Published online Dec 26, 2017. doi: 10.5662/wjm.v7.i4.117
Peer-review started: March 17, 2017
First decision: May 23, 2017
Revised: June 29, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 26, 2017
Processing time: 290 Days and 11.8 Hours
The symptom cluster of shortness of breath (SOB) contributes significantly to the outpatient workload of cardiology services. The workup of these patients includes blood chemistry and biomarkers, imaging and functional testing of the heart and lungs. A diagnosis of diastolic heart failure is inferred through the exclusion of systolic abnormalities, a normal pulmonary function test and normal hemoglobin, coupled with diastolic abnormalities on echocardiography. Differentiating confounders such as obesity or deconditioning in a patient with diastolic abnormalities is difficult. While the most recent guidelines provide more avenues for diagnosis, such as incorporating the left atrial size, little emphasis is given to understanding left atrial function, which contributes to at least 25% of diastolic left ventricular filling; additionally, exercise stress testing to elicit symptoms and test the dynamics of diastolic parameters, especially when access to the “gold standard” invasive tests is lacking, presents clinical translational gaps. It is thus important in diastolic heart failure work up to understand left atrial mechanics and the role of exercise testing to build a comprehensive argument for the diagnosis of diastolic heart failure in a patient presenting with SOB.
Core tip: Shortness of breath is a common clinical complaint. Etiologies such as systolic heart failure, obstructive airways disease or anemia have clear and reproducible physiological changes detectable through routine diagnostic tests. Diastolic heart failure (DHF) is often a diagnosis of exclusion. In the absence of directly demonstrating an elevation of left ventricular end diastolic pressures at rest or exercise, DHF is inferred by a combination of symptoms and resting echocardiography findings. We discuss the importance of a wider consideration, e.g., left atrium function and exercise stress testing, in DHF work-up.
- Citation: Iyngkaran P, Anavekar NS, Neil C, Thomas L, Hare DL. Shortness of breath in clinical practice: A case for left atrial function and exercise stress testing for a comprehensive diastolic heart failure workup. World J Methodol 2017; 7(4): 117-128
- URL: https://www.wjgnet.com/2222-0682/full/v7/i4/117.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i4.117
Most cardiological services are faced with a large number of referrals to diagnose and manage the symptom cluster of dyspnea or shortness of breath (SOB). Broadly the etiologies can be cardiac, respiratory, haematological, due to obesity or physical deconditioning. When a cardiac cause is considered likely, imaging modalities such as echocardiography and occasionally cardiac magnetic resonance imaging can rule out systolic heart failure or heart failure with reduced ejection fraction (SHF/HFrEF). Diastolic heart failure or heart failure with preserved ejection fraction (DHF/HFpEF) can be inferred, but requires greater analysis. Exercise stress protocols are also receiving greater attention for diagnosis of HFpEF.
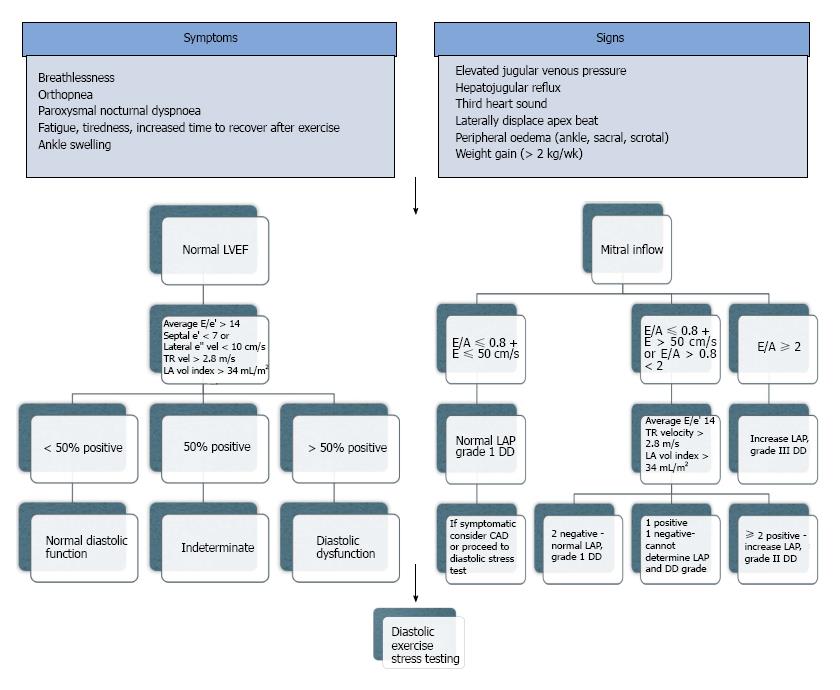
To understand the controversies in DHF it is important to go back to the basics. HF is defined as “a clinical syndrome characterized by typical symptoms (e.g., breathlessness, ankle swelling and fatigue) that may be accompanied by signs (e.g., elevated jugular venous pressure, pulmonary crackles and peripheral oedema) caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intracardiac pressures at rest or during stress”[1]. From this, four points are important in the work-up of patients suspected with DHF syndrome: (1) Chronic functional SOB, is the main reason for seeking medical care, however asymptomatic structural changes can also be detected. The correlation of changes at rest and with exercise with or without symptoms are yet to be adequately clarified; (2) in presentations with acute SOB admissions, risk stratifying diastolic abnormalities to a clinical course is also problematic[2,3]; for example are the observed changes age related or evidence of diastolic dysfunction contributing to DHF; (3) three conditions must be satisfied to rule in the diagnosis of HFpEF: Clinical symptoms of heart failure; normal or mildly abnormal systolic function [eft ventricular ejection fraction (LVEF) > 50%]; and demonstration of diastolic abnormalities in left ventricular (LV) relaxation and filling, and stiffness manifesting as increased LV filling pressures (invasively measured as LV end diastolic pressure > 16 mmHg (LVEDP) or mean pulmonary capillary wedge pressure or mean left atrial (LA) pressure > 12 mmHg), at rest or with exercise[2,4]; and (4) demonstrating altered LV pathophysiology in the “resting state” are better established, while evaluation of dynamic diastolic changes (i.e., during exercise) and alterations in left atrium (LA) metrics (i.e., volume or function parameters), have not be given enough emphasis.
The incidence of HFpEF appears to be increasing relative to HFrEF. Combined data among HF presentations reveals an average prevalence of 54% (range 40%-71%)[5]. The etiology and pathophysiological basis also appears different. Patients tend to be older with greater burden of co-morbidities[6,7]. Cardiovascular and non-cardiovascular mortality is increased, although lower than HFrEF. However, survival trends are improving with HFrEF but not HFpEF[8-11]. There have been numerous publications and guideline updates that provide a synopsis of pathophysiology[12-14], clinical correlation and pathways for assessment of DHF[1-3,15] and management[16]. This review is focused on establishing the importance of LA function and exercise testing in the workup of a patient presenting with SOB. We also explore the rationale for including LA metrics under the umbrella of the DHF syndrome focusing on published work using echocardiography as the imaging modality (DHF and HFpEF are used interchangeably, where DHF is used in context of the syndrome and HFpEF in the scientific commentary).
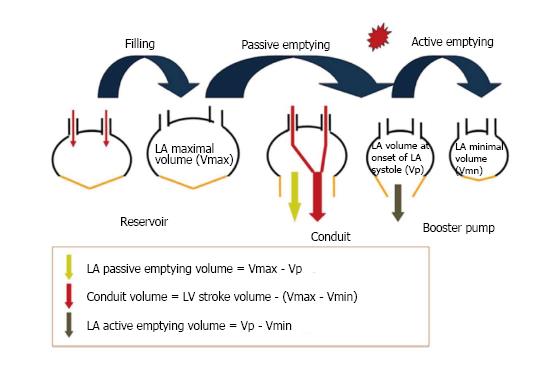
The LA is predominately composed of overlapping and varyingly aligned layers of muscle fibers that have marked variation in thickness but is overall, significantly thinner than the LV. The left coronary artery and oblique vein, which drain into the coronary sinus, are the main arterial and venous blood vessels. Specifics on LA anatomy have been previously detailed[17,18]. The LA has four important mechanical functions across three phases (Figure 1): (1) A reservoir (phase) to receive blood and store kinetic energy (as pressure) for LV filling that coincides with mitral valve closure to opening and ventricular events of isovolumic contraction, ejection and isovolumic relaxation; (2) a conduit (phase) for transiting blood (in early diastole) from the pulmonary veins to the LV after a pressure gradient develops to open the mitral valve and also passively during diastasis and is dependent on LV relaxation and preload; (3) a pump (contractile phase) to provide a “booster” depending on the preload, afterload, intrinsic contractility and electromechanical coupling [term defines the time between atrial electrical activation and mechanical activation (19)] to augment LV filling in late diastole; and (4) a suction effect to refill itself in early systole.
The LA contributes upto 30% of LV filling (The three phases can contribute around 40%, 35% and 25% respectively). LA flow from the pulmonary veins is continuous while LV filling is intermittent. The LA also acts as a volume sensor and regulates fluid balance by, neurohormonal function with production and regulation of natriuretic peptides, by regulatory (barometer) function via mechanoreceptors, and by interaction with renin angiotension aldosterone system/pathway (RAAS)[19-25].
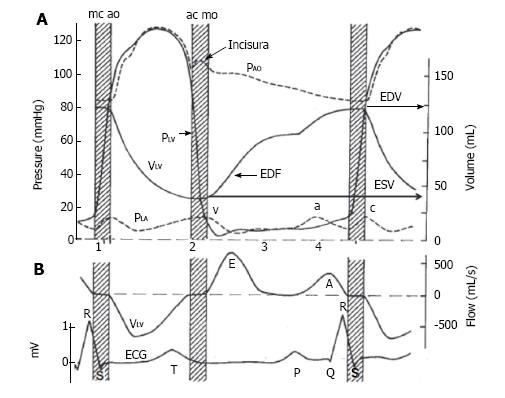
LV diastole coincides with LA systolic phase. Of the four parameters used to define diastolic function, three, LV relaxation, distensibility (restoring force) and stiffness (compliance) are predominately determined by LV characteristics and morphology. The fourth, LV filling or preload has significant LA contribution and is a compensation to maintain stroke volume (SV). Through LA and LV preload, afterload, contractility and electromechanical coupling passive and active atrioventricular connectivity are established. There are several publications that describe and evaluate LV aspects of DHF are cited[1,3,12,13,19,26,27]. Diastole is described in four phases and these phases can be related to phasic LA events (Figure 2)[28]: (1) Isovolumic relaxation during LA reservoir period; (2) rapid early filling; (3) diastasis during LA conduit phase; and (4) late filling, during atrial contraction phase.
When there is pressure and volume overload the process of atrial remodeling starts. In 220 healthy patients, age related LA indexed volumes changed only beyond the eight-decade[29]. In contrast, and without increasing LA size, changes in phasic atrial volumes and augmentation of LA contraction occur earlier, corresponding with age related alterations in LV diastolic relaxation[30,31]. Changes in the indexed LA volume (LAVi) appear to parallel the grade of diastolic diastolic dysfunction (DD)[22]. Atrial arrhythmias is an independent precipitant of atrial remodeling. The response of atrial cell to external stress incites hypertrophy, fibrosis and subsequently LA dilatation and hypocontractility[21]. LA dysfunction may alter the reservoir and conduit functions of the atrium and reduce the ability to absorb increases in LVEDP being transmitted to the pulmonary vasculature, for which there is a threshold similar to LV Frank-Starling mechanics[32]. Loss of phasic LA pump function can also lead to symptoms by reducing late LV diastolic filling, which is more marked when there is preexisting systolic impairment[25,33]. Pressure load to the LA can be seen in mitral stenosis and or increased LVEDP. Volume loading occurs in mitral regurgitation, intracardiac shunts or arteriovenous fistulae and high cardiac output states. These have to be factored in using LA metric when evaluating DD.
In a patient with SOB, echocardiography will firstly confirm LV systolic function (i.e., normal or mildly impaired ventricle (LVEF > 50%). A body of evidence is developing however to suggest that “sub clinical” systolic dysfunction such as reduced longitudinal LV shortening are present, and occur before the alteration seen in LVEF. At this stage the clinical context for DHF is evolving. Cardiac imaging with echocardiography however does not directly measure LVEDP and infers this by changes in volume, blood and tissue velocities. Invasive measures (LV pressure tracing or pressure volume data) and natriuretic peptides can provide direct information on myocardial stretch and hence diastolic abnormalities[15]. However, the noninvasively evaluated e/e’ (ratio of early diastolic transmitral velocity to early diastolic tissue velocity) serves as a surrogate of LV EDP.
Some patients manifest symptoms during exercise and this similarly can be assessed[27,34-37]. There is no single non-invasive index that confirms or rules out the diagnosis, however using a combination of parameters, this can be achieved (Figure 3). Furthermore it is unclear if any one parameter provides greater weight than another.
There is a volume of data to support LA enlargement and adverse cardiovascular outcomes independent of age, gender and the major comorbid cardiovascular risk factors[22,38,39]. In fact LA dilatation should be considered pathological before the eight decade[28]. Among 2042 residents in Olmstead County, Minnesota over 45 years of age, LAVi predicted all cause mortality, as did the grade of DD[40]. From the same community, retrospective analysis of 1160 participants (> 65 years) followed for 3.8 ± 2.7 years, LAVi > 32 mL/m2 predicted risk for first cardiovascular event (P = 0.003)[41]. Several studies with 851 and 1495 patients over 65 years of age, found that measures of LA size predicted new development of HF[42,43]. This risk was also demonstrated in 483 younger participants (mean age 47 years) followed for 6.8 years, where Leung et al showed that LAVi > 24 mL/m2 was the only independent echocardiographic predictor of cardiovascular death, congestive heart failure, myocardial infarction, stroke and atrial fibrillation. Using a variety of methods, studies show an increase in cardiac and all cause mortality in a general population[44,45], following myocardial infarction[46,47], and with dilated cardiomyopathy[48]; predicts ischemic heart disease[41,49,50], atrial fibrillation and stroke[40,41,44,49-56].
Alteration in LA mechanics (function), with or without LA dilatation, correlate with disease states such as hypertension, diabetes and renal impairment, and to adverse outcomes[57,58]. In 1802 participants of the Dallas Heart study imaged with magnetic resonance imaging, decreasing LA emptying fraction was independently associated with mortality but not LAVi[59]. In HF the reservoir and conduit functions are inversely related with Doppler parameters of DD and LVEDP. As HF progresses atrial contractility also gradually declines[60-62]. Early changes in LA mechanics, correlations with comorbidities and disease severity and recovery with treatments, have been demonstrated for hypertension[63-65], atrial fibrillation[66-70] and valvular heart disease, using a variety of methods[19,22].
LA changes particularly the LAVi reflects the chronicity and cumulative effects of changes in LV filling pressures. While the LAVi does not reflect acute changes in LV pressures it can be used as a barometer for chronically elevated LV filling pressures. This change can persist for some time after pressures have normalized. Increased LA volume can also be seen in athletes, bradycardia, anemia, high output states, atrial arrhythmias and mitral valve disease, independent of diastolic dysfunction. When these conditions are excluded LAVi > 34 mL/m2 should alert treating physicians to the possibility of DD and raised LV filling pressures[15].
To summarize the data, firstly LA size is a marker of health in a population; secondly a change in size highlights a remodeling process that predicts adverse outcomes; and thirdly alterations in LA size and mechanics potentially is caused by alterations in LV diastolic filling abnormalities due to atrioventricular interdependence[40].
Conventional echocardiography is sufficient to assess atrial size, but a combination of conventional and novel techniques are required to assess atrial mechanical functions.
M-mode and 2D echocardiography measuring the antero-posterior diameter, as performed in early studies, is now agreed to be an inadequate measure of LA size. Both the American and European Society of Echocardiography are in consensus that LAV using either the ellipsoid model or Simpson’s method in two and four chamber apical views is more accurate as LA enlargement occurs asymmetrically. When the LAV is indexed (LAVi) it provides the strongest association, most sensitive predictor and risk stratification tool for cardiovascular outcomes[2,3,22,38]. A detailed description of LAV is highlighted below[55].
LA passive volumes consist of: (1) Preatrial contraction volume (VpreA), measured at the onset of the P-wave on an electrocardiogram (ECG); (2) minimal LA volume (Vmin), measured at the closure of the mitral valve in end-diastole; and (3) maximal LA volume (Vmax), measured just before the opening of the mitral valve in end-systole.
LA active volumes are: (1) LA reservoir volume (Vmax - Vmin); (2) LA conduit volume (LV total SV - LA reservoir volume); (3) LA passive emptying volume (Vmax - VpreA); and (4) LA contractile volume (VpreA - Vmin).
Physiological associations of LA size have been noted with body size and gender, but these differences are not apparent once indexed to BSA. Age related changes are seen at the extremes but not with normal aging. Based on the sensitivity and specificity for predicting cardiac events, population studies have shown mean LAVi by biplane Simpsons or area length method was between 20-23 ± 6-7 mL/m2, giving a normal value of 22 ± 6 mL/m2[31,40,44,51,53]. In the guidelines, 1 standard deviation (SD) from the mean > 28 mL/m2 is considered LA enlargement and 2 SD from the mean > 34 mL/m2 for DD[3,18,21]. Pressure load to the LA can be seen in mitral stenosis and or increased LVEDP. Volume loading occurs in mitral regurgitation, intracardiac shunts or arteriovenous fistulae and high cardiac output states. These have to be factored in evaluating DD and LA changes. Factoring these conditions LAVi has been shown to strongly correlate with the degree of DD and even differentiate between normal and pseudonormal filling patterns[19,20,22,50,71,72].
The gold standard test atrial volume loop is invasive and not routinely available. Four established echocardiographic parameters can provide information on the varying phases of LA function with advantages and disadvantages (Table 1).
| LA function | Volumetric | Spectral Doppler | Tissue Doppler and deformation indexes | ||||
| LA volume fraction | Transmitral flow | PV flow | Composite indexes | TDI | Strain (ε) | Strain rate | |
| Global | LA EF [(LAmax - LAmin)/LAmax] | - | - | LAFI | - | - | - |
| Reservoir | Expansion index [(LAmax - LAmin)/LAmin] | - | S | - | S’ | S; total | S |
| Conduit | Passive EF [(LAmax - LApre-A)/LAmax] | E E/A | D | - | E’ | e-pos | E |
| Contractile (Booster) | Active EF [(LApre-A - LAmin)/LApre-A] | A E/A AFF | PVa | Ejection force (AEF) LAKE | A’ | a-neg | A |
2D volumetric analysis (the volume method) is the simplest but requires skill in obtaining the images and is time consuming. It uses LA volume at their maximum, minimum and just before LA systole to determine function.
Spectral (pulsed wave) Doppler of transmitral flow and pulmonary veins (sampled at mitral leaflet tips) are readily available, easy to use but only provides estimate of LA function. It is dependent on immediate loading conditions and can be affected by myocardial tethering acquisition angle, heart rates, atrial fibrillation, conduction system disease, age related reductions in LV diastolic compliance, altered hemodynamics and mitral valve disease. Peak transmitral A wave velocity, velocity time integral and atrial fraction can be used to measure LA contractile function and has been beneficial in following correction of atrial fibrillation with cardioversion, cathether ablation or surgery[53,71-78]. The atrial ejection force can be calculated with several assumptions of the density of blood and a circular mitral annulus area, where diameter is measured in 4-chamber view. This has found correlation with return of atrial function post cardioversion, adverse cardiovascular remodeling and cardiovascular events[79,80], although significant technical limitations persist[20]. Importantly all Doppler measurements can only be performed in sinus rhythm.
Tissue Doppler imaging of intrinsic myocardial velocity (e.g., mitral annulus), can provide regional and when averaged from several sites, global function. It is a low-velocity and high amplitude signal and has the advantage of being load independent. Tissue Doppler has deficiencies of angle dependency (acquisition angle - long axis), is dependent on cardiac motion and myocardial properties such as tethering and annular sampling site. A’ values has been shown to be a useful surrogate of global LA function, while all parameters (S’, E’ and A’) provide useful prognostic information[20,31,81-86].
Deformation analysis with strain and the speed of deformation with strain rate imaging can quantify regional and global function independent of tethering. Values however show regional variation[63,73]. Positive values are seen with chamber dilatation and wall stretch and negative values with contraction. Similarly this method has shown correlations with clinical outcomes and prognosis such as maintenance of sinus rhythm and atrial mechanics in atrial fibrillation[67-71,87], New York Heart Association Functional Class[97], LA contractile function[63,65], hypertensive heart disease[64,66] and valvular heart disease[19].
SOB and exercise intolerance due to HFpEF, should demonstrate an increased LVEDP with exercise. The proven exercise protocols are stress echocardiography, combined stress echocardiography and cardiopulmonary stress test, and right heart catheterization with exercise[27,34-36,88-98]. HFpEF is a systemic condition with an interaction of the primary cause coupled with secondary pathophysiological changes in the LV and LA. The continuity of the vasculature places the cardiac and peripheral endothelial beds at risk of injury when chronically exposed to risk factors. This loss of compliance or efficiency can see disproportionate rises in LV filing pressures, which can be buffered for, e.g., by changes in atrial function[27]. Thus a combination of deficits in arterial-ventricular-atrial function will be present in symptomatic individuals where a rise in LVEDP or LA pressure is the common denominator.
Burgess et al[91] studied 37 patients at baseline and after supine cycle ergometry, and found that the e/e’ of > 13 correlates with an elevated LVEDP during exercise. In another 166 patients post-exercise e/e’ > 13 was highly specific (90%) for stratifying an exercise capacity of < 8 METs or > 8 METs[91]. Nedeljkovic et al[89] studied 87 patients with HTN, exertional SOB and normal resting LV function with combined exercise stress echocardiography cardiopulmonary testing to identify masked HFpEF found correlations between e/e’ > 15 and reduced peak V02 and other parameters with high sensitivity and specificity. Maeder et al[36] identified 14 patients with diagnosed HFpEF and matched controls, who subsequently underwent supine cycle ergometer exercise, found that patients with HFpEF achieved a similar pulmonary capillary wedge pressure (PCWP) to asymptomatic controls at a much lower workload. However, contrary to Burgess et al[91], the e/e’ did not reflect the hemodynamic changes during exercise in HFNEF patients.
Pulmonary artery pressures, which can act as a surrogate for elevated left sided filling pressures can also be used. This spectral Doppler method measures the tricuspid regurgitation (TR) jet velocity and applies the formula 4V2 + right atrial pressure (V = Doppler velocity of regurgitant jet). Standardized measures of right atrial pressure are readily available from guideline and textbooks. While the non-invasive stress test is practical and translatable, translational gaps persists partly due discrepancies in role of e:e’ found in Burgess et al[91] and Maeder et al[36], identifying a suitable adjunct for pulmonary artery pressures when TR is absent, and establishing values that constitute elevated pressures across the spectrum of resting diastolic profiles, and baseline pulmonary artery pressures.
The current understanding of diastology does not allow us to definitively correlate symptoms to the varying changes in diastolic profiles. In addition no single parameter can be used to determine the diagnoses. In the process of grading diastolic abnormalities changes in the mitral valve velocity profiles and tissue Doppler occur as a normal part of aging. With the advent of exercise diastology and the inclusion of left atrial volume in the most recent guidelines, highlights the importance of looking for evidence that LV filling pressures are elevated in a patient with SOB. We thus feel that an important first step is to document an increase in intracardiac pressures and the subsequent steps should go on to explore the causes for this both in the LV and LA. The bases for the later is that many of the atrial derived parameters are used to define LV diastolic function, with little emphasis on how changes in LA function could alter this.
DHF syndrome is a broad categorisation of a complex syndrome with multiple contributors where the end result is SOB and clinical impairment. Unlike SHF where the entirety of the syndrome is coupled with an impairment of LV myocardial contractility, in HFpEF it remains unclear how the interplay between degrees of LV stiffness and LA dynamics contributes to symptoms. Thus terminology in HFpEF should reflect the atrioventricular interaction in LV diastole. Lets explore several hypothetical case examples: (1) HfpEF - With predominantly impaired LV relaxation. In this scenario a patient would have clinical symptoms and signs, abnormal LV diastolic parameters, has demonstrated elevation of LVEDP (at rest or exercise), without significant LA abnormalities, and a shift of LVEDP and volume curve to the left; and (2) HFpEF - secondary to atrial dysfunction/atrial fibrillation. In this scenario the patients have similar presentation as above, however despite rate control, remains symptomatic. Restoration of sinus rhythm correlates with clinical improvement of symptoms.
Part of establishing the terminology requires an improved understanding of all aspects of LV and LA abnormalities.
The premise of any future study should be based on consolidating the diagnosis with this point in mind: “In a patient with SOB and normal LVEF the diagnosis of HFpEF can only be consolidated by reproducibly demonstrating an elevation of LVEDP or LA pressure before treatment, that this elevation is outside a physiological norm and correlates with the patients symptoms”. The premise of therapeutic studies while not the aim of this paper should also focus on atrioventricular pathophysiological derangements. From this point we can explore the steps in cardiac investigations.
Screening: (1) Firstly all patients should have a screening echocardiogram; and (2) epidemiology studies are still needed to correlate the chronology of diastolic parameters with time and symptoms.
Demonstrating increased LVEDP: Firstly, we need to demonstrate an increase in LVEDP, and secondly we need to demonstrate the abnormality in the atrioventricular context. An important question then is should exercise stress testing be a routine part of DHF work-up? Due to cost, availability and the sheer volume of patients’ invasive tests seem unrealistic, however non-invasive exercise echocardiography could screen patients needing an invasive test. Secondly, what parameters to use? (1) Pulmonary artery pressure elevations detected by exercise stress echocardiography can be a surrogate for LVEDP. Excluding other causes for pulmonary hypertension is important. When TR is absent patients could go onto an invasive exercise right heart study; and (2) The role of e/e’ and other variations in spectral and tissue Doppler parameters requires further attention. There is conflicting data from studies in the former and a lack of data for the latter[36,99]. Thirdly, natriuretic peptides: Are secreted in response to atrial (atrial natriuretic peptides) or ventricular (brain natriuretic peptides) stretch. These factors have different biological properties such as chamber secreted and half-life can be exploited for diagnosis and monitoring. In clinical translation its utility with exercise stress echocardiography as a surrogate for an invasive right heart study derived LVEDP is yet to be defined[95].
Atrial function: Is difficult to assess both at baseline and with exercise, as there are no clinically friendly tools. As many of the echocardiographic derived DHF parameters correlate with atrial mechanics, understanding how these parameters change with LA disease will better inform LV diastology. Several examples: From an invasive study in dogs undergoing exercise, it is observed that reservoir and booster functions increase but not conduit function[96]; in 50 HFpEF patients, using late diastolic mitral annular velocity and calculated left atrial reserve index, found reduced LA function with exercise that could contribute to symptoms in addition to LV systolic and diastolic abnormalities[97]. An improved understanding could also help inform future therapies targeting the LA.
Reliability in monitoring: Issues that need to be addressed are inter and intraobserver variability and the correlation of diastolic parameters following treatment and with changes in clinical status over time[100].
Diastolic compensation and chronology: For patients who have abnormal baseline diastology who do not demonstrate increases in LVEDP with exercise, we will need to find satisfactory means to exclude HFpEF from the diagnosis. This will require improved understanding of diastolic compensation in the chronology of myocardial cellular function, where a different result could be elicited with different conditions.
SOB is a common symptom presentation to cardiology clinics. Clinical workup can point toward coronary artery disease, HFrEF, respiratory causes or anemia. There is also a sizable group where differentiation is required between deconditioning, obesity or HFpEF. Thus diagnosis of HFpEF has and still remains difficult where no one parameter we have is “a smoking gun”. Baseline echocardiographic parameters have translated into flow diagrams published in the latest guidelines. There remain however important gaps in the understanding of this syndrome: (1) Diastolic function is complex in that it requires functional mechanics of both the atrium and ventricle, where less importance has been placed in understanding LA function; (2) exercise stress echocardiography is underutilized in the diagnostic work-up; (3) our understanding of the baseline and subsequent parameters in its reproducibility and clinical translation requires more study; (4) the terminology defining the major contributor to HFpEF into atrial or ventricular dysfunction, should be explored; and (5) the translation of diagnostic findings into the clinical context such as relieving LVEDP, addressing myocardial stiffness with antifibrotics, correcting or augmenting atrial function and perhaps even devices to improve atrioventricular electrical or mechanical functions. To satisfactorily deliver optimal treatments more studies are needed to consolidate on our understanding and to confidently provide the diagnosis of HFpEF in a patient presenting with SOB.
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Teragawa H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 4913] [Article Influence: 545.9] [Reference Citation Analysis (4)] |
| 2. | Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 1845] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 3. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD; Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 3822] [Article Influence: 424.7] [Reference Citation Analysis (0)] |
| 4. | Kovács SJ. Diastolic function in heart failure. Clin Med Insights Cardiol. 2015;9:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070-3077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 6. | Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3103] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 8. | Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 9. | Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 603] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 10. | Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 548] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 11. | Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 524] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 13. | Kovács Á, Papp Z, Nagy L. Causes and pathophysiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Phan TT, Shivu GN, Abozguia K, Sanderson JE, Frenneaux M. The pathophysiology of heart failure with preserved ejection fraction: from molecular mechanisms to exercise haemodynamics. Int J Cardiol. 2012;158:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Flachskampf FA, Biering-Sørensen T, Solomon SD, Duvernoy O, Bjerner T, Smiseth OA. Cardiac Imaging to Evaluate Left Ventricular Diastolic Function. JACC Cardiovasc Imaging. 2015;8:1071-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Corradi D, Maestri R, Macchi E, Callegari S. The atria: from morphology to function. J Cardiovasc Electrophysiol. 2011;22:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:1525-1533. [PubMed] |
| 19. | Todaro MC, Choudhuri I, Belohlavek M, Jahangir A, Carerj S, Oreto L, Khandheria BK. New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging. 2012;13:973-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 737] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 23. | Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TS. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 24. | Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9:191-196. [PubMed] |
| 25. | Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 837] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 26. | Asrar Ul Haq M, Mutha V, Rudd N, Hare DL, Wong C. Heart failure with preserved ejection fraction - unwinding the diagnosis mystique. Am J Cardiovasc Dis. 2014;4:100-113. [PubMed] |
| 27. | Asrar Ul Haq M, Goh CY, Levinger I, Wong C, Hare DL. Clinical utility of exercise training in heart failure with reduced and preserved ejection fraction. Clin Med Insights Cardiol. 2015;9:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mitchell JR, Wang JJ. Expanding application of the Wiggers diagram to teach cardiovascular physiology. Adv Physiol Educ. 2014;38:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Boyd AC, Schiller NB, Leung D, Ross DL, Thomas L. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc Imaging. 2011;4:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Spencer KT, Mor-Avi V, Gorcsan J 3rd, DeMaria AN, Kimball TR, Monaghan MJ, Perez JE, Weinert L, Bednarz J, Edelman K, Kwan OL, Glascock B, Hancock J, Baumann C, Lang RM. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85:272-277. [PubMed] |
| 31. | Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630-1635. [PubMed] |
| 32. | Blondheim DS, Osipov A, Meisel SR, Frimerman A, Shochat M, Shotan A. Relation of left atrial size to function as determined by transesophageal echocardiography. Am J Cardiol. 2005;96:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1143] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 34. | Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 525] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Huis In ‘t Veld AE, de Man FS, van Rossum AC, Handoko ML. How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J. 2016;24:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 573] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 39. | Katayama T, Fujiwara N, Tsuruya Y. Factors contributing to left atrial enlargement in adults with normal left ventricular systolic function. J Cardiol. 2010;55:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 41. | Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study). Am J Cardiol. 2006;97:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 43. | Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 44. | Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 352] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 539] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 47. | Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H, Matetzky S, Behar S, Eldar M, Feinberg MS. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, Enriquez-Sarano M. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 50. | Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD Jr, Kopecky SL, Tsang TS, Seward JB. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26:2556-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 52. | Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 53. | Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 819] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 55. | Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 789] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 56. | Caplan LR, D’Cruz I, Hier DB, Reddy H, Shah S. Atrial size, atrial fibrillation, and stroke. Ann Neurol. 1986;19:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Leischik R, Littwitz H, Dworrak B, Garg P, Zhu M, Sahn DJ, Horlitz M. Echocardiographic Evaluation of Left Atrial Mechanics: Function, History, Novel Techniques, Advantages, and Pitfalls. Biomed Res Int. 2015;2015:765921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 59. | Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, Narita H, Kimura G. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 61. | Sirbu C, Herbots L, D’hooge J, Claus P, Marciniak A, Langeland T, Bijnens B, Rademakers FE, Sutherland GR. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr. 2006;7:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 63. | Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 298] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Kokubu N, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, Ura N, Nagao K, Tsuzuki M, Wakabayashi C. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res. 2007;30:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Saha SK, Anderson PL, Caracciolo G, Kiotsekoglou A, Wilansky S, Govind S, Mori N, Sengupta PP. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr. 2011;24:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 67. | Kaya EB, Tokgözoglu L, Aytemir K, Kocabas U, Tülümen E, Deveci OS, Köse S, Kabakçi G, Nazli N, Ozkutlu H. Atrial myocardial deformation properties are temporarily reduced after cardioversion for atrial fibrillation and correlate well with left atrial appendage function. Eur J Echocardiogr. 2008;9:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, Antz M, Ernst S, Kuck KH. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. 2008;29:1397-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Thomas L, McKay T, Byth K, Marwick TH. Abnormalities of left atrial function after cardioversion: an atrial strain rate study. Heart. 2007;93:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Barberato SH, Pecoits-Filho R. Usefulness of left atrial volume for the differentiation of normal from pseudonormal diastolic function pattern in patients on hemodialysis. J Am Soc Echocardiogr. 2007;20:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 847] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 73. | Cianciulli TF, Saccheri MC, Lax JA, Bermann AM, Ferreiro DE. Two-dimensional speckle tracking echocardiography for the assessment of atrial function. World J Cardiol. 2010;2:163-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Manning WJ, Silverman DI, Katz SE, Riley MF, Doherty RM, Munson JT, Douglas PS. Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol. 1995;75:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, Munson JT, Douglas PS. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 357] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 76. | Thomas L, Thomas SP, Hoy M, Boyd A, Schiller NB, Ross DL. Comparison of left atrial volume and function after linear ablation and after cardioversion for chronic atrial fibrillation. Am J Cardiol. 2004;93:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Choong CY, Herrmann HC, Weyman AE, Fifer MA. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 499] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 78. | Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, Seward JB. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994;69:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 215] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Manning WJ, Silverman DI, Katz SE, Douglas PS. Atrial ejection force: a noninvasive assessment of atrial systolic function. J Am Coll Cardiol. 1993;22:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Chinali M, de Simone G, Roman MJ, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Devereux RB. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005;18:1570-1576; discussion 1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 82. | Hesse B, Schuele SU, Thamilasaran M, Thomas J, Rodriguez L. A rapid method to quantify left atrial contractile function: Doppler tissue imaging of the mitral annulus during atrial systole. Eur J Echocardiogr. 2004;5:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, Lam PK, Sanderson JE. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 309] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 84. | Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr. 2003;4:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Lindström L, Wranne B. Pulsed tissue Doppler evaluation of mitral annulus motion: a new window to assessment of diastolic function. Clin Physiol. 1999;19:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1127] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 87. | Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, D’Onofrio A, Severino S, Calabró P, Pacileo G. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 88. | Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55:1701-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Nedeljkovic I, Banovic M, Stepanovic J, Giga V, Djordjevic-Dikic A, Trifunovic D, Nedeljkovic M, Petrovic M, Dobric M, Dikic N. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur J Prev Cardiol. 2016;23:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 92. | Mezzani A, Agostoni P, Cohen-Solal A, Corrà U, Jegier A, Kouidi E, Mazic S, Meurin P, Piepoli M, Simon A. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16:249-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 93. | Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Cardiopulmonary exercise testing variables reflect the degree of diastolic dysfunction in patients with heart failure-normal ejection fraction. J Cardiopulm Rehabil Prev. 2010;30:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 95. | Hamasaki H. The Effects of Exercise on Natriuretic Peptides in Individuals without Heart Failure. Sports. 2016;4:32. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Nishikawa Y, Roberts JP, Tan P, Klopfenstein CE, Klopfenstein HS. Effect of dynamic exercise on left atrial function in conscious dogs. J Physiol. 1994;481:457-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Tan YT, Wenzelburger F, Lee E, Nightingale P, Heatlie G, Leyva F, Sanderson JE. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. 2010;96:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Pagel PS, Kehl F, Gare M, Hettrick DA, Kersten JR, Warltier DC. Mechanical function of the left atrium: new insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiology. 2003;98:975-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 99. | Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Køber L, Bourgoun M, McMurray JJ, Velazquez EJ, Maggioni AP. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J. 2009;30:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol. 2008;102:70-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |