Published online Jun 26, 2014. doi: 10.5662/wjm.v4.i2.99
Revised: January 1, 2014
Accepted: March 13, 2014
Published online: June 26, 2014
Processing time: 270 Days and 2.9 Hours
Generally, the dental pulp needs to be removed when it is infected, and root canal therapy (RCT) is usually required in which infected dental pulp is replaced with inorganic materials (paste and gutta percha). This treatment approach ultimately brings about a dead tooth. However, pulp vitality is extremely important to the tooth itself, since it provides nutrition and acts as a biosensor to detect the potential pathogenic stimuli. Despite the reported clinical success rate, RCT-treated teeth are destined to be devitalized, brittle and susceptible to postoperative fracture. Recently, the advances and achievements in the field of stem cell biology and regenerative medicine have inspired novel biological approaches to apexogenesis in young patients suffering from pulpitis or periapical periodontitis. This review mainly focuses on the benchtop and clinical regeneration of root apex mediated by adult stem cells. Moreover, current strategies for infected pulp therapy are also discussed here.
Core tip: Compared with traditional root canal therapy, stem cell-based therapies initiate a new approach to treating dental pulp diseases. The development of teeth depends on many kinds of stem cells and some of which still exist after the formation of the root, creating a chance for the tooth to regenerate itself when it stops developing due to infection or trauma. This article provides an interesting view on the benchtop and clinical regeneration of root apex mediated by adult stem cells. Moreover, current strategies for infected pulp therapy are also discussed.
- Citation: Li Y, Shu LH, Yan M, Dai WY, Li JJ, Zhang GD, Yu JH. Adult stem cell-based apexogenesis. World J Methodol 2014; 4(2): 99-108
- URL: https://www.wjgnet.com/2222-0682/full/v4/i2/99.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i2.99
When the dental pulp is infected, traditionally, the dental pulp must be replaced with inorganic materials (paste and gutta percha) via root canal therapy (RCT). However, for RCT-treated teeth, the loss of pulp vitality, which primarily provides nutrition and acts as a biosensor to detect the potential pathogenic stimuli, will bring about various problems including the decreased strength and increased fragility; teeth are destined to be dead, devitalized, brittle and susceptible to postoperative fracture. Therefore, crowns are suggested to protect the non-vital tooth, but subsequently bring about other complications, including food impaction, recurrent caries, gingivitis, coronal leakage or microleakage, etc.
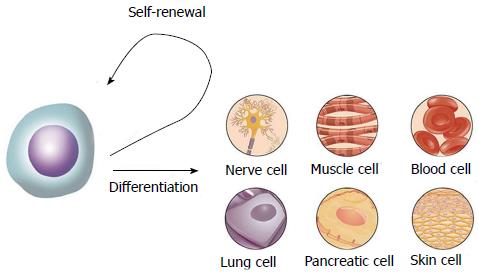
As a result, a better way to treat dental pulp diseases is needed. Nowadays stem cell-based therapies represent a promising potential to improve the life of patients with conditions ranging from neurodegenerative and traumatic diseases to regenerative disorders requiring replacement of complex structures such as bones and teeth[1-3]. Stem cells have the capacity of self-renewal and multiple-differentiation (Figure 1). They can be divided into four types, including totipotent stem cells, pluripotent stem cells, multipotent stem cells and unipotent or progenitor stem cells[2]. Due to the ethical and legal issues, the clinical application of embryonic stem cells is still controversial and restricted, although they can differentiate into almost every cell type in the human body[1]. Adult stem cells, however, become valuable because they can be isolated from many different adult tissues and demonstrate the potential to give rise to cells of various lineages[4]. The typical adult stem cells, like neural stem cells (NSCs), hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), are considered as multipotent because they can give birth to different cell types in the tissue of origin[5]. NSCs are present in the subventricular zone and the subgranular zone of the hippocampal dentate gyrus and are able to generate three cell types of the brain including neurons, astrocytes, and oligodendrocytes. HSCs were first named by Cohneim about 130 years ago[6]. They can be isolated from bone marrow and differentiate into all blood cells of the myeloid and lymphoid cell lineages[3,7]. HSCs are the best-studied stem cells for transplantation. They have been used in stem cell-based therapies for decades, especially in an allogeneic setting[8]. Hematopoietic disorders or patients with other malignancies undergoing intensive chemotherapy or radiation therapy are usually treated with this kind of stem cells[9]. Another kind of adult stem cells were first described by Friedenstein in the 1970s and were later defined as “MSCs” by Caplan and others[10-13]. MSCs have the potential to differentiate towards lineages of mesenchymal origin, including bone, cartilage, fat, connective tissue, muscle and marrow stroma. They can be isolated from diverse organs and tissues, such as bone marrow, adipose tissue, umbilical cord blood and stroma, placenta, amniotic membrane, synovium, lung, dental pulp tissue and so on[3,5]. Recently, more attention was paid to the clinical use of MSC transplantation to treat diseases that affect the host organ, including kidney injury, liver failure, myocardial infarction, articular cartilage defect and spinal cord injury, etc.[14-18]. However, the application in the treatment of a young infected tooth remains unclear.
During tooth development, enamel is formed by ameloblasts derived from the oral epithelium, while dentin and dental pulp originate from the dental papilla. The development of tooth depends on many kinds of stem cells, some of which still exist after the formation of the root. It offers a chance for the tooth to regenerate itself when the tooth stops developing because of infection or trauma. Generally, apexification by calcium hydroxide (CH) is the most traditional method to treat the immature tooth suffering from infection, usually bringing about a calcified barrier in the root with an open apex or an incompletely formed root with necrotic pulp[19,20]. However, the root often stops developing, leading to an imbalanced crown and root containing thin root dentin and wide apex, which may cause root fracture during functional movements, and it also takes a long period to complete the treatment. Recently, another method called apexogenesis has been developed with the emergence of mineral trioxide aggregate (MTA), which refers to a vital pulp therapy procedure that encourages physiological development and formation of the root end[21,22]. Compared to apexification, teeth after apexogenesis with vital pulp therapy develop a normal thickness of dentin, root length and apical morphology with fewer follow-up appointments[23,24]. In cases of Jung et al[25], he observed a separately growing root after the apexification treatment, which indicated the coexistence of apexification and apexogenesis. It is believed that some stem cells play important roles in the continuing root development of infected immature tooth.
MSCs with the capacity of self-renewal and multi-lineage differentiation are regarded as attractive progenitor cell sources for tissue engineering and regeneration[26]. To date, several kinds of MSCs have been identified as promising candidates for dental tissue engineering in the dentistry field such as bone marrow MSCs (BMMSCs), dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs), periodontal ligament stem cells (PDLSCs), dental follicle precursor cells (DFPCs) and stem cells from apical papilla (SCAPs)[26-30]. Which MSCs are mostly suitable for apexogenesis? On one hand, a tooth derives from a tooth germ consisting of various MSCs which can develop certain parts of the tooth. On the other hand, MSCs of different origins may present various differentiation abilities. Previous studies have revealed that MSCs can differentiate into certain tissues after specific induction. Accumulating evidence demonstrates that MSCs have the potential to cross lineage boundaries, even able to differentiate into specific cells of tissues beyond their origin[31] (Tables 1 and 2 describe the adult stem cell candidates and their in vivo transplantation outcomes from different research groups).
| BMMSCs | DPSCs | SCAPs | SHEDs | PDLSCs | DFPCs | |
| Location | Bone marrow | Permanent tooth pulp | Apical papilla of developing root | Exfoliated deciduous tooth pulp | Periodontal ligament | Dental follicle |
| Specific markers | ND | ND | CD24 | ND | ND | GoPro49 |
| Proliferation rate | Moderate | High | High | High | High | High |
| Multi-potentiality | Odontoblasts, osteoblasts, adipocytes, chondrocytes, myocytes, neurocytes, tenocytes | Odontoblasts, osteoblasts, adipocytes, chondrocytes, myocytes, neurocytes | Odontoblasts, osteoblasts, adipocytes, chondrocytes, neurocytes | Odontoblasts, osteoblasts, adipocytes, chondrocytes, myocytes, neurocytes | Osteoblasts, chondrocytes, adipocytes, neurocytes, cementoblasts | Osteoblasts, chondrocytes, cementoblasts, adipocytes, PDL fibroblasts, neuron-like cells |
| In vivo transplantation | Dentin-pulp-like tissue, bone, cementum, PDL | Dentin-pulp complex, bone | Dentin-pulp-like complex, bone | Dentin-like tissue, bone | Cementum/PDL-like structure | Cementum-like structure, bone |
| Ref. | MSCs | Outcomes |
| Ohazama et al[42] | BMMSCs | Tooth structures and associated bone |
| Li et al[28] | BMMSCs | Tooth-like structures surrounded by bone and soft tissues |
| Kawaguchi et al[43] | BMMSCs | Histologically produced cementum, PDL and alveolar bone |
| Gronthos et al[44] | DPSCs | Pulp-dentine like tissue complexes lined with odontoblast-like cells |
| Carinci et al[45] | DPSCs | Bone-like tissues |
| Sonoyama et al[49] | SCAPs | A typical dentin structure with connective tissues |
| Battula et al[39] | SCAPs | Bone-/dentin-like mineralized tissues |
| Miura et al[53] | SHEDs | Dentin-like tissue, but not a dentin-pulp-like complex; not differentiate directly into osteoblasts but only induce new bone formation |
| Wang et al[55] | SHEDs | Exhibit an enhanced potential to form bone |
| Park et al[57]/Seo et al[58] | PDLSCs | A typical cementum/PDL-like structure |
| Morsczeck et al[64] | DFPCs | A structure consists of fibrous or rigid tissue, no dentin, cementum, or bone formation |
| Handa et al[65] | DFPCs | Cementum-like matrix |
| Honda et al[66] | DFPCs | Bone |
BMMSCs are the first isolated MSCs with a spindle-shaped morphology which have the ability to adhere to a plastic surface with high proliferative potential[32]. BMMSCs possess the self-renewal capacity to form colonies in vitro and are capable of differentiating into multiple mesenchymal cell lineages such as osteoblasts, adipocytes, chondrocytes, muscle cells, tenocytes, and nerve cells[33-35]. However, BMMSCs are limited to a growth potential of 30 to approximately 50 population-doublings (PDs) following ex vivo expansion[30]. BMMSCs express the Oct-4, Nanog, STRO-1, CD73, CD90, CD105, CD146[36,37] and are negative for CD14, CD34, CD45 and human leukocyte antigen-DR[38-40]. Based on their multi-lineage differentiation potential and their high proliferative capacity, BMMSCs have a great potential for stem cell-based regenerative therapies. For instance, the intracoronary transplantation of autologous BMMSCs for ischemic cardiomyopathy has shown the promising results[41]. Furthermore, after transplantation of BMMSCs into regions of central nervous injury, an improved functional recovery was observed in the injured rodent brain or spinal cord[3]. Ohazama et al[42] have reported that the combination of adult BMMSCs and embryonic oral epithelium can stimulate an odontogenic response in BMMSCs, and transfer of the complex into adult renal capsules can result in the development of tooth structures and associated bone. Li et al[28] also have demonstrated that the combination of oral epithelial cells from rat embryos with BMSSCs can generate tooth-like structures expressing dentin sialophosphoprotein (DSPP) and dentine matrix protein 1 (DMP1) surrounded by bone and soft tissue. Kawaguchi et al[43] have shown complete regeneration of periodontal defects after BMMSC transplantation, and histologically produced cementum, periodontal ligament (PDL), and alveolar bone.
DPSCs were first isolated and characterized from dental pulp tissue by Gronthos et al[44] in 2000. Similar to MSCs, DPSCs are positive for CD29, CD44, CD59, CD90, CD106, and CD146, and negative for CD34, CD45, and CD11b. DPSCs are described as a highly proliferative cell population with the self-renewal ability and multi-lineage differentiation potential[3]. DPSCs possess mesenchymal stem cell properties such as a fibroblast-like morphology, adherence to a plastic surface, and the ability to form colonies when cultured in vitro[3] and they are able to differentiate into chondrocytes, adipocytes, odontoblasts and neural-like cells under appropriate inductive conditions[2]. Previous studies have shown that DPSCs are capable of differentiating into odontoblastic lineages in vitro and form ectopic pulp-dentine like tissue complexes lined with odontoblast-like cells expressing DSPP when transplanted subcutaneously into immunocompromised mice in vivo[44]. DPSCs can also form bone-like tissues when transplanted into immunocompromised mice[45]. Some studies have demonstrated that DPSCs are able to differentiate into endothelial-like cells and express blood vessel markers and neural markers, but the in vivo differentiation potential is still under debate[46-48]. Due to their easy obtainment and the potential of multi-lineage differentiation, DPSCs are thought to be an ideal cell source for tissue regeneration and engineering.
Apical papilla means the soft tissue at the apices of developing permanent teeth[49] and stem cells from apical papilla (SCAPs) are a population of MSCs residing in the apical papilla of incompletely developed teeth[50]. The surface makers are similar to those of BMMSCs and DPSCs, but CD24 is only detected in SCAPs. The expression of CD24 is down-regulated following osteogenic induction. It has been reported that SCAPs display a higher proliferation rate than DPSCs, probably because they are derived from a developing tissue[51]. Similar to DPSCs, SCAPs are able to differentiate into a variety of cell types, but appear to have greater dentinogenic potential than DPSCs. An in vivo study has shown that SCAPs with hydroxyapatite/tricalcium phosphate particles that were transplanted into immunocompromised mice can generate a typical dentin structure with a layer of dentin tissue formed on the surface of the hydroxyapatite/tricalcium phosphate along with connective tissue[49]. SCAPs also demonstrate the capacity to undergo adipogenic differentiation after induction and express neural markers with or without stimulation when cultured in vitro[30]. Besides, SCAPs are able to form a bio-root with the use in combination with PDLSCs[52]. All the above findings suggest that SCAPs can be used for tissue regeneration and engineering. Based on previous findings, SCAPs appear to be the source of primary odontoblasts that are responsible for the formation of root dentin[49]. This may explain the clinic phenomenon that apexogenesis can occur in infected immature permanent teeth suffering from apical periodontitis or abscess[23,51].
Stem cells from human exfoliated deciduous teeth (SHEDs) were first isolated by Miura et al[53] from dental pulp tissue derived from exfoliated deciduous teeth. SHEDs exhibit a higher proliferation rate than DPSCs. They express similar surface markers as compared to DPSCs and BMMSCs, but the expression of CD105 and CD146 is higher, suggesting higher capacity for differentiation. SHEDs have the potential to differentiate into neurons, adipocytes, osteoblasts, and odontoblasts as well as DPSCs[54]. When SHEDs are subcutaneously transplanted into immunocompromised mice, they can form ectopic dentin-like tissue, but are not able to regenerate a dentin-pulp-like complex[2]. Wang et al[55] have revealed that SHEDs exhibit an enhanced potential to form bone, while Miura et al[53] have suggested that SHEDs can not differentiate directly into osteoblasts but only induce new bone formation. These findings imply that deciduous teeth may be involved in bone formation during the eruption of permanent teeth. SHEDs can also differentiate into neural cells after neuronal induction and express early neuronal markers. As a result, SHEDs can be a promising source of stem cells for regenerative medicine.
PDL is the soft connective tissue interposed between the cementum and the inner wall of the alveolar socket[56], which is derived from the dental follicle. Previous studies have suggested that the PDL space may contain stem cells that exhibit osteogenic, cementoblastic, adipogenic, chondrogenic and neurogenic characteristics under certain culture conditions[30,57], which are defined as PDL stem cells (PDLSCs). When cultured in vitro, PDLSCs can form mineralized nodules, express the bone-associated markers including alkaline phosphatase and bone sialoprotein (BSP) in response to bone-inductive factors such as insulin-like growth factor 1 and express high level of scleraxis, which is a specific transcription factor associated with tendon cells[58,59]. Although PDLSCs express a range of cementoblastic/osteoblastic markers, they do not form dentin and its associated haemopoietic components in vivo. Previous studies have demonstrated that PDLSCs transplanted into immunocompromised mice can generate a typical cementum/PDL-like structure, in which a thin layer of cementum-like tissue is formed on the surface of the carrier, along with condensed collagen fibres with sparse cells that resemble PDL structures[57,58]. But the cemetum/PDL-like structures appeared totally different from typical bone/marrow structures generated by BMMSCs and dentin/pulp-like structures generated by DPSCs[58]. Due to their capacity to form periodontal structures, PDLSCs can be used as a cell source for the treatment of periodontal diseases, tissue engineering and stem cell-based therapies.
Dental follicle is an ectomesenchymal tissue that surrounds the enamel organ and the dental papilla of the developing tooth germ prior to eruption. This tissue contains progenitor cells that form the periodontium including cementum, PDL, and alveolar bone. Dental follicle precursor cells (DFPCs) are isolated from human dental follicles of impacted third molars, expressing typical mesenchymal stem cell markers such as STRO-1, CD13, CD44, CD73, Notch1, and nestin[60,61]. Moreover, GoPro49, a novel Golgi protein, has been identified as a specific marker for DFPCs[62]. DFPCs possess the ability to differentiate into osteoblasts/cementoblasts, chondrocytes, adipocytes, and neuron-like cells when growing under the appropriate culture conditions in vitro[3,60,61]. Recent studies have revealed that DFPCs cultured at 38 to 40 °C demonstrate greater osteogenesis, indicating that appropriate heat-stress treatments can promote their differentiation[63]. When DFPCs were transplanted into immunocompromised mice, a structure comprised of fibrous or rigid tissue was generated, expressing BSP, osteocalcin (OCN) and collagen type I[64]. However, there were no dentin, cementum, or bone formation observed in the transplants in vivo. But some studies[65,66] have demonstrated that DFPCs can form cementum-like matrix or bone structures in the subcutaneous area of immunodeficient mice. More work still has to be performed to explore their potential capability during the cellular therapies for periodontal diseases.
In addition to stem cells, adequate inductive materials that can promote the differentiation of stem cells are essential to the success of stem cell-based treatment. Previous studies have investigated the effects of several materials on different stem cells, which may guide us to pair the right stem cells with the most compatible inductive material.
CH (pH = 12.5) has a good antimicrobial characteristic and can inhibit tooth resorption and induce the hard tissue formation[67]. It has been successfully utilized in various endodontic treatments, such as apexification, apexogenesis, pulp capping, pulpotomy, and routine root canal therapy in infected canals for its potential to induce hard tissue repair at the site of pulp exposure[68,69], An earlier study has suggested osteo-inductive properties of CH[70], however, no significant changes were observed in vitro[71]. When CH is placed at the exposed pulp site, damaged primary odontoblasts are replaced with newly differentiated odontoblast-like cells. Ji et al[68] have exhibited that CH can increase the recruitment, migration, proliferation, and mineralization of DPSCs and PDLSCs. Besides, Ruparel’s work has suggested that CH can promote survival of SCAPs[72]. Moreover, low concentrated CH induces the proliferation of pulp fibroblasts. However, there exist several disadvantages of CH used in apexification, including multiple visits, the long treatment time and the risk of root fracture as a result of long-term use of CH[67,73-75]. It is also suggested that direct contact of CH with the tissue will induce the formation of calcified tissue in the pulp space, thus preventing pulp tissue from regeneration[23]. Another problem is that CH may damage the Hertwig’s epithelial root sheath and thereby destroy its ability to induce the nearby undifferentiated cells to become odontoblasts[73].
In 1993, mineral trioxide aggregate (MTA) was firstly introduced into endodontics and now has been widely used in diverse endodontic therapies, including pulp capping, pulpotomy, apical barrier formation, apexogenesis in developing teeth, repair of root perforations and root canal filling. MTA is a cement mixture that consists of different oxide compounds, including sodium and potassium oxides, calcium oxide, silicon oxide, ferric oxide, aluminum oxide, and magnesium oxide[76]. Compared with CH, MTA is a better choice for direct pulp capping because of its lower solubility, improved mechanical strength, better marginal adaptation, and better sealing ability, but no significant histological difference is established[77,78]. Some data also suggest that MTA is more predictable with consistent hard-tissue formation[79] as a result of the release of a large number of Ca2+ ions or the secretion of bone morphogenetic protein 2 and transforming growth factor-beta 1 by periodontal fibroblasts[80]. Some studies have reported that MTA can induce the formation of hard tissue in a shorter period of time than CH[81,82]. Further study revealed that MTA can induce tissue regeneration via the promotion of mesenchymal stem cell adhesion, proliferation, and migration[83]. Recent studies have shown that MTA stimulates the odontogenic differentiation of DPSCs, with the up-regulation of OCN and DSP[84-86]. The significantly increased levels of the angiogenic factors such as vascular endothelial growth factor and fibroblastic growth factor-2 of DPSCs are observed following the treatment with MTA in vitro[84,85], which play a critical role in tissue development, cell migration, inflammation and wound repair.
Generation of well-vascularized pulp-like tissue by using a tooth slice model has been reported. Huang et al[30] suggested that DPSCs differentiate into odontoblast-like cells with a cellular process extending into dentinal tubules when seeded onto the existing dentine. Cordeiro et al[87] have demonstrated similar findings that odontoblast-like cells arose from the stem cells and localized against the existing dentine surface in their in vivo study model. Previous studies have shown that human DPSCs, SCAPs and SHEDs, in combination with synthetic scaffolds or human root segments, are able to generate vascularized pulp-like tissues and form dentin-like mineral structures depositing onto the existing dentinal wall in the root canal space[88-90]. The mechanism behind this phenomenon has been speculated to be related with the released growth factors by dentine, such as TGF-β, which attract and induce the differentiation of odontoblasts[45]. Chemical disinfection of the root canal space may destroy these embedded growth factors.
The concept of “revascularization” describes the clinical healing of periapical abscesses and continued root formation in immature teeth with nonvital pulps[91]. However, it does not encompass the actual healing and repair process that takes place in these clinical cases[92].
The revascularization method assumes that the root canal space has been disinfected and the formation of blood clot can produce a matrix (e.g., fibrin) that traps cells capable of initiating new tissue formation. Its treatment effect is different from apexification because not only is the apex closed but the canal walls are thicker as well. It is also different from apexogenesis which also comes up with a closed apex and thicker dentinal walls resulting from the function of remaining vital root pulp.
With regard to revascularization, all the studies report the continued thickening of the dentinal walls and subsequent apical closure. The root length is increased by the growth of cementum. Connective tissue similar to PDL is also present in the canal space[93].
The success of root canal revascularization is mainly due to several factors. First, the immature avulsed tooth has an open apex, short root and intact but necrotic pulp tissue, so that the new tissue has easy access to the root canal system and a relatively short distance for proliferation to reach the coronal pulp horn. The speed with which the tissue completely revascularizes the pulp space is important because bacteria from outside are continually attempting to enter the pulp space. The ischemically necrotic pulp acts as a scaffold into which the new tissue grows, and the usually intact crown slows bacterial penetration because the only access for bacteria to the pulp is through cracks or enamel defects. Thus, the race between proliferation of new tissue and infection of the pulp space favors the new tissue formation. Second, minimum instrumentation preserves the viable pulp tissue which contributes to further development of open apex root. Third, young patients have greater healing capacity and more stem cell regenerative potential[94].
The greatest benefit of such biological approaches for dental tissue restoration over many conventional dental materials lies in the fact that reparative matrices become an integral part of the tooth, avoiding any of the problems arising from restoration retention and possible marginal bacterial microleakage. Moreover, this treatment approach strengthens the root walls of immature teeth.
Pulp regeneration is not only to solve the aesthetic issues of the conventional root canal filling materials, but also to achieve the regeneration of the whole tooth vitality and restore the normal function of teeth. Dental pulp nerve regeneration can produce a protective response to maintain long-term survival of teeth when they were stimulated by mechanical, temperature, or chemical stimuli.
Apexogenesis should be performed in three kinds of dental diseases of immature teeth, including reversible pulpitis, irreversible pulpitis and apical periodontitis. Pulp capping is usually applied for treating reversible pulpitis. The treatment of exposed vital pulp is accomplished by sealing the pulpal wound with CH or MTA to facilitate the reparative dentin formation. Irreversible pulpitis is often cured by pulpotomy following the steps below: (1) cervical pulpotomy to remove diseased pulp; 2) root canal disinfection with sodium hypochlorite; (3) placement of a thin layer of MTA in the crown aspect of the canal with a moist cotton pellet for 1 wk; (4) removal of the cotton pellet and sealing the root canal access with resin-modified glass ionomer; and (5) restoring the tooth with composite resin.
Traditional multiple-visit apexification with CH is the treatment choice of immature teeth suffering from periapical periodontitis, which can induce the formation of an apical hard tissue barrier. Due to the disadvantages listed above, regeneration management is recommended. Here are the protocols: (1) disinfect the root canal with sodium hypochlorite; (2) apply antibiotic paste (ciprofloxacin, metronidazole and minocycline) for 4 wk; (3) stir a file beyond the tooth apex to cause bleeding in the canal; (4) place a thin layer of MTA in the crown aspect of the canal; (5) seal the root canal access with resin-modified glass ionomer; and (6) restore the tooth with composite resin[33,95-97]. It is recommended that pulp regeneration should not be delivered to deciduous teeth as it may risk the retaining teeth and impair the eruption pattern of adult teeth[38,98].
Nowadays, various clinical studies are conducted using MSCs as transplants for treatment or to improve the functional outcomes. Stem cell-based therapies have drawn more attention to healing dental diseases. With the application of effective dental materials, stem cell-based apexogenesis may help a number of immature teeth develop. However, further work is required to increase the success rate of apexogenesis, so that this method can be widely used in the clinic.
P- Reviewer: Gopinath SCB, Mauro V S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Huo N, Tang L, Yang Z, Qian H, Wang Y, Han C, Gu Z, Duan Y, Jin Y. Differentiation of dermal multipotent cells into odontogenic lineage induced by embryonic and neonatal tooth germ cell-conditioned medium. Stem Cells Dev. 2010;19:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Fawzy El-Sayed KM, Dörfer C, Fändrich F, Gieseler F, Moustafa MH, Ungefroren H. Adult mesenchymal stem cells explored in the dental field. Adv Biochem Eng Biotechnol. 2013;130:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig. 2013;17:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] |
| 5. | Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2010;109:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 770] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 8. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Verfaillie CM, Pera MF, Lansdorp PM. Stem cells: hype and reality. Hematology Am Soc Hematol Educ Program. 2002;369-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 947] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 11. | Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83-92. [PubMed] |
| 12. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3298] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 13. | Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3486] [Cited by in RCA: 3347] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 14. | Shin DA, Pennant WA, Yoon do H, Ha Y, Kim KN. Co-transplantation of bone marrow-derived mesenchymal stem cells and nanospheres containing FGF-2 improve cell survival and neurological function in the injured rat spinal cord. Acta Neurochir (Wien). 2014;156:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | de Almeida DC, Donizetti-Oliveira C, Barbosa-Costa P, Origassa CS, Câmara NO. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin Biochem Rev. 2013;34:131-144. [PubMed] |
| 17. | Hughey CC, James FD, Ma L, Bracy DP, Wang Z, Wasserman DH, Rottman JN, Shearer J. Diminishing impairments in glucose uptake, mitochondrial content, and ADP-stimulated oxygen flux by mesenchymal stem cell therapy in the infarcted heart. Am J Physiol Cell Physiol. 2014;306:C19-C27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yuan S, Jiang T, Sun L, Zheng R, Ahat N, Zhang Y. The role of bone marrow mesenchymal stem cells in the treatment of acute liver failure. Biomed Res Int. 2013;2013:251846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Seltzer S, Krasner P. Endodontology: biologic considerations in endodontic procedures. Philadelphia: Lea and Febiger 1988; 1–30. |
| 20. | Sheehy EC, Roberts GJ. Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth: a review. Br Dent J. 1997;183:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Nosrat A, Asgary S. Apexogenesis treatment with a new endodontic cement: a case report. J Endod. 2010;36:912-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Forghani M, Parisay I, Maghsoudlou A. Apexogenesis and revascularization treatment procedures for two traumatized immature permanent maxillary incisors: a case report. Restor Dent Endod. 2013;38:178-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Huang GT. A paradigm shift in endodontic management of immature teeth: conservation of stem cells for regeneration. J Dent. 2008;36:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Shabahang S. Treatment options: apexogenesis and apexification. J Endod. 2013;39:S26-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Jung IY, Kim ES, Lee CY, Lee SJ. Continued development of the root separated from the main root. J Endod. 2011;37:711-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56:709-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Li ZY, Chen L, Liu L, Lin YF, Li SW, Tian WD. Odontogenic potential of bone marrow mesenchymal stem cells. J Oral Maxillofac Surg. 2007;65:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100. [PubMed] |
| 30. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1321] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 31. | Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15195] [Article Influence: 584.4] [Reference Citation Analysis (0)] |
| 33. | Moreno-Hidalgo MC, Caleza-Jimenez C, Mendoza-Mendoza A, Iglesias-Linares A. Revascularization of immature permanent teeth with apical periodontitis. Int Endod J. 2014;47:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1011] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 35. | Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1015] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 36. | Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1628] [Article Influence: 90.4] [Reference Citation Analysis (13)] |
| 37. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2866] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 38. | Sakai VT, Moretti AB, Oliveira TM, Fornetti AP, Santos CF, Machado MA, Abdo RC. Pulpotomy of human primary molars with MTA and Portland cement: a randomised controlled trial. Br Dent J. 2009;207:E5; discussion 128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, Abele H, Schewe B, Just L, Skutella T. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Gronthos S, Zannettino AC. A method to isolate and purify human bone marrow stromal stem cells. Methods Mol Biol. 2008;449:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552-556. [PubMed] |
| 42. | Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 43. | Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 44. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3363] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 45. | Carinci F, Papaccio G, Laino G, Palmieri A, Brunelli G, D'Aquino R, Graziano A, Lanza V, Scapoli L, Martinelli M. Comparison between genetic portraits of osteoblasts derived from primary cultures and osteoblasts obtained from human pulpar stem cells. J Craniofac Surg. 2008;19:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Marchionni C, Bonsi L, Alviano F, Lanzoni G, Di Tullio A, Costa R, Montanari M, Tazzari PL, Ricci F, Pasquinelli G. Angiogenic potential of human dental pulp stromal (stem) cells. Int J Immunopathol Pharmacol. 2009;22:699-706. [PubMed] |
| 47. | Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 427] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 49. | Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 800] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 50. | Wu J, Huang GT, He W, Wang P, Tong Z, Jia Q, Dong L, Niu Z, Ni L. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod. 2012;38:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 504] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 53. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1983] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 54. | Gosau M, Götz W, Felthaus O, Ettl T, Jäger A, Morsczeck C. Comparison of the differentiation potential of neural crest derived progenitor cells from apical papilla (dNC-PCs) and stem cells from exfoliated deciduous teeth (SHED) into mineralising cells. Arch Oral Biol. 2013;58:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, Liu SY, Chen L, Ding Y, Xuan K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012;57:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 56. | Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997;13:20-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 380] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Park JC, Kim JM, Jung IH, Kim JC, Choi SH, Cho KS, Kim CS. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: in vitro and in vivo evaluations. J Clin Periodontol. 2011;38:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2502] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 59. | Choi HD, Noh WC, Park JW, Lee JM, Suh JY. Analysis of gene expression during mineralization of cultured human periodontal ligament cells. J Periodontal Implant Sci. 2011;41:30-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Drees J, Felthaus O, Gosau M, Morsczeck C. Butyrate stimulates the early process of the osteogenic differentiation but inhibits the biomineralization in dental follicle cells (DFCs). Odontology. 2013;Jul 9; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Aonuma H, Ogura N, Takahashi K, Fujimoto Y, Iwai S, Hashimoto H, Ito K, Kamino Y, Kondoh T. Characteristics and osteogenic differentiation of stem/progenitor cells in the human dental follicle analyzed by gene expression profiling. Cell Tissue Res. 2012;350:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Takatalo MS, Tummers M, Thesleff I, Rönnholm R. Novel Golgi protein, GoPro49, is a specific dental follicle marker. J Dent Res. 2009;88:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Rezai Rad M, Wise GE, Brooks H, Flanagan MB, Yao S. Activation of proliferation and differentiation of dental follicle stem cells (DFSCs) by heat stress. Cell Prolif. 2013;46:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 624] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 65. | Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, Sampath Narayanan A. Cementum matrix formation in vivo by cultured dental follicle cells. Bone. 2002;31:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Lee Y. Effect of calcium hydroxide application time on dentin. Restor Dent Endod. 2013;38:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010;16:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Wang X, Jong G, Lin LM, Shimizu E. EphB-EphrinB interaction controls odontogenic/osteogenic differentiation with calcium hydroxide. J Endod. 2013;39:1256-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | MITCHELL DF, SHANKWALKER GB. Osteogenic potential of calcium hydroxide and other materials in soft tissue and bone wounds. J Dent Res. 1958;37:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | da Silva RA, Leonardo MR, da Silva LA, de Castro LM, Rosa AL, de Oliveira PT. Effects of the association between a calcium hydroxide paste and 0.4% chlorhexidine on the development of the osteogenic phenotype in vitro. J Endod. 2008;34:1485-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 73. | Huang GT. Apexification: the beginning of its end. Int Endod J. 2009;42:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Cotti E, Esposito S, Jacobs R, Slagmolen P, Bakland LK. Comprehensive management of a complex traumatic dental injury. Dent Traumatol. 2013;Sep 2; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 76. | Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Dammaschke T, Stratmann U, Wolff P, Sagheri D, Schäfer E. Direct pulp capping with mineral trioxide aggregate: an immunohistologic comparison with calcium hydroxide in rodents. J Endod. 2010;36:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Mente J, Geletneky B, Ohle M, Koch MJ, Friedrich Ding PG, Wolff D, Dreyhaupt J, Martin N, Staehle HJ, Pfefferle T. Mineral trioxide aggregate or calcium hydroxide direct pulp capping: an analysis of the clinical treatment outcome. J Endod. 2010;36:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 79. | Shabahang S, Torabinejad M, Boyne PP, Abedi H, McMillan P. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011;37:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Accorinte Mde L, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E, Souza V, Alessandro LD. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 82. | Mohammadi Z. Strategies to manage permanent non-vital teeth with open apices: a clinical update. Int Dent J. 2011;61:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | D’Antò V, Di Caprio MP, Ametrano G, Simeone M, Rengo S, Spagnuolo G. Effect of mineral trioxide aggregate on mesenchymal stem cells. J Endod. 2010;36:1839-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Paranjpe A, Smoot T, Zhang H, Johnson JD. Direct contact with mineral trioxide aggregate activates and differentiates human dental pulp cells. J Endod. 2011;37:1691-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod. 2010;36:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Seo MS, Hwang KG, Lee J, Kim H, Baek SH. The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod. 2013;39:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 88. | Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1420] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 89. | Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 90. | Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 91. | Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 92. | Huang GT, Lin LM. Letter to the editor: comments on the use of the term “revascularization” to describe root regeneration. J Endod. 2008;34:511; author reply 511-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 94. | Bansal R, Bansal R. Regenerative endodontics: a state of the art. Indian J Dent Res. 2011;22:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011;37:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 96. | Wigler R, Kaufman AY, Lin S, Steinbock N, Hazan-Molina H, Torneck CD. Revascularization: a treatment for permanent teeth with necrotic pulp and incomplete root development. J Endod. 2013;39:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 97. | Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 582] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 98. | Cardoso-Silva C, Barbería E, Maroto M, García-Godoy F. Clinical study of Mineral Trioxide Aggregate in primary molars. Comparison between Grey and White MTA--a long term follow-up (84 months). J Dent. 2011;39:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |