
| Test | Guideline indication | Sensitivity | Specificity | Stress modality | Advantage | Disadvantage |
| ECG | 1st L-PTP | 45-50 | 85-90 | Physical | Simple and safe | Accuracy |
| 2nd I-PTP | Availability | ECG artifact | ||||
| Lower cost | False positives | |||||
| Echo | 1st L-PTP | 80-85 | 80-88 | Physical | Simple and safe | Suboptimal image quality e.g., resting wall motion defects, lung disease, respiratory artifact, |
| 2nd L-PTP | Pharma* | Availability | Image capture within 90 sec of peak HR | |||
| Lower cost | Cost of contrast | |||||
| No radiation | ||||||
| ECG independent | ||||||
| Mobility independent* | ||||||
| Ischemia: Quantify and localize; greater spatial resolution (subendocardial) | ||||||
| Myocardial perfusion scintigraphy (SPECT, PET) | 1st L-PTP | 73-92 | 63-87 | Physical | Accurate quantification ischemic area | Cost |
| 2nd L-PTP | 90% | 75-87 | Pharma* | Ischemia: Quantify and localize; greater spatial resolution (subendocardial) | Availability | |
| ↑ accuracy with septal defects | Radiation and retesting | |||||
| Ischemia: ↓spatial resolution e.g. for subendocardial ischemia | ||||||
| Pharma: CI, SE, ↓ sensitivity for multivessel disease | ||||||
| ↑ acquisition time | ||||||
| Artifacts: Lung motion, breast tissue, diaphragm attenuation | ||||||
| MRI | ||||||
| Ischemia | 1st I-PTP | 79-88 | 81-91 | Pharma* | Body habitus/lung window independent | Cost |
| Perfusion | 2nd L-PTP | 67-94 | 61-85 | Accurate | Availability | |
| No radiation | Expertise | |||||
| Operator independence | ↓ Gating: Rhythm and rate | |||||
| High spatial resolution | ||||||
| Can perform absolute quantification of perfusion | ||||||
| CA Score | 1st L-PTP | 95-99 | 64-83 | Direct visualization coronary artery | Availability | Radiation |
| CTCA | 2nd I-PTP | Non-invasive | Cost | |||
| Anatomical information | Ca score role | |||||
| FFR | No functional information | |||||
| Contrast | ||||||
| ↓ Gating: Rhythm and rate | ||||||
Ca Score statements support its use as a one off, in selected patients of low to intermediate risk to complement an existing functional assessment or modify risk to a higher or lower grade. We cannot advocate a role as a standalone investigation for CAD[62,63]. The utility of CTCA is now clear, with ongoing research areas for risk stratification in acuity[64,65]. As we are still unclear as to the boundaries of complimentary or additive information with functional tests and traditional risk scores, the patient cost and pretest counselling required, the role for non-specialist referrals without patients driven intent is open for further review.
Once a baseline test is done, the choice of future tests must be reproducible complimentary and provide additive information. Inter and intratest reproducibility is best achieved with stress echocardiography. All limitations of baseline test must be adequately documented for future references. The most important factor in future tests is pretest planning, i.e., access to previous test findings, what additional information is required and the targets to be met. Reassessment of prognosis and diagnosis (if required must) also be documented in test or consultations reports. Any changes to baselines risk must also be recalculated.
Typical presentations of angina or angina equivalents with or without classical findings on diagnostics can often be related to syndromes involving the coronary vasculature or heart muscle. On diastolic heart issues we refer to our recent publication[66]. Numerous authoritative publications have explored the dynamism of the large and small coronary arteries. Important points to consider are the low rates of community and primary care recognition of this syndrome, higher frequency of representation and resource utilization, higher morbidity and prognostic considerations and lack of formally labelling patients with the diagnosis. Many of these issues can be negated by increasing first presentation diagnosis or labeling of non-cardiac chest pains following specialist encounters. Formalizing a treatment or action plan is also critical[53,66-70].
Ambulatory silent myocardial ischemia is demonstrated in nearly 50% of stable CAD, 15% with either hypertension or 12 and 33 with diabetes alone or with another risk factor[71-74]. Functional diagnostics induced silent ischemia; with at least mild to moderate CAD shares similar risk to symptomatic patients. As silent ischemia can occur with or without CAD the boundaries with vasospastic angina require greater exploration. In patients with risk factors identifying further fixed risk, e.g., family history of premature CAD (before 60 years) in first degree relative, modifiable risk, e.g., sedentary lifestyle, end organ changes, e.g., proteinuria or early large or small vessel atherosclerosis, e.g., retinopathy, peripheral or carotid artery disease. The choice of test favors an imaging based functional test, while imaging of a vascular bed must also be considered complimentary early in the screening process[75-79], although improved outcomes may yet to be defined[80,81]. Among women who are more likely to have ECG changes, prognosis is unclear and more work is needed[82].
While the decisions for either functional or anatomical test are clear, the decisions for both are less clearly stated. Several important parameters guide the need for angiography. When PTP score approaches 85% e.g., male > 60 years with typical angina. Similarly there are categories of risk at young age amongst some population with risk factor clusters e.g., diabetics and chronic renal impairment[82-98]. Should risk scoring algorithms be strengthened, more evidence develop, clearer positions for warranting both types of information could be forthcoming.
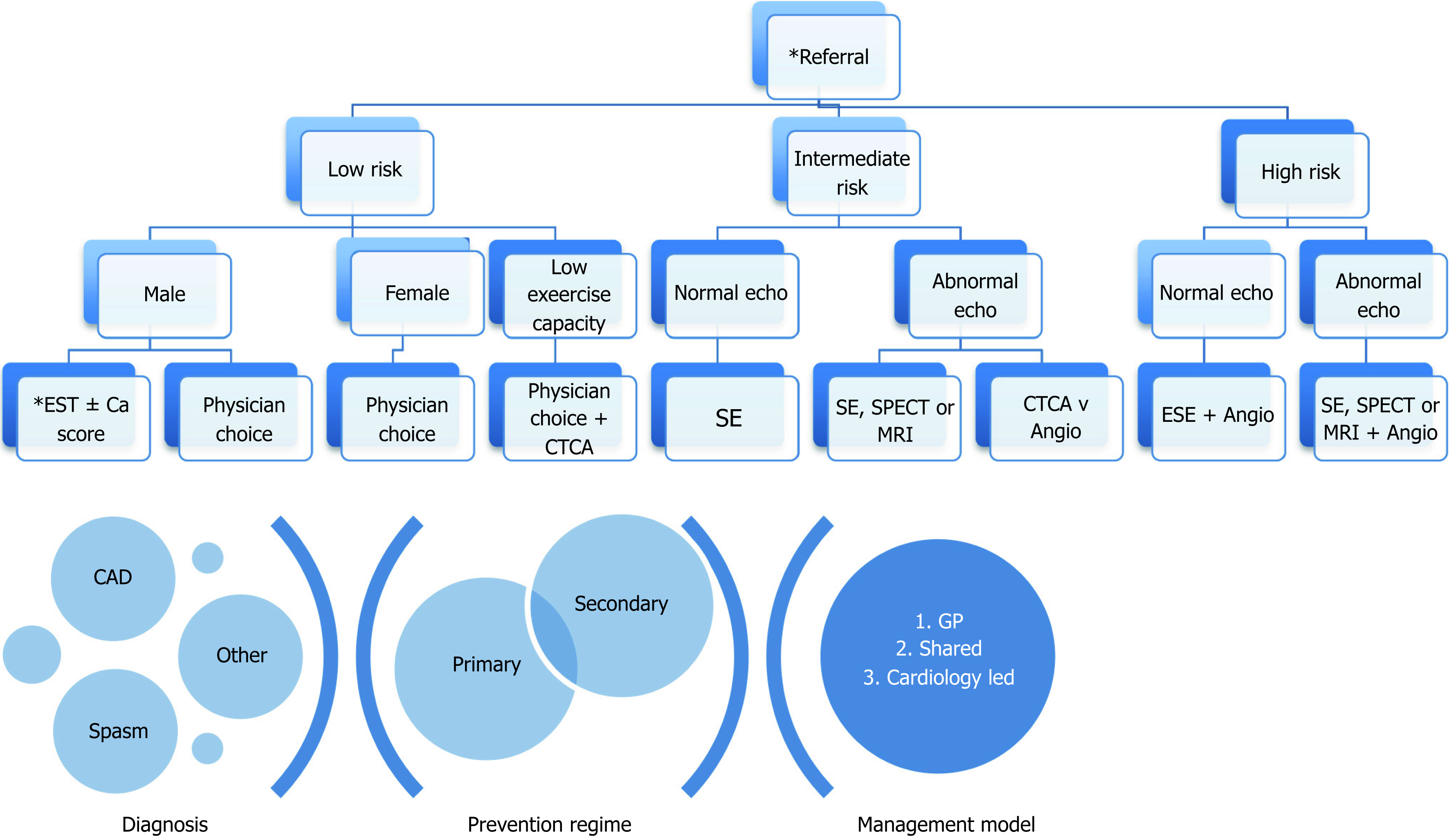
It is always premature to rest when a plateau in knowledge is assumed to have been achieved. But in all developed health systems it is premature to invest public resources to understand variations in established observations that do not meet robust requirements of need, especially cost-efficiency. We accept differentials exist in phase 1-3 trials that assume largely private capital, so this argument is largely for phase 4 trials or post translational observations[99-102]. The observations presently is “the cost efficiency of using unimodal risk stratification of CAD within health clusters that serve a multiethnic patient population with varying stages of epidemiological transition based on findings and guidelines from homogenous populations”. Presently true costings can’t be evaluated without further understanding of some care domains and processes within them. Broadly however, CAD assessment requires acknowledgement of four fundamental goals (Figure 4).
Family history and ethnicity are examples of fixed risk that remains difficult to quantify. Clinical judgement is a difficult area to research, but plays a strong role in management. Further avenues to explore are: (A) Avenues to improve translated and cultural descriptors in history for anginal symptoms; (B) Standardizing multiethnic population level risk scores; (C) Factoring in the burden of disease in risk scores; (D) Referral forms with PTP screening tools or PTP done in waiting rooms prior to test; (E) What constitutes a baseline cardiac risk score? e.g., biomarkers like hsTrop; and how often to do? Does one test supersede another? and (F) With higher risk scores do we need both functional and anatomical information?
Without directly visualizing atherosclerosis, most conclusions are inferred. Gaps exist when CAD is not excluded and with non-critical CAD, greater risk factors or microvascular disease. (A) Redefining adequate physiological endpoints that account for the ischemic threshold, with varying grades of exercise; and (B) Visualizing alternate vascular beds as surrogate for coronary vasculature in selected cases.
Once significant CAD is established management including rehabilitation and long term care pathways are well defined with solid health infrastructure in place. When patients are not cleared of CAD nor have nontraditional risks, grey areas emerge. Such areas include: (A) Factoring in the burden of disease and providing post-test risk scores; (B) Minimizing readmission and utilization of diagnostics for vasospastic CAD; (C) Licensing and return to work issues; and (D) Ensuring framework for longer term follow-up is documented, even when only a diagnostic test is requested.
Ongoing health education and engagement of specialty, tertiary and regulatory bodies are important. The jurisdictions for many of these are still poorly defined. (A) Health Clusters: (1) Jurisdictions: understanding of the boundaries, shared resources and negotiation of clinical scope could be better defined. State and federal funding tend to promote non-cooperative working relationships. Honorary acknowledgements of regional cardiologist in public institutions are examples to consider; and (2) Shared clinical and education models – research in health economics is largely a process of testing different working models. We suggest hybrid models which supports continuous information to and from all stakeholders; for providers’ flexibility of management, with accountability; and public bodies the most effective resource sharing models as important areas to explore; (B) Audit and Research Importance of audits – while regulation from authorities create a blanket rule, it is preferable that physicians using public funds should provide evidentiary data to governing bodies. Several options include: firstly, continuous professional development – to include questionnaires on selected key performance indicators across a range of issues in that community. This way accumulation of knowledge by a specialist is also transferred back to governing bodies; secondly, a mandatory requirement for audit, via governing body attached institutes across a range of domains at stipulated intervals. Health trainees could be assigned to assist. This will ensure a robust standard in documentation and management. As an important source for variations are referral biases, while structures have to be in place to align outcomes, punitive approaches must be avoided, as would providing sufficient time alerts to align practices; (C) Terminology - standardizing the minimum information required and the terminology to assist inter-observer interpretation. For example, define: (1) Nature of CAD - e.g., atherosclerotic, vasospastic, undefined; (2) Symptom - e.g., angina, angina equivalent, silent; (3) Etiology - e.g., main cause, CAD in women; (4) Structure - e.g., associated myopathies and anomalies; (5) Risk - e.g., main etiology and future risks; and (6) Monitoring - e.g., define suitable chronology of reinvestigations; and (D) Conflicting evidence in medicine - translational research questions should focus on answering many broad questions rather than, “yes” and “no” question such as mortality. We must continue to reinforce while hard major adverse cardiovascular outcome are not produced for all ethnicities, this should be a secondary outcome in phase-4 studies. The primary focus are delivery models and cost-efficiency across established health service Taxonomy domains. Nesting this question within larger studies could also broaden the funding appeal[102-105].
CAD work-up in multiethnic populations requires a more considered approach. While it is unlikely significant CAD is missed further considerations could reveal opportunities to improve cost-efficiency through the entirety of a patient’s health journey. Established pathways provide a good foundation to work from. Fine tuning this approach based on improving the translation of: ischemic symptom, “angina and equivalents” across language and cultures; risk scores when factoring ethnicity and country of birth; observable myocardial ischemia factoring individual variations and combinations of workload, stenosis severity and ischemia duration and its influence on the ischemic constellation. We have also highlighted potential options to explore. It is also becoming apparent that new paradigms in medical practice within well-defined disease processes such as CAD are developing. Such observations, calls for greater strengthening of collaboration within health clusters for education and research, while maintaining healthy competitive clinical service models to ensure uninterrupted and efficient care for patients, from all stakeholders in public and private sectors.
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Schoenhagen P, Ciccone MM S- Editor: Dou Y L- Editor: A E- Editor: Song H
| 1. | Heberden W. Some account of a disorder of the breast. Medical Transactions - The Royal College of Physicians of London. 1772;2:59-67. |
| 2. | Warren J. Remarks on angina pectoris. N Engl J Med. 1962;266:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Story C, Cherney K, Nall R. The History of Heart Disease. Retrieved 16 November. 2017; Available from: URL: https://www.healthline.com/health/heart-disease/history. |
| 4. | van Tellingen C. Chest pain and angina pectoris - or the ugly swan and the beautiful duckling. Neth Heart J. 2010;18:561-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1078] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 6. | Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 7. | Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 8. | Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 9. | Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization 2011; . |
| 10. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9579] [Article Influence: 736.8] [Reference Citation Analysis (0)] |
| 11. | Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8018] [Cited by in RCA: 7442] [Article Influence: 572.5] [Reference Citation Analysis (0)] |
| 12. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3027] [Cited by in RCA: 3578] [Article Influence: 325.3] [Reference Citation Analysis (0)] |
| 13. | Institute of Medicine. Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries. Fuster V, Kelly BB. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: The National Academies Press 2010; . [PubMed] [DOI] [Full Text] |
| 14. | O’Flaherty M, Bishop J, Redpath A, McLaughlin T, Murphy D, Chalmers J, Capewell S. Coronary heart disease mortality among young adults in Scotland in relation to social inequalities: time trend study. BMJ. 2009;339:b2613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Beltrame JF, Dreyer R, Tavella R. Epidemiology of Coronary Artery Disease. Gaze D. Coronary Artery Disease - Current Concepts in Epidemiology, Pathophysiology, Diagnostics and Treatment. Rijeka: InTech 2012; . |
| 16. | Tunstall-Pedoe H, Vanuzzo D, Hobbs M, Mähönen M, Cepaitis Z, Kuulasmaa K, Keil U. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000;355:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 280] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Nichols M, Peterson K, Alston L, Allender S. Australian heart disease statistics 2014. Melbourne: National Heart Foundation of Australia, 2014. Available from: URL: https://www.heartfoundation.org.au/images/uploads/publications/HeartStats_2014_web.pdf. |
| 18. | Australian Institute of Health and Welfare, editor. Trends in Coronary heart disease mortality: age groups and populations. Canberra: Australian Institute of Health and Welfare 2014; . |
| 19. | Kaski JC. Stable Angina Pectoris: Definition, Clinical Presentation and Pathophysiologic Mechanisms. In: Essentials in Stable Angina Pectoris. Springer, Cham 2016; . [DOI] [Full Text] |
| 20. | Cassar A, Holmes DR Jr, Rihal CS, Gersh BJ. Chronic coronary artery disease: diagnosis and management. Mayo Clin Proc. 2009;84:1130-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Maas AH. The clinical presentation of “angina pectoris” in women. Available from: URL: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/The-clinical-presentation-of-angina-pectoris-in-women. |
| 23. | Lowth M. Disease and Different Ethnic groups. Available from: URL: https://patient.info/doctor/diseases-and-different-ethnic-groups. |
| 24. | Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1440] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 25. | Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 622] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 26. | Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3702] [Cited by in RCA: 3718] [Article Influence: 265.6] [Reference Citation Analysis (0)] |
| 27. | Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, van der Velde G, Tu JV, Lee DS, Goodman SG, Petrella R, OâFlaherty M, Krahn M, Capewell S. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994-2005. JAMA. 2010;303:1841-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Hu FB, Stampfer MJ, Manson JE, Grodstein F, Colditz GA, Speizer FE, Willett WC. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. N Engl J Med. 2000;343:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2075] [Cited by in RCA: 1908] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 30. | Ferreira-González I. The epidemiology of coronary heart disease. Rev Esp Cardiol (Engl Ed). 2014;67:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015;101:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Shafiq A, Arnold SV, Gosch K, Kureshi F, Breeding T, Jones PG, Beltrame J, Spertus JA. Patient and physician discordance in reporting symptoms of angina among stable coronary artery disease patients: Insights from the Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study. Am Heart J. 2016;175:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Chew DP, MacIsaac AI, Lefkovits J, Harper RW, Slawomirski L, Braddock D, Horsfall MJ, Buchan HA, Ellis CJ, Brieger DB, Briffa TG. Variation in coronary angiography rates in Australia: correlations with socio-demographic, health service and disease burden indices. Med J Aust. 2016;205:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, Naghavi M, Salomon JA, Shibuya K, Vos T, Wikler D, Lopez AD. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 798] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 36. | Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL; Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG). Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 762] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 37. | Jensen JM, Voss M, Hansen VB, Andersen LK, Johansen PB, Munkholm H, Nørgaard BL. Risk stratification of patients suspected of coronary artery disease: comparison of five different models. Atherosclerosis. 2012;220:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Koskinas KC. Appropriate use of non-invasive testing for diagnosis of stable coronary artery disease. Available from: URL: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-12/Appropriate-use-of-non-invasive-testing-for-diagnosis-of-stable-coronary-artery. |
| 39. | Maznyczka A, Sen S, Cook C, Francis DP. The ischaemic constellation: an alternative to the ischaemic cascade-implications for the validation of new ischaemic tests. Open Heart. 2015;2:e000178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Jeetley P, Burden L, Senior R. Stress echocardiography is superior to exercise ECG in the risk stratification of patients presenting with acute chest pain with negative Troponin. Eur J Echocardiogr. 2006;7:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Raposeiras-Roubin S, Garrido-Pumar M, Pubul-Nuñez V, Peña-Gil C, Argibay-Vázquez S, Agra-Bermejo RM, Abu-Assi E, Martínez-Monzonís A, Vega M, Ruibal-Morell A, González-Juanatey JR. Discrepancy between stress electrocardiographic changes and nuclear myocardial perfusion defects in the prognostic assessment of patients with chest pain. Rev Port Cardiol. 2013;32:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, Kontos MC, McCord J, Miller TD, Morise A, Newby LK, Ruberg FL, Scordo KA, Thompson PD; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 442] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 43. | Pinkstaff S, Peberdy MA, Kontos MC, Finucane S, Arena R. Quantifying exertion level during exercise stress testing using percentage of age-predicted maximal heart rate, rate pressure product, and perceived exertion. Mayo Clin Proc. 2010;85:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol. 2014;63:2569-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 45. | Melki D, Lugnegård J, Alfredsson J, Lind S, Eggers KM, Lindahl B, Jernberg T. Implications of Introducing High-Sensitivity Cardiac Troponin T Into Clinical Practice: Data From the SWEDEHEART Registry. J Am Coll Cardiol. 2015;65:1655-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Lipinski MJ, Baker NC, Escárcega RO, Torguson R, Chen F, Aldous SJ, Christ M, Collinson PO, Goodacre SW, Mair J, Inoue K, Lotze U, Sebbane M, Cristol JP, Freund Y, Chenevier-Gobeaux C, Meune C, Eggers KM, Pracoń R, Schreiber DH, Wu AH, Ordoñez-Llanos J, Jaffe AS, Twerenbold R, Mueller C, Waksman R. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J. 2015;169:6-16.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Smulders MW, Kietselaer BL, Schalla S, Bucerius J, Jaarsma C, van Dieijen-Visser MP, Mingels AM, Rocca HP, Post M, Das M, Crijns HJ, Wildberger JE, Bekkers SC. Acute chest pain in the high-sensitivity cardiac troponin era: A changing role for noninvasive imaging? Am Heart J. 2016;177:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, Ridker PM, Pradhan AD. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women’s Health Study. Circulation. 2011;123:2811-2818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh RJ, Rouleau JL, Pfeffer MA, Braunwald E; Prevention of Events with Angiotensin Convertin+g Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 675] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 50. | Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, Ford I, Trompet S, Stott DJ, Kearney PM, Mooijaart SP, Kiechl S, Di Angelantonio E, Sattar N. High-Sensitivity Cardiac Troponin Concentration and Risk of First-Ever Cardiovascular Outcomes in 154,052 Participants. J Am Coll Cardiol. 2017;70:558-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 51. | Shaw LJ, Hendel RC, Cerquiera M, Mieres JH, Alazraki N, Krawczynska E, Borges-Neto S, Maddahi J, Bairey Merz CN. Ethnic differences in the prognostic value of stress technetium-99m tetrofosmin gated single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;45:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED; American College of Cardiology-National Cardiovascular Data Registry Investigators. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 53. | Delcour KS, Khaja A, Chockalingam A, Kuppuswamy S, Dresser T. Outcomes in patients with abnormal myocardial perfusion imaging and normal coronary angiogram. Angiology. 2009;60:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Sanchis J, Bodí V, Llácer A, Núñez J, Consuegra L, Bosch MJ, Bertomeu V, Ruiz V, Chorro FJ. Risk stratification of patients with acute chest pain and normal troponin concentrations. Heart. 2005;91:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States: an analysis of repeated cross-sectional data. Ann Intern Med. 2014;161:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | Carlisle DM, Leape LL, Bickel S, Bell R, Kamberg C, Genovese B, French WJ, Kaushik VS, Mahrer PR, Ellestad MH, Brook RH, Shapiro MF. Underuse and overuse of diagnostic testing for coronary artery disease in patients presenting with new-onset chest pain. Am J Med. 1999;106:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Winchester DE, Meral R, Ryals S, Beyth RJ, Shaw LJ. Appropriate use of myocardial perfusion imaging in a veteran population: profit motives and professional liability concerns. JAMA Intern Med. 2013;173:1381-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 868] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 59. | Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1343] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 60. | Hamilton-Craig C, Boga T, West C, Kelly N, Anscombe R, Burstow D, Platts D. Contrast echocardiography in Australian clinical practice. Heart Lung Circ. 2010;19:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Hamilton-Craig C, Strugnell WE, Raffel OC, Porto I, Walters DL, Slaughter RE. CT angiography with cardiac MRI: non-invasive functional and anatomical assessment for the etiology in newly diagnosed heart failure. Int J Cardiovasc Imaging. 2012;28:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Hamilton-Craig C, Chan J. The clinical utility of new cardiac imaging modalities in Australasian clinical practice. Med J Aust. 2016;205:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Hamilton-Craig CR, Chow CK, Younger JF, Jelinek VM, Chan J, Liew GY. Cardiac Society of Australia and New Zealand position statement executive summary: coronary artery calcium scoring. Med J Aust. 2017;207:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Hamilton-Craig CR, Friedman D, Achenbach S. Cardiac computed tomography--evidence, limitations and clinical application. Heart Lung Circ. 2012;21:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Markham R, Murdoch D, Walters DL, Hamilton-Craig C. Coronary computed tomography angiography and its increasing application in day to day cardiology practice. Intern Med J. 2016;46:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Iyngkaran P, Anavekar NS, Neil C, Thomas L, Hare DL. Shortness of breath in clinical practice: A case for left atrial function and exercise stress testing for a comprehensive diastolic heart failure workup. World J Methodol. 2017;7:117-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Melikian N, De Bruyne B, Fearon WF, MacCarthy PA. The pathophysiology and clinical course of the normal coronary angina syndrome (cardiac syndrome X). Prog Cardiovasc Dis. 2008;50:294-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Maseri A, Beltrame JF, Shimokawa H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ J. 2009;73:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Arthur HM, Campbell P, Harvey PJ, McGillion M, Oh P, Woodburn E, Hodgson C. Women, cardiac syndrome X, and microvascular heart disease. Can J Cardiol. 2012;28:S42-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Pepine CJ, Geller NL, Knatterud GL, Bourassa MG, Chaitman BR, Davies RF, Day P, Deanfield JE, Goldberg AD, McMahon RP. The Asymptomatic Cardiac Ischemia Pilot (ACIP) study: design of a randomized clinical trial, baseline data and implications for a long-term outcome trial. J Am Coll Cardiol. 1994;24:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Stramba-Badiale M, Bonazzi O, Casadei G, Dal Palù C, Magnani B, Zanchetti A. Prevalence of episodes of ST-segment depression among mild-to-moderate hypertensive patients in northern Italy: the Cardioscreening Study. J Hypertens. 1998;16:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Milan Study on Atherosclerosis and Diabetes (MiSAD) Group. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1997;79:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Cosson E, Guimfack M, Paries J, Paycha F, Attali JR, Valensi P. Are silent coronary stenoses predictable in diabetic patients and predictive of cardiovascular events? Diabetes Metab. 2003;29:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Zhang L, Li H, Zhang S, Jaacks LM, Li Y, Ji L. Silent myocardial ischemia detected by single photon emission computed tomography (SPECT) and risk of cardiac events among asymptomatic patients with type 2 diabetes: a meta-analysis of prospective studies. J Diabetes Complications. 2014;28:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Weiner DA, Ryan TJ, McCabe CH, Ng G, Chaitman BR, Sheffield LT, Tristani FE, Fisher LD. Risk of developing an acute myocardial infarction or sudden coronary death in patients with exercise-induced silent myocardial ischemia. A report from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol. 1988;62:1155-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Conti CR, Bavry AA, Petersen JW. Silent ischemia: clinical relevance. J Am Coll Cardiol. 2012;59:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Barthelemy O, Le Feuvre C, Timsit J. Silent myocardial ischemia screening in patients with diabetes mellitus. Arq Bras Endocrinol Metabol. 2007;51:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Ahmed AH, Shankar K, Eftekhari H, Munir M, Robertson J, Brewer A, Stupin IV, Casscells SW. Silent myocardial ischemia: Current perspectives and future directions. Exp Clin Cardiol. 2007;12:189-196. [PubMed] |
| 80. | De Lorenzo A. Screening for silent coronary artery disease in diabetics- or not? Curr Diabetes Rev. 2015;11:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Tavares CA, Wajchjenberg BL, Rochitte C, Lerario AC. Screening for asymptomatic coronary artery disease in patients with type 2 diabetes mellitus. Arch Endocrinol Metab. 2016;60:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Park KE, Richard Conti C. Prognostic Significance of Asymptomatic Myocardial Ischemia in Women vs. Men. Curr Pharm Des. 2016;22:3871-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Moralidis E, Didangelos T, Arsos G, Athyros V, Mikhailidis DP. Myocardial perfusion scintigraphy in asymptomatic diabetic patients: a critical review. Diabetes Metab Res Rev. 2010;26:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Valensi P, Lorgis L, Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | De Vriese AS, Vandecasteele SJ, Van den Bergh B, De Geeter FW. Should we screen for coronary artery disease in asymptomatic chronic dialysis patients? Kidney Int. 2012;81:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Sharma R, Pellerin D, Brecker SJ. The detection of myocardial ischemia in end-stage renal disease. Curr Opin Investig Drugs. 2007;8:232-236. [PubMed] |
| 87. | Le Feuvre C, Jacqueminet S, Barthelemy O. Myocardial ischemia: a silent epidemic in Type 2 diabetes patients. Future Cardiol. 2011;7:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Shirani J, Dilsizian V. Screening asymptomatic patients with type 2 diabetes mellitus for coronary artery disease: does it improve patient outcome? Curr Cardiol Rep. 2010;12:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Cuocolo A, Concilio C, Acampa W, Ferro A, Evangelista L, Daniele S, Petretta M. Cardiovascular risk stratification of diabetic patients. Minerva Endocrinol. 2009;34:205-221. [PubMed] |
| 90. | Zellweger MJ. Prognostic significance of silent coronary artery disease in type 2 diabetes. Herz. 2006;31:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: subclinical atherosclerosis: the memory of lifetime risk factor exposure. Eur Heart J. 2012;33:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Gonçalves Pde A, Rodríguez-Granillo GA, Spitzer E, Suwannasom P, Loewe C, Nieman K, Garcia-Garcia HM. Functional Evaluation of Coronary Disease by CT Angiography. JACC Cardiovasc Imaging. 2015;8:1322-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Gutterman DD. Silent myocardial ischemia. Circ J. 2009;73:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Aizenberg DJ. Cardiovascular Testing in Asymptomatic Patients: Carotid Duplex, Cardiac Stress Testing, Screen for Peripheral Arterial Disease. Med Clin North Am. 2016;100:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Vermeltfoort IA, Teule GJ, van Dijk AB, Muntinga HJ, Raijmakers PG. Long-term prognosis of patients with cardiac syndrome X: a review. Neth Heart J. 2012;20:365-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Pathak LA, Shirodkar S, Ruparelia R, Rajebahadur J. Coronary artery disease in women. Indian Heart J. 2017;69:532-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Chiha J, Mitchell P, Gopinath B, Plant AJH, Kovoor P, Thiagalingam A. Gender differences in the severity and extent of coronary artery disease. Int J Cardiol Heart Vasc. 2015;8:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Sharma K, Gulati M. Coronary artery disease in women: a 2013 update. Glob Heart. 2013;8:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Trisvetova E. Likely features of female coronary artery disease. E J Cardiology Practice. 2014;12:No. 22. |
| 100. | Zacharias K, Ahmadvazir S, Ahmed A, Shah BN, Acosta D, Senior R. Relative diagnostic, prognostic and economic value of stress echocardiography versus exercise electrocardiography as initial investigation for the detection of coronary artery disease in patients with new onset suspected angina. Int J Cardiol Heart Vasc. 2015;7:124-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Bourque JM, Beller GA. Value of Exercise ECG for Risk Stratification in Suspected or Known CAD in the Era of Advanced Imaging Technologies. JACC Cardiovasc Imaging. 2015;8:1309-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Iyngkaran P, Liew D, McDonald P, Thomas MC, Reid C, Chew D, Hare DL. Phase 4 Studies in Heart Failure - What is Done and What is Needed? Curr Cardiol Rev. 2016;12:216-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Iyngkaran P, Thomas MC, Johnson R, French J, Ilton M, McDonald P, Hare DL, Fatkin D. Contextualizing Genetics for Regional Heart Failure Care. Curr Cardiol Rev. 2016;12:231-242. [PubMed] |
| 104. | Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, Yancy CW, Faxon DP; American Heart Association Disease Management Taxonomy Writing Group. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114:1432-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 105. | Iyngkaran P, Tinsley J, Smith D, Haste M, Nadarajan K, Ilton M, Battersby M, Stewart S, Brown A. Northern Territory Heart Failure Initiative-Clinical Audit (NTHFI-CA)-a prospective database on the quality of care and outcomes for acute decompensated heart failure admission in the Northern Territory: study design and rationale. BMJ Open. 2014;4:e004137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Netw Open. 2018;1:e183605. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |