Published online Dec 26, 2013. doi: 10.5662/wjm.v3.i4.45
Revised: October 3, 2013
Accepted: October 18, 2013
Published online: December 26, 2013
Translational medicine pursues the conversion of scientific discovery into human health improvement. It aims to establish strategies for diagnosis and treatment of diseases. Cancer treatment is difficult. Radio-pharmaceutical research has played an important role in multiple disciplines, particularly in translational oncology. Based on the natural phenomenon of necrosis avidity, OncoCiDia has emerged as a novel generic approach for treating solid malignancies. Under this systemic dual targeting strategy, a vascular disrupting agent first selectively causes massive tumor necrosis that is followed by iodine-131 labeled-hypericin (123I-Hyp), a necrosis-avid compound that kills the residual cancer cells by crossfire effect of beta radiation. In this review, by emphasizing the potential clinical applicability of OncoCiDia, we summarize our research activities including optimization of radioiodinated hypericin Hyp preparations and recent studies on the biodistribution, dosimetry, pharmacokinetic and, chemical and radiochemical toxicities of the preparations. Myocardial infarction is a global health problem. Although cardiac scintigraphy using radioactive perfusion tracers is used in the assessment of myocardial viability, searching for diagnostic imaging agents with authentic necrosis avidity is pursued. Therefore, a comparative study on the biological profiles of the necrosis avid 123I-Hyp and the commercially available 99mTc-Sestamibi was conducted and the results are demonstrated. Cholelithiasis or gallstone disease may cause gallbladder inflammation, infection and other severe complications. While studying the mechanisms underlying the necrosis avidity of Hyp and derivatives, their naturally occurring fluorophore property was exploited for targeting cholesterol as a main component of gallstones. The usefulness of Hyp as an optical imaging agent for cholelithiasis was studied and the results are presented. Multiple uses of automatic contrast injectors may reduce costs and save resources. However, cross-contaminations with blood-borne pathogens of infectious diseases may occur. We developed a radioactive method for safety evaluation of a new replaceable patient-delivery system. By mimicking pathogens with a radiotracer, we assessed the feasibility of using the system repeatedly without septic risks. This overview is deemed to be interesting to those involved in the related fields for translational research.
Core tip: Translational medicine converts scientific discovery into clinical applications. Radiopharmacy has played a multidisciplinary role. Based on unique necrosis avidity, OncoCiDia presents a generic approach for management of cancers, on which recent results on its optimization are summarized. Myocardial infarction is a clinical problem. A comparative study between infarct avid iodine-131 labeled-hypericin and commercial 99mTc-Sestamibi is presented. Cholelithiasis may cause biliary complications. The usefulness of Hyp as an optical imaging agent for cholelithiasis is demonstrated. Multiple uses of automatic contrast injectors may reduce costs but can cause cross-contaminations. We developed a radioactive method for safety evaluation of a new replaceable patient-delivery system.
- Citation: Cona MM, Witte P, Verbruggen A, Ni Y. An overview of translational (radio)pharmaceutical research related to certain oncological and non-oncological applications. World J Methodol 2013; 3(4): 45-64
- URL: https://www.wjgnet.com/2222-0682/full/v3/i4/45.htm
- DOI: https://dx.doi.org/10.5662/wjm.v3.i4.45
Translational medicine refers to the creativity of joining knowledge from “bench to bedside” or from laboratory experiments to clinical trials for producing new drugs, devices, diagnostic and therapeutic options for patients. Hence, translational research is identified as a crucial interface between basic science and clinical medicine. It intends to discover better ways to solve real practical problems enhancing human health and well-being. Another current trend in translational medical research is to hybrid diagnosis and therapy into a combined approach as newly termed a “theragnostic” modality.
In the area of cancer therapeutics, transforming basic research results into clinical practice is becoming increasingly important. Cancer is one of the leading causes of mortality worldwide and little progress has been achieved in treating most of the solid tumors, which could be resistant to common therapies. Based on necrosis-avidity, OncoCiDia is a generic and unconventional theragnostic strategy recently introduced as a complementary modality to improve cancer treatability[1,2]. Unlike other cancer therapies directly attacking multimutant and refractory cancer cells, OncoCiDia may selectively treat solid malignancies by massively necrotizing the tumors plus radioactively cleansing their microenvironments, meanwhile the tumors under treatment can be visualized by nuclear scintigraphy. Thus, a dedicated acronym OncoCiDia is created to portray this cancer (Onco) management approach with both tumoricidal (Ci) and diagnostic (Dia) effects. It consists of two sequential complementary treatments involving the intravenous application of a vascular disrupting agent (VDA) followed by systemic targeted radiotherapy (STR) using a potent necrosis-avid compound iodine-131 labeled-monoiodohypericin (131I-Hyp). Furthermore, to make this novel anticancer strategy clinically applicable, optimizations of the procedures for labeling, purification and formulation of the radioiodinated hypericin (Hyp) have been performed and the outcomes are summarized and commented in this overview article. Results on biodistribution, dosimetry, pharmacokinetic and, chemical and radiochemical toxicities of OncoCiDia have been also presented, which hopefully may boost the advance of this strategy into the clinic.
Myocardial infarction constitutes a health problem with large morbidity, mortality and economic burden[3]. Nuclear imaging based on myocardial perfusion tracers, which distribute in proportion to the regional myocardial blood flow, has played an important role in the assessment of tissue viability[4,5]. However, the development of diagnostic imaging agents with authentic specific avidity for necrosis has been a desired goal due to the numerous utilities they may offer. In earlier studies, the necrosis avid 123I-Hyp has shown its potential usefulness as a diagnostic cardiac agent due to its notable uptake in necrotic tissues[6,7]. In a more recent experiment, its biodistribution and targetability have been compared to those of the commercially available myocardial perfusion tracer 99mTc-Sestamibi and the results have been herein summarized.
Cholelithiasis is the medical term for gallstone disease. Gallstones are hard, rock-like collections, mainly from cholesterol and bile salts that build up in the gallbladder or bile duct. Eventually, they can cause gallbladder inflammation resulting in pain, jaundice, infection and other serious complications. Because the high affinity between cholesterol and the naturally occurring fluorophore hypericin has been reported[8,9], a preliminary in vitro study for assessing the potential suitability of Hyp as an optical diagnostic imaging agent in patients with gallstones was performed and the results are presented in this work.
Multiple uses of automatic contrast injection systems during imaging procedures can reduce costs and save resources. However potential outbreak associated with cross-contaminations with blood-borne pathogens of infectious diseases through the contrast medium may occur. The Transflux contrast delivery system is a simple tube delimited by two one-way valves intended to deliver contrast media from a reservoir to the patient and with the need to only change the tubing in direct contact with the patient blood. It incorporates a safety zone and a one-way valve in the patient line that allow the delivery system and the vein to be flushed and the blood reflux to be prevented. By mimicking microbial pathogens with a particulate radiotracer, we developed a radioactive method for quantitative safety evaluation of this new replaceable patient-delivery system and the main findings are reported here.
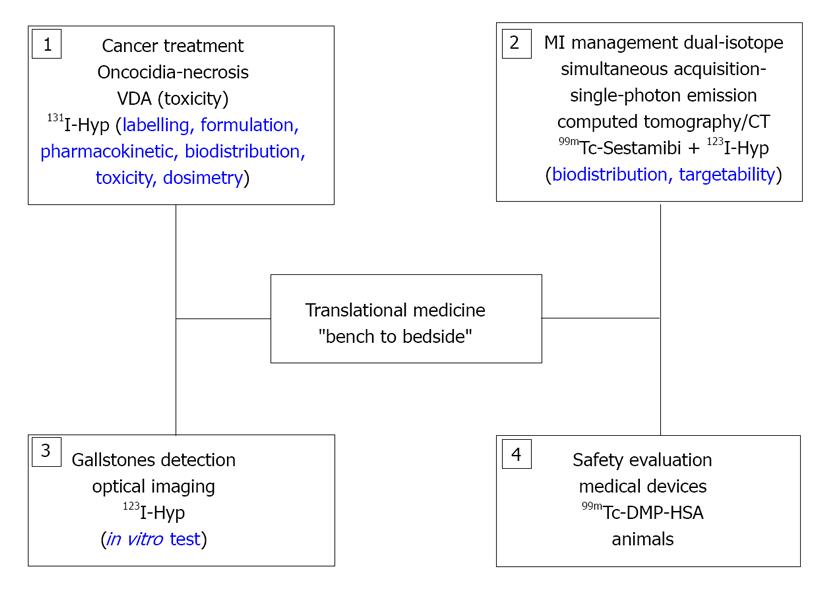
This overview paper is in the framework of the full 4-year doctoral training, in which translational research has been attempted as a key component for resolving important problems in diverse medical fields (Figure 1).
Cancer is a complex group of malignant diseases influenced by genetic and environmental factors. Over the past decades, the incidence and prevalence of cancer have raised with an overall estimation of about 20 million new cases by 2030[10]. The costs associated with cancer diagnosis, therapy, and follow-up have drastically soared. Conventional therapies including surgery, chemotherapy and external beam radiotherapy are often ineffective for treating resistant and disseminated solid malignancies. Novel and cost-effective approaches, once available, are essential to improve cancer treatability and curability.
STR is a radiotherapy that makes use of systemically administered radioactive compounds for delivering lethal radiation doses to the tumor while preserving normal tissues. Several radioactive agents have been clinically used, for instance, radioiodine for thyroid cancer owing to its specific uptake by thyroid glandular tissues[11]; iodine-131 metaiodobenzylguanidine for treating pheochromocytoma[12] and neuroblastoma[13]; Metastron (strontium-89 chloride) as a palliative treatment in patients with bone metastases[14]; and radioactive microspheres for radioembolization of liver cancer[15]. Anti-CD20 monoclonal antibody (MoAb) conjugated to I-131 (tositumomab, Bexxar®)[16] or yttrium-90 (ibritumomab tiuxetan, Zevalin®)[17] for treating non-Hodgkins lymphoma, and somatostatin derivatives labeled with bound indium-111, lutetium-177 or yttrium-90 for neuroendocrine tumors (NETs)[18,19] are lately introduced, constituting a step forward in tumor specific targeting.
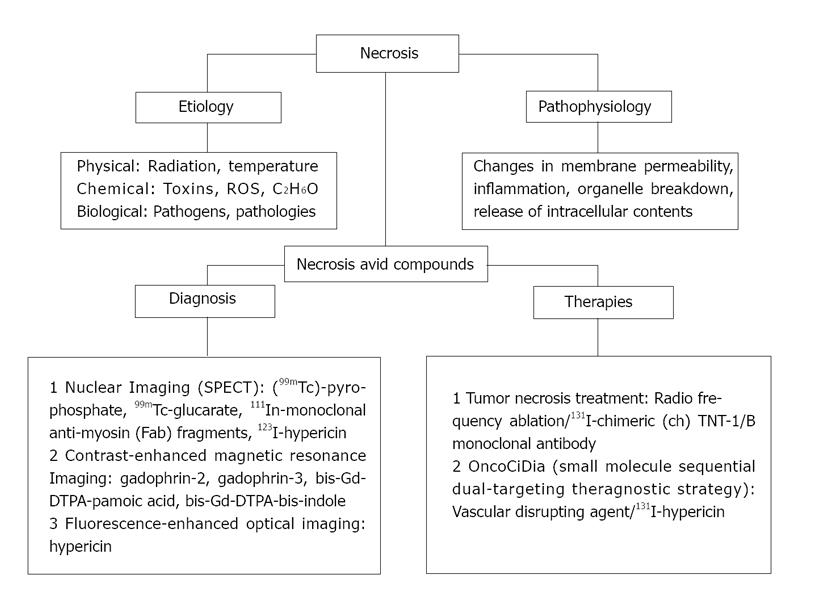
However, most of the above-mentioned cancer types represent a small proportion among the overall cancer cases. Malignant solid tumors, which represent the major cancer incidence worldwide, have been difficult to treat due to their histological diversity, disorganized angiogenesis and unpredictable mutations. Once carcinogenesis is established, tumor cells become resistant to therapies due to the multiple escape mechanisms facilitated by intrinsic mutations and/or overlapping molecular pathways. Even if a proper radioactive MoAb is chosen, in most of the cases, only small amounts of injected dose (0.001%-0.1% /g) could accumulate in the tumor[20]. Low absorbed doses (1500 cGy) are subsequently reached in cancer cells that are much lower than the usually required doses (5000 cGy) for getting therapeutic responses[21,22]. With somatostatin derivatives-based radiopharmaceuticals characterized by high affinity for distinct receptors overexpressed in the tumor, short-term accumulation in the tumor and retention in normal tissues have also been reported[23]. Therefore, necrosis as a generic alternative target has been utilized for potential theragnostic applications (Figure 2).
Rather than hitting cancer cells undergoing numerous mutations[24,25] that cause uncontrollable growth and escape from annihilation, leading to post-therapeutic cancer resistant clones[24], an innovative anticancer approach called tumor necrosis treatment (TNT) was introduced[26,27]. Since the proportion of dead tissue in fast-growing tumors can be more than 50% of the total cancer volume due to tumor vascular deformation or insufficient blood supply[28,29], necrosis could become a generic target in almost all solid tumors. TNT approach uses radiolabeled MoAbs that spare normal tissue and target naturally occurring intracellular antigens (a complex of double-stranded DNA and histone H1-antigens) present throughout tumor necrosis[30,31]. Unlike conventional STR, it is a crossfire-dose therapeutic modality, in which the radiation dose deposited to cancer cells only comes from radionuclides on surrounding necrosis.
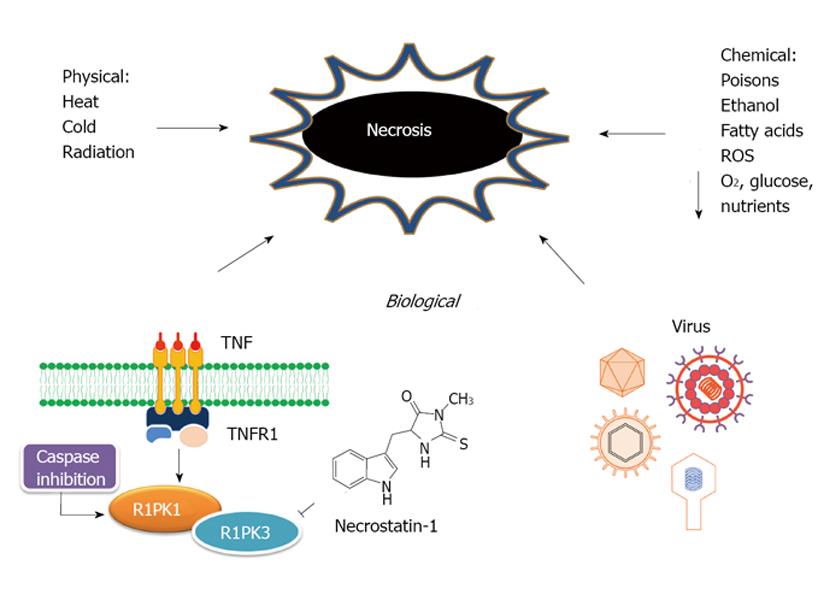
Definition, etiology and pathophysiology of necrosis: The term of necrosis is originated from the Greek prefix “necros”, meaning “dead”. It constitutes an irreversible process or “no return” status in the cell life[32]. Cell death by necrosis has been historically stereotyped as an unregulated process[33]. Any severe lesions caused by physical stresses, toxins, infections or genetically programmed injuries if reaching a certain degree and receiving no intervention may alter physiological homeostasis, eventually leading to tissue or organ necrosis[34]. However, it turns increasingly evident that the multi-pathway cell-death program apoptosis may not be the only cellular mechanism involved in regulating cell death[35]. Programmed necrosis or necroptosis has emerged as a specialized biochemical mechanism that can be induced by different stimuli such as tumor-necrosis factor receptors (TNFR1, TNFR2)[36], inactivation of cysteine-aspartic acid proteases (caspase)[37] and caspase-8 mutations[38]. It becomes clear that necroptosis could be regulated by the kinase receptor-interacting protein 1 (RIPK1), which constitutes the molecular target of necrostatins, an emerging class of cytoprotective drugs inhibiting specifically necroptotic cells[37]. Recently, the kinase activity of RIPK3, a family member of RIPK1, has also been related to programmed necrosis[39] (Figure 3).
Necrosis is commonly believed to be a passive process since it involves no protein production, is not restrained by any homeostatic mechanisms, and includes almost negligible energy requirements. The necrotic cells can no longer retain the integrity of the cell or cytoorganelle membranes and perform inherent functions. After losing the ability to maintain homeostasis, biological fluids from the blood cross the damaged cell membrane and enter the intracellular space, leading to organelles enlargement, production of toxins and activating enzymes associated to the degradation of cellular life molecules. The swollen organelles become nonfunctional, ceasing the synthesis of proteins and ATP. The mitochondrial swelling causes cytolysis and the debris is discharged into the surroundings, which triggers tissue inflammation regulated by small proteins-cytokines, reactive oxygen species and certain immune system cells[40]. The organism interprets the presence of the debris as signal of tissue injury and reacts to defend itself. In response, immune system cells migrate into the site of damage and combat the supposed invading microorganisms[40]. Dissociation of ribosomes from the endoplasmic reticulum and nucleus disintegration with chromatin condensation take place in turn[41]. The dead cells eventually fade away because of the combination of enzymatic denaturation and fragmentation process, followed by polymorphonuclear leukocyte phagocytosis of solid particles[42].
Preclinical studies and clinical trials on TNT: In a pioneer pre-clinical study conducted on a ME-180 human cervical carcinoma model with 131I-labeled TNT-1 MoAb, Chen et al[31] proved the effective and preferential targeting of this radiotherapeutics within the tumor, which established the potential clinical usefulness of TNT. To date, about 200 patients have been treated with TNT worldwide. A phase I study of 131I-chimeric(ch) TNT-1/B MoAb for the treatment of advanced colon cancer was performed. The infusion of 131I-chTNT-1/B MoAb was well tolerated and showed no significant non-hematologic effects. Based on tumor cross-product response criteria, however, none of the patients exhibited complete or partial response[43]. Phase I and II trials of convection-enhanced delivery of 131I-chTNT-1/B MoAb were conducted on patients with high-grade adult gliomas, showing promising therapeutic outcomes[44]. Similar results were found in a pivotal study in patients with advanced lung cancer treated with 131I-chTNT-1/B MoAB[45]. More recently, genetically engineered Fab’ and F(ab’) 2 constructs of chimeric TNT (chTNT)-3 antibody labeled with indium-111 were prepared and preclinically evaluated. The conjugates showed faster body clearance, better biodistribution but lower tumor uptake than the parental 111In-labeled chTNT-3 in tumor-bearing mice[46].
To increase the amount of MoAb binding-necrotic sites in the tumor, necrosis-inducing treatments (NITs) such as radiofrequency ablation were also used as starting complementary techniques[47].
However, myelosuppression due to unfavorable pharmacokinetic properties of MoAbs constitutes an important dose limiting factor that prevents substantial improvement of TNT-based modality[43,44].
OncoCiDia, also known as small molecule sequential dual-targeting theragnostic strategy[1], is a novel anticancer approach with great potential for treating solid tumors. Relying on a soil-to-seeds concept, it offers a one-stop-shop for diagnostic imaging, treatment and follow-up[2]. Similar to the TNT approach, it is based on the natural phenomenon of necrosis. However, instead of using radioactive MoAb with large molecular size (150 kDa) and complex pharmacokinetics, it involves two small compounds (< 1 kDa) with pre-identified high and divert but complementary targetability. The intravenously (IV) administered VDA triggers selective tumor vascular shutdown and subsequent central necrosis. However, a viable rim of tumor cells in the periphery always exists as seeds for repopulation of cancer cells[48]. 131I-Hyp is then IV injected, which preferentially localizes at the newly generated necrotic sites and acts as a cleansing shot to lethally irradiate residual tumor cells through a crossfire effect[2]. The small molecular size of 131I-Hyp makes it possible to permeate fast through tissues and target less accessible sites throughout the solid tumor. This may overcome the initial barriers faced by the systemic delivery of MoAb, which limits diffusion from blood vessels and inhibits drug tumor penetration[21,49].
Vascular disrupting agents: Vascular disrupting agents (VDAs) are a novel category of potential anticancer drugs that induce tumor vascular shutdown by destroying the endothelium of tumor vasculature. It has been reported that blood vessels in tumors proliferate more rapidly than those in normal tissues[49]. Newly formed endothelial cells are more sensitive than mature ones that own a well-developed actin cytoskeleton and may retain the cell shape in spite of depolymerization of the tubulin cytoskeleton caused by the VDA[50]. After VDA administration, the occlusion of blood-supplying vessels and capillary sprouts obstructs oxygen and nutrient supply to the tumor cells, compromising cellular integrity and eventually leading to hemorrhagic tumor necrosis[51]. Different groups of VDAs have been developed, e.g., tubulin-binding agents cause microtubule depolymerization by binding either the colchicine or vinblastine sites, whereas flavonoid derivatives selectively obstruct tumor-related vessels due to their indirect pharmacodynamic effects[51]. VDAs can be obtained from nature such as combretastatins (CA4P, OXi-4503, and AVE-8062), colchicines (ZD6126) and phenylahistin (NPI-2358), whilst others are synthetic compounds (DMXAA, MN-029 and EPC2407)[51].
Hyp: Hyp is a red-colored anthraquinone derivative (naphthodianthrone), which is one of the principal active compounds of the genus Hyp (Clusiaceae) comprising roughly 450 species worldwide[52]. Hyp was initially found in the dark glands of the flowering parts from Hyp perforatum L (St. John’s Wort)[53], an aromatic, perennial plant. Hyp can be also obtained from fungi Dermocybe[54] or from endophytic fungi growing in different plant species[52]. However, the most commercially available Hyp compounds are synthesized.
Hyp has been considered a vinylogous carboxylic acid. Its deprotonations are likely at the phenolic hydroxyl groups at the peri- and bay-regions having different acidities. In aqueous system the bay- and peri-regions show estimated pKa values of 1.7 and 12.5[55], respectively. Hyp showed a non-planar conformation owing to the repelling interactions among the side chains of the aromatic skeleton[56]. The proximity of acidic and basic functional groups allows the formation of intramolecular hydrogen bonds, which influence the tautomeric equilibria and acid-base properties[55]. Hyp has 16 conceivable tautomers[57]. Among them, the most stable is the 7, 14-dioxoisomer[58]. Hyp dissolves in polar solvents over concentrations of 10-3 mol/L, producing red fluorescent solutions. It is soluble in Dimethyl sulfoxide (DMSO), ethanol, pyridine, methanol, acetone, butanone, ethyl acetate and aqueous alkaline solutions[59]. It has been found in soluble form under physiological conditions due to the complex formation with biological macromolecules, mainly low-density lipoprotein (LDL)[60].
Hyp is a natural product of pharmaceutical interest due to its ever-expanding anti-inflammatory[61], antiretroviral[62], antimicrobial[63], antitumor[64] and antidepressive[65] activities. Recently, it has been found with a highly selective affinity for necrosis[6,7] (Figure 4).
The mechanisms associated with the necrosis avidity of Hyp remain unknown and a number of hypotheses have been proposed. Hyp specifically accumulates in exposed sites of degraded life molecules in the necrotic cell debris[66]. Binding to LDL[60] and serum or interstitial albumins[67] have been put forward as potential interaction pathways. Hyp has also been found to show highly selective avidity for lipid components including cholesterol[8], phosphatidylserine and phosphatidylethanolamine[68] present in the cell membrane bilayer.
Iodine isotopes: Iodine-123 (123I) is a halogen with a physical half-life of 13.1 h. It decays by electron capture to tellurium-123, emitting gamma radiation with a main energy of 159 kEV, which is exploitable for nuclear scintigraphy, biodistribution and radiodosimetry studies.
Iodine-131 (131I) with a decay half-life of 8.02 d is the most common iodine radioisotope utilized in medical applications owing to its relatively easy availability and low cost. It decays by emission of beta minus electrons with a maximal energy of 606 kEV (89% abundance) and a tissue penetration of 0.6-2.0 mm[69] as well as 364 kEV gamma rays of 81% abundance. 131I destroys tissue by short-range beta radiation, causing DNA damage and cell death to the cell that takes up the tracer by self-dose effect and to other cells up to several micrometers away by cross-fire effect.
Due to radioprotection reasons, 123I is frequently used as surrogate of 131I for labeling optimization, biodistribution and dosimetry studies with the mutually interpretable outcomes.
Preparation of 123/131I -labeled-monoiodohypericin: Radioiodination via direct electrophilic substitution is a simple method based on in situ formation of positively charged iodine (I+) using mild oxidants such as N-chloro para-toluenesulfonylamide (chloramine T), peracetic acid and 1,3,4,6-tetrachloro-3α, 6α-diphenyl glycoluril (Iodogen). The radioactive iodine atom in an oxidized form replaces a hydrogen atom of an activated aromatic ring. Since Hyp is a polycyclic aromatic quinone having hydroxyl substituents, it can be efficiently radioiodinated.
Two main methods for direct radiolabelling of Hyp with iodine isotopes have been described. Bormans et al[70] reported a radioiodination procedure of Hyp in ethanol using phosphoric acid and peracetic acid as oxidant for 30 min. On high performance liquid chromatography (HPLC), radiochemical yields ranging between 70%-97% were achieved[70]. Sun et al[71] described a simple method, in which Hyp in DMSO is labeled with 131/123I using iodogen as oxidant (either in a pre-coated tube or in powder form), at pH value between 6.5-7.5 for 2 to 10 min. Labeling yields higher than 99% were attained as indicated by paper chromatography (PC).
However, although PC is useful for the purpose of identification due to its convenience and simplicity, we investigated the method developed by Sun and Ni using HPLC. This technique provides high resolution and allows identifying and quantifying small amounts of substances. Labeling conditions were screened for varying reaction parameters such as Hyp mass, Hyp/iodogen molar ratio and reaction time. Stability over time of the radioactive Hyp was also checked. For radiochemical yield determination and purification, the effect of different mobile phases either in gradient or isocratic modes was studied on a quaternary HPLC system equipped with a Ultraviolet (UV) absorbance (254 nm) and radiometric detector. An XTerra® C18 column (4.6 mm × 150 mm, 5.0 μm) and a flow rate of 1.0 mL/min were used for radiochemical yield analysis. On the other hand, an XTerra® C18 semi-preparative column (10 mm × 250 mm, 10 μm) and a flow rate of 3.0 mL/min were set for purification. The peak areas of Hyp and radioiodinated Hyp were considered as response variables in this optimization test.
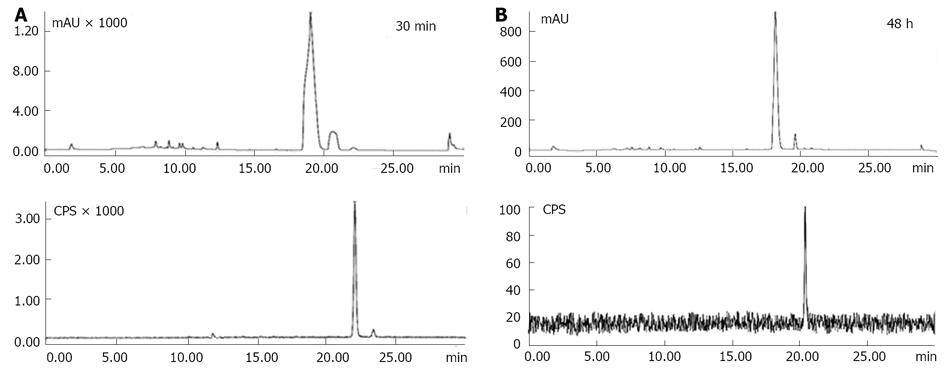
The preferred conditions for Hyp radioiodination were 2.0 mg Hyp in a molar ratio (Hyp: iodogen) of (3.4:1); 90/10, mL/L DMSO/50 mmol/L sodium phosphate buffer at pH 7.4 for 20 min. For radiochemical yield determination, a mobile phase consisting of acetonitrile/5 mmol/L ammonium acetate buffer pH 7.0 in gradient mode (0 min: 5:95 v/v, 25 min: 95:5 v/v, 30 min: 5:95 v/v) provided the best resolution between adjacent peaks. UV/radio-chromatograms showed unlabeled Hyp and iodine-123-labeled monoiodohypericin (123I-Hyp) with tR of 19.18 ± 0.15 min and 22.13 ± 0.05 min, respectively. Free iodide was not observed (Figure 5A). 123I-Hyp was prepared with specific activity above 50 GBq/μmol in a radiochemical yield of 95.4% and remained stable over 48 h at room temperature (Figure 5B). As confirmed by mass spectrometry, the small differences between the labeling yield obtained by PC and HPLC were due to the concurrent formation of di-[(123I) iodohypericin in low percentage (approximately 3%), which was detected together with mono-(123I)] iodohypericin by PC.
After labeling, excessive reagents (unlabeled Hyp and iodogen) were removed by HPLC using acetonitrile/5 mmol/L ammonium acetate buffer at pH 7.0 as mobile phase in a gradient mode (0 min: 75:25, v/v, 5 min: 75:25, v/v, 30 min: 90:10, v/v). Good separation between Hyp and radioiodinated Hyp peaks was achieved. 123I-Hyp was obtained with a radiochemical purity above 99.0%. However, broad peaks with long retention times for Hyp and radioiodinated Hyp were typically observed during the purification process. It seems once radioiodinated Hyp is mixed with unlabeled Hyp in a total mass of about 2 mg, they might undergo partial retention in the HPLC system due to aggregate formation. Under physiological conditions, such aggregates may show reduced necrosis affinity and increased uptake in organs of the mononuclear phagocyte system (MPS), hampering the potential clinical usefulness of 131I-Hyp for OncoCiDia.
Confronted problems: OncoCiDia has shown the best results using a mixture of radioiodinated Hyp/unlabeled Hyp[1]. To overcome the limitations related to the purification process, we recommended the clinical use of the non-purified radioiodinated Hyp, which is attained with high labeling yields (> 95%). However, conditions for Hyp radioiodination require excess of starting material at high concentrations of DMSO, which also dissolved the oxidizing agent iodogen. As a result, both reagents remain in the formulation of the non-HPLC purified radioiodinated Hyp, giving toxicity concerns. Moreover, the unlabeled Hyp present in the mixture is in a concentration range of 10-3 mol/L, in which it may aggregate in biocompatible aqueous formulations. Regarding 123/131I-Hyp, the incorporation of an iodine atom into a molecule can also result in a more lipophilic and less water soluble derivative[72]. Under these circumstances, a proper delivery system is essential for preventing aggregate formation and subsequently ensuring efficient targeting to necrotic tumor. Another potential issue arises with the co-injection of unlabeled Hyp which could influence 123/131I-Hyp on the biodistribution and targetability over time. Since the treatment of solid tumors requires the preferential delivery of a radiotherapeutic dose to the tumor while preventing normal tissues from undesired side effects[73], the dosimetry of this co-injection approach has to be estimated, as well. These above-mentioned problems have been assessed or addressed below.
Formulation: For 123/131I-Hyp/Hyp, the co-solvency approach seems to be a good alternative due to its rapidness and simplicity. In preclinical investigations, a formulation consisting of water/ polyethylene glycol (PEG 400) (80/20, v/v) has been reported[1]. Pure DMSO as solvent for the poorly water soluble 123/131I-Hyp/Hyp has also been used[74]. However, further optimizations are needed.
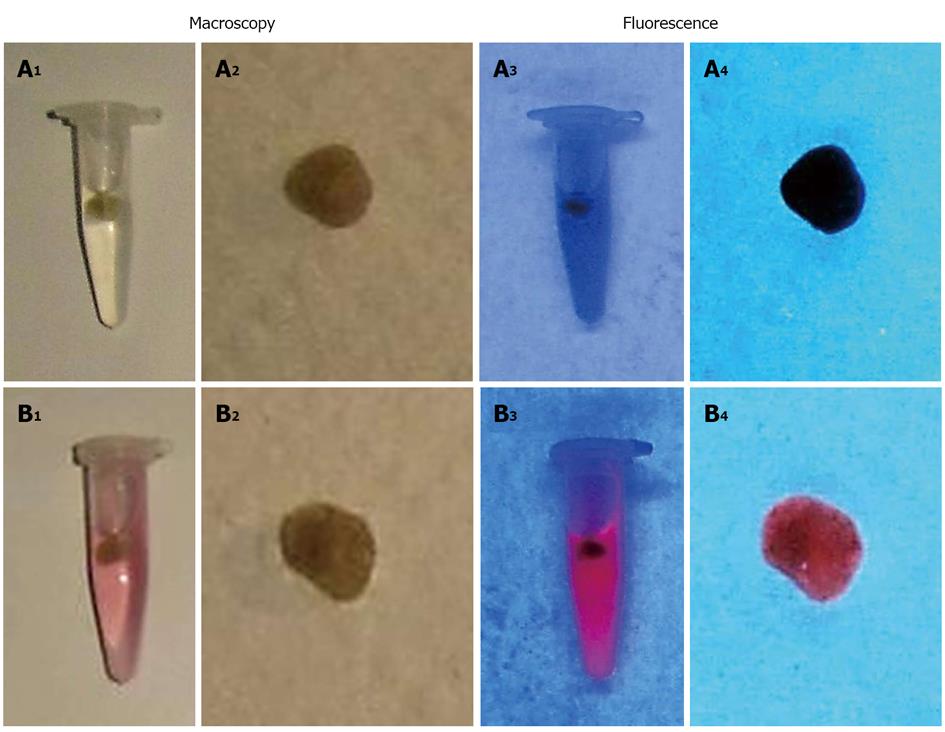
In a recent study, we tested several delivery systems for 123/131I-Hyp/Hyp using macroscopic and microscopic techniques and the results are summarized in Table 1[75]. Overall, formulations with a water content below 40% showed red fluorescent solutions without aggregate formation. In contrast, formulations containing around 70% water appeared as cloudy brownish solutions with reduced fluorescent properties. Animal studies confirmed the previous in vitro observations. For instance, when DMSO/PEG 400/water (25:60:15, v/v/v) was used as a vehicle, 123/131I-Hyp/Hyp showed low uptake in MPS organs, high necrosis affinity and striking tumoricidal effects days after OncoCiDia application. With 123/131I-Hyp/Hyp in DMSO/saline (20:80, v/v), instead, radioactivity accumulation in MPS organs but low uptake in necrotic tumor were found. Consequently, poor radiation dose was deposited in the tumor, leading to disease progression because of rapid repopulation of residual cancer cells at the tumor periphery after VDA attack[75].
| Formulation | Macroscopic digital imaging | Microscopy |
| DMSO/saline (20/80, v/v) | Cloudy brownish solution, no fluorescence | Massive formation of aggregates reduced fluorescent intensity |
| DMSO/water (25/75, v/v) | Cloudy brownish solution, no fluorescence | Massive formation of aggregate reduced fluorescent intensity |
| DMSO/D10W (25/75, v/v) | Cloudy brownish solution, no fluorescence | Massive formation of aggregates reduced fluorescent intensity |
| DMSO/D20W (25/75, v/v) | Cloudy brownish solution, no fluorescence | Massive formation of aggregates reduced fluorescent intensity |
| DMSO/serum (25/75, v/v) | Cloudy brownish solution, moderate fluorescence signal | Massive formation of aggregates moderate fluorescent intensity |
| DMSO/PEG 400/water (25/60/15, v/v/v) | Bright red solution, highly fluorescent | No aggregates formation; strong, homogeneous fluorescence |
| EtOH/PEG 400/water (10/50/40, v/v/v) | Dark red solution, moderate fluorescence | Aggregate formation; moderate, heterogeneous fluorescence, |
| EtOH/PEG 400/water (10/60/30, v/v/v) | Red solution, minimum aggregation, high fluorescence | Some aggregates; strong, homogeneous fluorescence |
| PEG 400/water (60/40, v/v) | Red solution, high fluorescence | Some aggregates; strong, homogeneous fluorescence |
| PEG 400/water (70/30, v/v) | Bright red solution, highly fluorescent | Some aggregates; strong, homogeneous fluorescence |
| PVP-10000 | Dark red solution, moderate fluorescence | Aggregate formation; reduced fluorescence intensity |
| PVP-29000 | Dark red solution, moderate fluorescence | Aggregate formation; reduced fluorescence intensity |
| β-Cyclodextrins | Cloudy brownish solution, no fluorescence | Massive formation of aggregates reduced fluorescent intensity |
However, earlier studies have reported that the common pharmaceutical solvents may have biological and pharmacological activity mainly when given undiluted[76-78]. Alternatively, the water-soluble sodium cholate (NaCh), a naturally occurring liver-produced surfactant with low toxicity, was assessed as a potential solubilizing agent for 123I-Hyp/Hyp in an animal model of acute myocardial infarction (MI) (Cona et al[79]). The amphiphilic NaCh molecule with hydrophilic and hydrophobic sides of different solubility properties forms micelles, which act as emulsifier above the critical micellar concentration. Necrosis avidity of 123I-Hyp/Hyp dissolved in a NaCh solution and its favorable biodistribution were demonstrated (Figure 6). The suitability of NaCh as a solubilizing agent of 123I-Hyp for hotspot imaging of acute MI could be demonstrated (Cona et al[79]).
Biodistribution and dosimetry studies: Tissue distribution of 123I-Hyp/Hyp was studied on animal models either of reperfused partial liver infarction (RPLI)[80] or ethanol-induced muscle necrosis. Dosimetric extrapolations of 131I-Hyp from animals to humans were attempted using biodistribution data of 123I-Hyp in RPLI animals in combination with Organ Level Internal Dose Assessment/Exponential Modeling software, microsphere model and human phantoms of both genders.
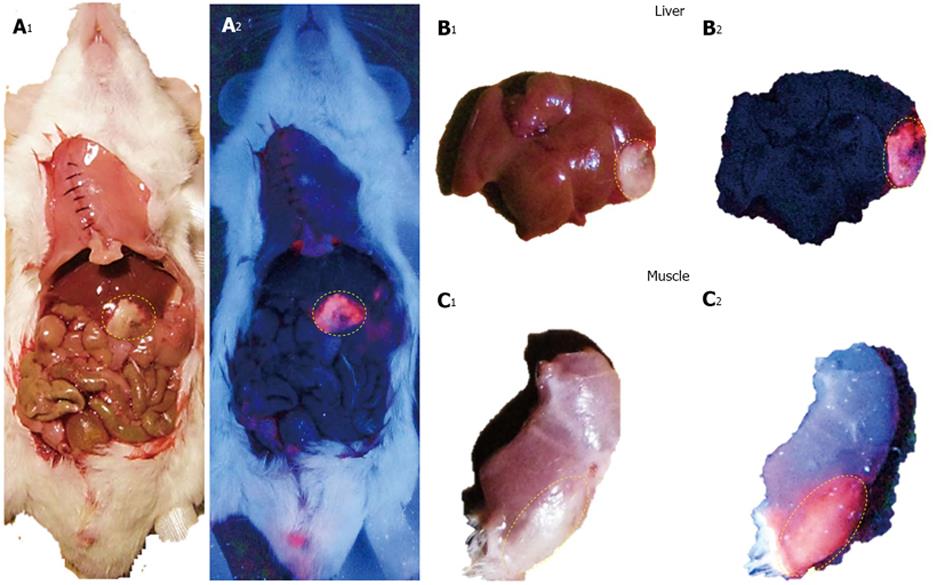
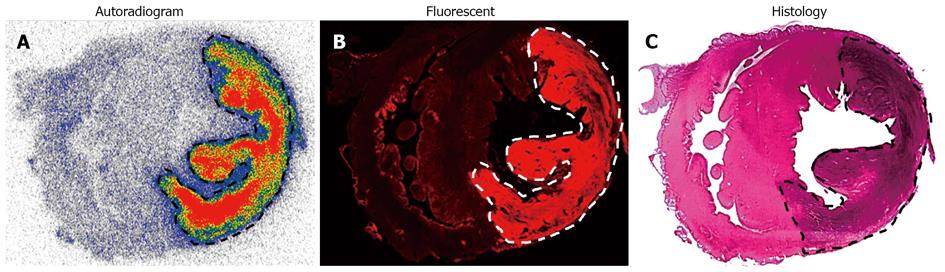
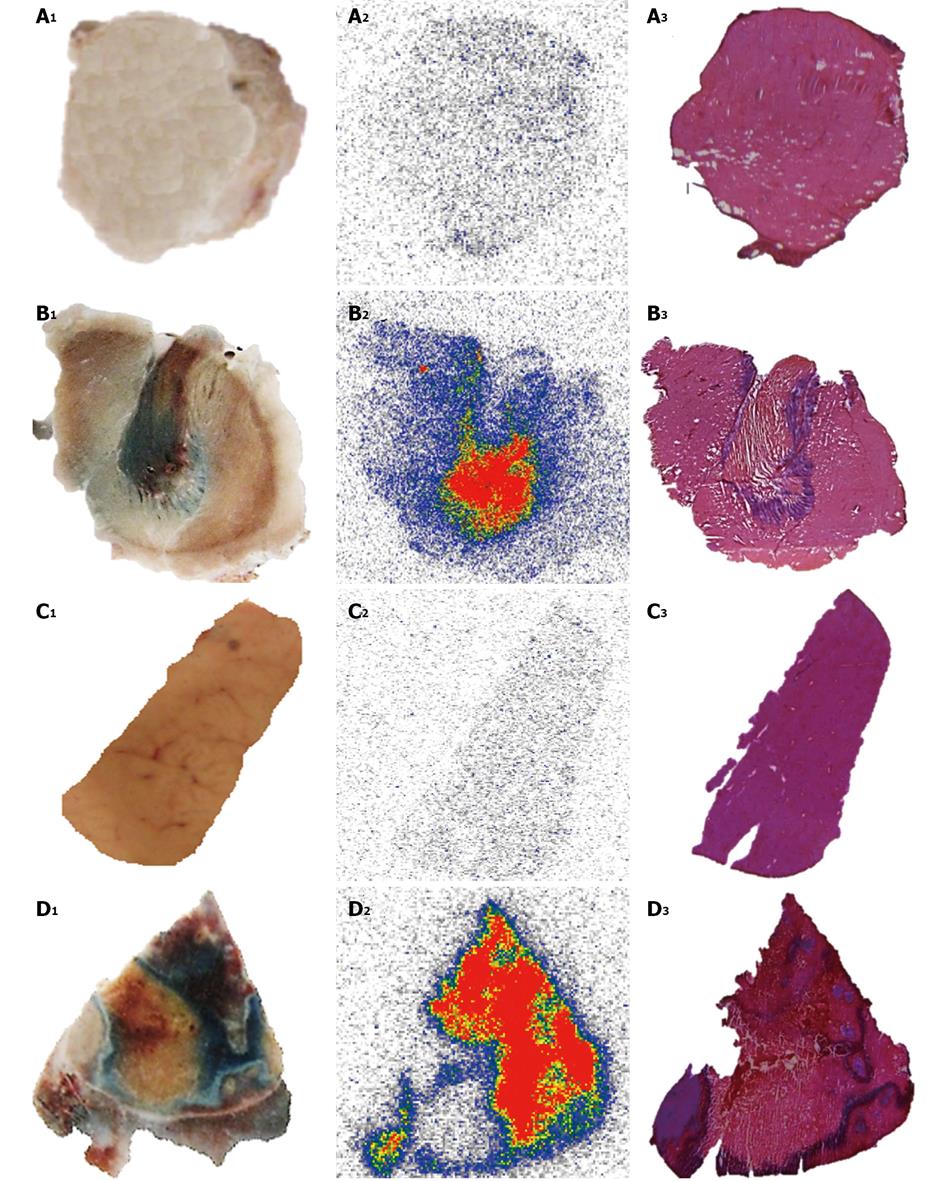
123I-Hyp was accumulated at high concentrations in hepatic infarction and muscle necrosis but low uptake either in viable liver or muscle was detected (Figure 7), as previously reported[1,7,74,75]. Dosimetry studies revealed much higher (> 100 times) absorbed doses of 131I-Hyp in hepatic infarction than in normal liver (Cona et al[79]). Based on this finding, such doses seem to be much higher than those estimated with other radiotherapeutics under investigation or currently used in clinic (Table 2)[31,81-88]. This corroborates the high affinity for tumor necrosis as well as the tumor shrinkage and growth delay previously observed in animals bearing different engrafts tumors after a single treatment with OncoCiDia (Figure 8)[1,75,89].
| Therapeutics | Dosimetry calculations | Species | Targeting tissue | Pathology | Dose to tumor(mGy/MBq) | Ref. |
| 131I-Hyp | OLINDA/EXM software | RPLI rats | Necrotic tissue | Solid tumors | 276–93600 | [79] |
| 131I-Labeled TNT-1 monoclonal antibody | Organ uptake-time integration by trapezoid method Whole body image analysis | Nude mice bearing ME-180 human cervical tumors | Histone fraction H1 in necrotic tissues | Cervical carcinoma cell | 366–3610 | [31] |
| 131I-m-iodobenzylguanidine (MIBG) | MicroPET/CT 124I-MIBG OLINDA/EXM software | Mice bearing A431 human epithelial carcinoma xenografts | Norepinephrine transporter | Neuroblastoma | 97–380 | [80] |
| 131I- labeled monoclonal antibody MN-14 | MIRDOSE3 software | Nude mice with intraperitoneal LS174T tumors | Carcinoembryonic antigen | Peritoneal metastases of colorectal origin | -16200 | [89] |
| 131I-tositumomab | SPECT/CT Imaging DPM Monte Carlo electron and photon transport program | Humans | CD20-positive B-cells | Refractory B-cell NHL | 2.81 (mean) | [81] |
| 177Lu-DOTA-AE105 | Organ uptake-time integration by trapezoid method Sphere model | Nude mice bearing colorectal HT-29 tumor | uPAR-positive HT-29 xenograft | Colorectal cancer | 5.8 | [82] |
| 177Lu-pertuzumab | Organ uptake-time integration by trapezoid method Sphere model | BALB/c (nu/nu) Mice with HER-2–overexpressing xenografts | HER-2 tyrosine kinase receptor | Breast cancer | -6900 | [83] |
| 186Re-1-hydroxy- ethylidene-1,1 diphosphonic acid | MIRDOSE 3.1 software | Humans | Bone mineral metabolite | Skeletal metastases | 23–34 | [84] |
| 90Y-ibritumomab tiuxetan | PET/CT Imaging DPM 89Zr-ibritumomab tiuxetan OLINDA/EXM software | Humans | CD20-positive B-cells | Relapsing NHL | 8.6–28.6 | [85] |
| 90Y- DOTA0-DPhe1-Tyr3- octreotide | SPECT/CT Imaging DPM 111In-DOTA-TOC | Humans | Somatostatin receptor subtype 2 | NETs | 4-31 (mean 10) | [86] |
In biodistribution studies, 123I-Hyp was cleared within 24 h with reduced blood pool radioactivity. Thyroid, the dose limiting organ for 131I-labeled products, showed almost no radioactivity concentration due to the absence free iodide at earlier time points, suggesting in vivo stability of 123/131I-Hyp. However, an increased uptake in the gland was detected, starting at the second day after tracer administration. In the lungs, a persistent 123I-Hyp uptake was found, leading to a moderately absorbed radiation dose of 131I-Hyp. The highest levels of radioactivity were found in the intestines, which constitute the major elimination pathway of this radioactive compound. As a consequence, bowel structures received a high radiation dose, being identified as one of the dose limiting organs for OncoCiDia.
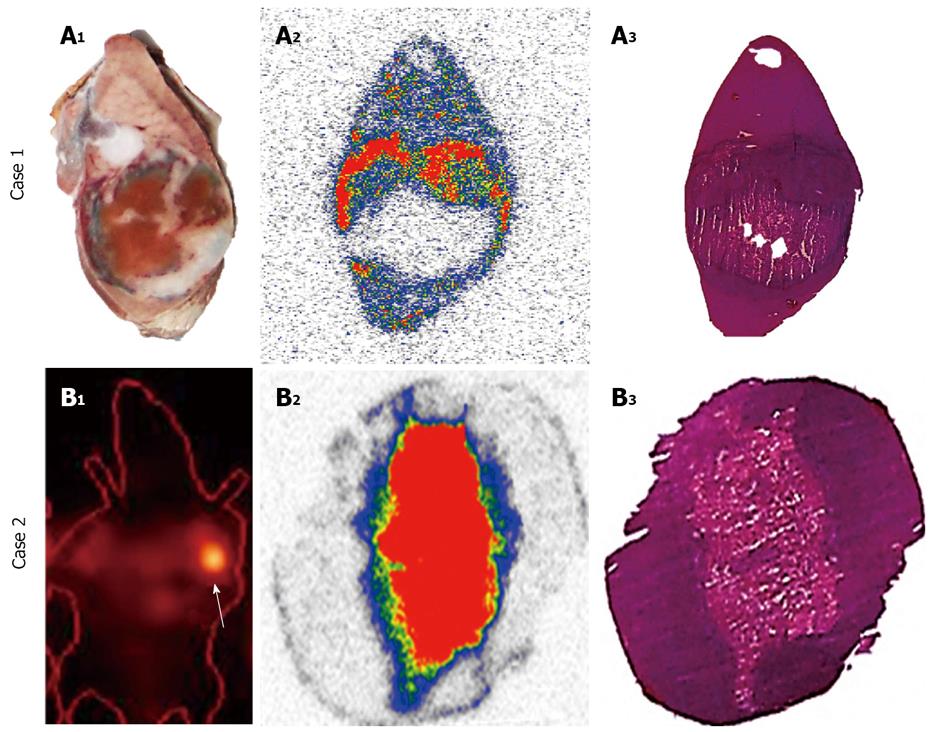
Effect of added Hyp on biodistribution and targetability of 123/131I-Hyp: In STR, it is known that the mass of the unlabelled (carrier) compound present in the final radioactive solution can be critical for high specific activities that are required for maximal radioactivity accumulation in the disease site. In a recent investigation for OncoCiDia, we proved that the co-injection of unlabelled Hyp positively affected the necrosis uptake of the radioiodinated Hyp in RPLI rats[90]. Although both preparations of 123I-Hyp with micro- or Hyp-added dosing showed similar tissue distributions and major hepatobiliary excretion, it was found that the carrier-added 123I-Hyp accumulated at higher concentrations in necrosis. Similarly, long retention into tumor necrosis for several weeks could characterize the carrier-added 131I-Hyp (Figure 8 case 2), which explains the striking therapeutic effects observed in the previous experiments[1,75,89].
Toxicity studies: OncoCiDia is a two-step anticancer strategy involving different compounds with potential chemical and/or radiochemical toxicities, which have been investigated, and possible solutions are herein proposed.
Toxicity from VDAs can happen as a result of the effect of VDAs either on tumor blood vessels or normal tissues. Lack of complete specificity for tumor-related vasculature or downstream effects induced by cytokines and other released factors can contribute to the toxic signs or effects. A distinctive transient and acute toxicity pattern with minor cumulative side effects such as tumor pain, nausea and vomiting, headaches, vision changes, symptoms associated to serotonin release, neuromotor abnormalities and cerebellar ataxia, acute hemodynamic disturbances, abdominal pain, hypertension, and tachycardia have been reported after VDA administration in clinical trials[91]. The degree and types of the side effects might differ among flavonoids and tubulin-binding agents[91].
So far, combretastatin A-4 phosphate (CA4P), a synthetic phosphorylated derivative of the natural product combretastatin A-4, is a tubulin-binding agent that has been most extensively used in OncoCiDia experiments. In preclinical studies, intravenous CA4P at a dose of 10 mg/kg has shown a complete and rapid vascular shutdown of the tumor with minimal effects at the well-perfused periphery[1,89]. However, some discrepancies have been noted in animal studies and clinical trials concerning the anticancer effect of VDAs[92]. According to the Food and Drug Administration-approved rules for the correct dose calculation[93], such a dose of 10 mg/kg in rodents would be equivalent to 60 mg/m2 in humans. However, phase I clinical trials of CA4P have demonstrated a minimal objective tumor response by using similar doses of 52 to 68 mg/m2[94]. The reason for this difference is not yet clear but could be caused by either miscalculations of the dose based on body surface area in mg/m2 or due to inaccurate dose translation from animal (mg/kg) to humans (mg/m2) in addition to the less likely interspecies differences. Based on our experiences, we believe that it may hamper the actual potentialities of VDAs for getting desired anticancer effects in human patients if the dose issues are not properly settled. To overcome the problem, an alternative option could be to increase the total injected dose of CA4P but in an approach of multiple small doses for preventing acute side effects[95]. Moreover, since cardiovascular toxicity seems to be with the most toxicity concerns for CA4P[96], pretreatments with high doses of intravenous diltiazem for preventing hypertension accompanied with amlodipine for secondary prophylaxis or nifedipine and atenolol for blocking tachycardia can be also applied[97,98]. Patients taking QT prolonging drugs, or with a history of significant cardiovascular disease, hypokalemia or hypomagnesemia should be handled with great caution[99].
A study on plasma pharmacokinetics and cerebrospinal fluid penetration of Hyp in rhesus monkeys was conducted. Intravenous administration of 2 mg/kg Hyp was well tolerated. At a dose of 5 mg/kg, a transient severe photosensitivity rash was seen at 12 h that resolved within 12 d[100]. In a phase I study to evaluate the safety and antiretroviral activity of Hyp in thirty HIV-infected patients, weekly repeated IV doses of 0.25 or 0.5 mg/kg were tested. Eleven out of twenty-three patients developed severe cutaneous phototoxicity[101]. In patients with recurrent malignant glioma, a newly developed water soluble formulation of Hyp was IV given (0.1 mg/kg) for tumor visualization. Hyp application proved to be safe with no side effects[102]. Therefore, a low single dose of Hyp at < 0.2 mg/kg for OncoCiDia should be free of noticeable side effects.
I-131 has been used successfully for over 70 years to treat hyperthyroidism and papillary or follicular thyroid cancer and its proper therapeutic use is almost without side effects[103].
However, since accumulation of radioactive iodine was observed in the thyroid, potential damage to the gland could occur due to unnecessary radiation overexposure. Therefore, patients undergoing OncoCiDia treatment should take a thyroid-blocking agent on a daily basis before, during and after radiopharmaceutical administration. Either Lugol’s solution consisting of elemental iodine and potassium iodide in water or supersaturated potassium iodide solution or oral potassium iodide can be used for this purpose[104].
In a toxicity study with Hyp labeled with non-radioactive iodine (127I), the animals tolerated well IV injections of 0.1 and 10 mg/kg without any signs of clinical toxicity or obvious side effects. The median lethal dose (LD50) of 20.26 mg/kg for 127I-Hyp was above 1000 times of the experimental chemical dose of 131I-Hyp used in OncoCidia, suggesting a wide safety margin with insignificant chemotoxicity[105].
On the other hand, radiation toxic effects could be an issue for 131I-Hyp. Experimental evidence indicated that persistent radioactivity retention mainly occurred in the intestines within few days after injection due to its hepatobiliary excretion[70,75,90]. The acute gastrointestinal syndrome is a main concern following irradiation of the intestinal tract[106]. In a recent study, we tested the suitability of using a newly designed catheter to reduce intestinal retention of radioactivity after IV administration of 131I-Hyp. Biodistribution and pharmacokinetics of radioiodinated Hyp were also investigated in animals with and without catheterization. In general, the total radioactivity accumulated in the intestines was dramatically reduced by ten times in those animals with catheter placement. Improved tissue biodistribution and kinetic parameters of 123I-Hyp were also seen in cannulated animals. By using this approach, radiation overexposure because of prolonged excretion of 131I-Hyp can be prevented in the future clinical practice[107].
A safety study of IV administered iodogen in DMSO was conducted in mice of both sexes. LD50 was determined with iodogen/DMSO doses ranging from 40.0 to 70.0 mg/kg. Toxicity at 30.0 mg/kg was tested for changes in behavior, body weight, and serum biochemistry over 14 d. Due to the toxicity concerns associated with the use of DMSO, a high dose of the solvent was also concurrently tested.
Good safety profile was demonstrated with iodogen in DMSO with LD50 values above 50.0 mg/kg and pure DMSO. No animal deaths, pathologies or clinical toxicities were recorded after 30.0 mg/kg iodogen in DMSO, which is 3000 times the dose intended for possible human applications[108].
Overall these data put forward a solid indication for the manageable and tolerable safety of OncoCiDia and potential upcoming clinically applicable formulations in terms of both radiotoxicity and chemotoxicity.
Coronary heart disease, in which MI is a major component, represents the most common cause of death in the Western world. To assess myocardial viability, nuclear imaging uses radiolabelled compounds recognizing specific structures, receptors or antigens to scrutinize the molecular process under physiological conditions in a noninvasive manner. Several myocardial perfusion tracers for single photon emission computed tomography (SPECT) such as thallium-201 (201Tl) and technetium-99m (99mTc) labeled agents (e.g., sestamibi and tetrofosmin) are currently available in the clinic. They evenly distribute throughout the normal myocardium in proportion to the blood flow, depicting the dead/ischemic tissues as “black spot”. However, the development of specific targeting agents with genuine necrosis affinity constitutes an important goal in the management of cardiac pathologies. They may allow early detection, delineation of the infarcted or ischemic area, patient follow-up over-time and evaluation of the response to revascularization therapies[109]. Other cardiovascular diseases related to cardiac cell death could be identified including diverse cardiomyopathy[110], myocardial inflammation, acute myocarditis[111]. Acute or chronic diffuse myocardial damage due to cardiac transplant rejection could also be detected[112].
Various “hot spot” imaging tracers have been exploited for the visualization of MI. Technetium (99mTc)-pyrophosphate accumulates in necrotic myocardium by targeting the calcium phosphate present in the mitochondria of infarcted or harshly damaged myocardium[113]. 99mTc-glucarate complex preferentially localizes into basic protein histones within denatured nuclei and subcellular organelles in the dead cardiomyocytes[114]. 111In-labelled monoclonal anti-myosin Fab specifically recognizes the intracellular heavy chain of the exposed cardiac myosin of severely damaged cells[115]. Unfortunately, overestimation of the infarct size due to poor specificity for distinguishing ischemic and necrotic tissues[116,117], and reduced diagnostic accuracy and low target to background ratio on scintigraphic images because of the prompt dissociation of the tracer in vivo and short-term accumulation at the damage site[7] have been noticed.
By micro-single photon emission computed tomography (μSPECT), the potential usefulness of the necrosis avid 123I-Hyp for detection and quantification of acute MI has been reported[118], which is essential for clinical management of ischemic heart disease. In a more recent study[119], 123I-Hyp was compared with the commercial myocardial perfusion agent technetium-99m-labeled-hexakis (2-methoxyisobutylisonitrile) (99mTc-Sestamibi) in organ distribution and targetability in rabbits with acute MI using dual-isotope simultaneous acquisition-μSPECT/computed tomography and postmortem methods. 123I-Hyp underwent hepatobiliary excretion whereas 99mTc-Sestamibi distribution was characterized by more rapid hepatorenal elimination. 99mTc-Sestamibi preferentially accumulated in the normal myocardium, whereas 123I-Hyp confirmed to be a necrosis specific agent that allowed hot spot imaging of irreversibly damaged myocardium or acute MI. Therefore, 99mTc-Sestamibi and 123I-Hyp can be considered as a pair of complementary tracers for DISA-SPECT/CT in nuclear cardiology[119].
Cholelithiasis refers to the presence of gallstones in the gallbladder. Although these supersaturated deposits of bile are initially formed within the gallbladder, they may distantly pass into other parts of the biliary tract, reaching the common bile duct, the cystic duct and the pancreatic duct. They can broadly vary in size and appear as a single stone or as an assortment of stones with different sizes. Gallstones generally come in three different types including cholesterol stones that represent about 80%, pigment stones composed of bilirubin, the yellow breakdown product of normal heme catabolism found in bile, and mixed stones. Gallstones in the gallbladder may cause acute cholecystitis[120], an inflammatory condition distinguished by bile retention leading to secondary infection by intestinal microorganisms, mainly Escherichia coli, Klebsiella, Enterobacter, and Bacteroides species[121]. Presence of gallstones in the biliary tract can produce obstruction of the bile ducts, leading to severe ascending cholangitis or pancreatitis, which can be life-threatening. Eventually, they can be very painful and may require surgical intervention to remove the gallbladder and/or stones.
Since Hyp is primarily excreted via bile and its interaction with cholesterols has been proved[8,9], the potential use of Hyp as an optical imaging agent for fluorescent detection of gallstones in the clinic was explored. Cholesterol, pigment and mixed gallstones were derived from cholecystectomy patients. In vitro studies were conducted by incubating the gallstones with Hyp solutions at increasing concentrations (0-0.01 mg/mL) either in solvent or bile. Under UV light at 254 nm wavelength, red fluorescence was seen on stones previously incubated with Hyp, but this was not observed on only solvent or bile-treated gallstones (Figure 9). The intensity of stone fluorescence depended on Hyp concentration. Although other techniques like ultrasonography or cholescintigraphy scan with 99mTc-hepatobiliary iminodiacetic acid can usually detect gallstones, the use of Hyp may aid fluorescent detection and removal of gallstones during open and/or endoscopic cholecystectomy and cholangiotomy. In vivo studies are needed to prove whether it is a vital and applicable approach.
Multiple uses of automatic contrast injection systems for automatic delivery of contrast media during enhanced imaging procedures can reduce costs and save resources. However, cross-contaminations with blood-borne pathogens of infectious diseases may occur[122,123]. To avoid possible nosocomial outbreaks, the injection system including the power syringes, filling and injecting set and the patient line has to be completely changed for each patient. However, this proves expensive and time consuming due to the wasted surplus contrast materials in the setup from each exam, the consumptions of disposable devices, and the long pauses for changing the entire set-up per patient. To reduce material and costs, several institutions worldwide have been applying multiple usages of the syringes with automatic injectors for serial patients. Generally, these commercially available injection systems contain a special one-way-valve tube device. However, nosocomial outbreaks between patients are still a problem because of contamination of the injection system with blood-borne pathogen[124].
The purpose of this experiment was to develop a radioactive method for quantitative safety evaluation of a new replaceable patient-delivery system[125]. This system (Transflux™ Diepenbeek, Belgium) contains a safety zone composed by a tube and two one-way valves. It permits to flush the whole injector system and the vein but prevents blood reflux during contrast-enhanced imaging. This system is replaced for each new patient, whereas the power syringes need to be changed only once a day after multiple uses for a series of patients. It has been applied for years in many radiology units without any contaminative infections reported, which though has to be experimentally justified.
By mimicking pathogens with a diffusible radiotracer, we evaluated the feasibility of using this system repeatedly without septic risks. The experiment was performed by intravenous injection of 99mTc-dimercaptopropionyl-human serum albumin in rabbits previously connected via an endovenous catheter to an automatic contrast injection system. Protocols with normal saline and contrast agent plus saline loaded in the injection system were compared. By sampling and analyzing aliquots from the filling and injecting set, patient line and blood, it was checked if the radiotracer from the patient line in contact with animal blood was able to cross the safety zone and reach the power syringes.
Overall, with both protocols, radioactivity was found in blood and in patient line but in none of the samples from the filling-injecting set. This radioactive method appears accurate and reliable. The patient-delivery system proves safe and convenient, which is in line with the clinical experiences collected to date. By replacing the patient delivery system, cross-contamination risks can be avoided without changing the main part of injection system. This method can be applied for evaluation of similar devices before human use.
Translational medicine aims at identifying solutions to specific health problems. Different but important difficulties faced in cancer treatment, identification of cardiac infarction, detection of cholelithiasis and safety evaluation of medical devices might be considerably tackled by well-designed laboratory experiments.
OncoCiDia presents an unconventional but general approach based on the necrosis avidity for treating multifocal and multitype malignant tumors. It uses a combined sequence of a vascular disrupting agent for triggering massive tumor necrosis followed by the necrosis avid 131I-Hyp to destroy remaining tumor cells. Some technical optimizations have been performed and herein demonstrated to assist in introducing OncoCiDia to the possible clinical practice. The feasible Hyp radioiodination with good radiochemical yields and a proper formulation for in vivo applications have been investigated and discussed. The favorable biodistribution, dosimetry and pharmacokinetic patterns as well as good in vivo tolerance and low toxicity of radioiodinated Hyp have been exhibited in animal experiments. In general, the genuine benefits of 131I-Hyp distinguished by high and unprecedented long-term accumulation in tumor necrosis in the vicinity of cancer cells and its convenient clearance mechanism through bile without renal retention could noticeably impact on cancer theragnostic management or open doors for handling a wide diversity of cancers in future clinical practice.
Targeting necrosis may offer new opportunities for the management of cardiac pathologies. The clinical introduction of radioactive necrosis-specific agents like 123I-Hyp might play a complementary role in detection and quantification of acute myocardial infarction. The combination of the genuine 123I-Hyp necrosis avidity with the preferential uptake of the currently used commercial myocardial perfusion agent by normal myocardium might offer additional information in the clinical management of this life-threatening pathology.
On the other hand, the use of the fluorophore hypericin as an optical imaging agent with low in vivo toxicity could be an excellent diagnostic tool for the detection and removal of gallstones in patients suffering from such common clinical conditions.
Finally, the risk of accidental cross-contamination in medical devices can be minimized through safety evaluation studies based on the inherent sensitivity of radioactive methods in preclinical animal experiments.
P- Reviewers: Guo ZS, Leitman IM S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Li J, Sun Z, Zhang J, Shao H, Cona MM, Wang H, Marysael T, Chen F, Prinsen K, Zhou L. A dual-targeting anticancer approach: soil and seed principle. Radiology. 2011;260:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 2. | Li J; Aschylus. OncoCidia. Available from: http: //www.aeschylus-philanthropy.eu/index.php/b/project/oncocidia. |
| 3. | Roger VL. Epidemiology of myocardial infarction. Med Clin North Am. 2007;91:537–552. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Bauer A, Mehilli J, Barthel P, Müller A, Kastrati A, Ulm K, Schömig A, Malik M, Schmidt G. Impact of myocardial salvage assessed by (99m)Tc-sestamibi scintigraphy on cardiac autonomic function in patients undergoing mechanical reperfusion therapy for acute myocardial infarction. JACC Cardiovasc Imaging. 2009;2:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Lombardo A, Rizzello V, Galiuto L, Natale L, Giordano A, Rebuzzi A, Loperfido F, Crea F, Maseri A. Assessment of resting perfusion defects in patients with acute myocardial infarction: comparison of myocardial contrast echocardiography, combined first-pass/delayed contrast-enhanced magnetic resonance imaging and 99mTC-sestamibi SPECT. Int J Cardiovasc Imaging. 2006;22:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ni Y, Bormans G, Marchal G, Verbruggen A. Tissue infarction and necrosis specific compounds (of hypericin derivatives). Available from: http://www.google.com/patents/EP1651201B1. |
| 7. | Ni Y, Huyghe D, Verbeke K, de Witte PA, Nuyts J, Mortelmans L, Chen F, Marchal G, Verbruggen AM, Bormans GM. First preclinical evaluation of mono-[123I]iodohypericin as a necrosis-avid tracer agent. Eur J Nucl Med Mol Imaging. 2006;33:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Ho YF, Wu MH, Cheng BH, Chen YW, Shih MC. Lipid-mediated preferential localization of hypericin in lipid membranes. Biochim Biophys Acta. 2009;1788:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Eriksson ESE, Eriksson LA. The influence of cholesterol on the properties and permeability of hypericin derivatives in lipid membranes. J. Chem Theory Comput. 2011;7:560–574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Cancer Research UK. Cancer Worldwide - the global picture. Future trends. Available from: http: //www.cancerresearchuk.org/cancer-info/cancerstats/world/cancer-worldwide-the-global-picture. |
| 11. | Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, Tennvall J, Bombardieri E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Gedik GK, Hoefnagel CA, Bais E, Olmos RA. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2008;35:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Polishchuk AL, Dubois SG, Haas-Kogan D, Hawkins R, Matthay KK. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117:4286-4293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | McEwan AJ, Amyotte GA, McGowan DG, MacGillivray JA, Porter AT. A retrospective analysis of the cost effectiveness of treatment with Metastron (89Sr-chloride) in patients with prostate cancer metastatic to bone. Nucl Med Commun. 1994;15:499-504. [PubMed] |
| 15. | Rhee TK, Lewandowski RJ, Liu DM, Mulcahy MF, Takahashi G, Hansen PD, Benson AB, Kennedy AS, Omary RA, Salem R. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Smith K, Byer G, Morris CG, Kirwan JM, Lightsey J, Mendenhall NP, Hoppe BS, Lynch J, Olivier K. Outcomes of patients with non-Hodgkin’s lymphoma treated with Bexxar with or without external-beam radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Micallef IN. Ongoing trials with yttrium 90-labeled ibritumomab tiuxetan in patients with non-Hodgkin’s lymphoma. Clin Lymphoma. 2004;5 Suppl 1:S27-S32. [PubMed] |
| 18. | Virgolini I, Traub T, Novotny C, Leimer M, Füger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M. Experience with indium-111 and yttrium-90-labeled somatostatin analogs. Curr Pharm Des. 2002;8:1781-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53-R73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 20. | Paganelli G, Chinol M. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Sharkey RM. Radioimmunotherapy against the tumor vasculature: A new target? J Nucl Med. 2006;47:1070-1074. [PubMed] |
| 22. | Govindan SV, Goldenberg DM, Hansen HJ, Griffiths GL. Advances in the use of monoclonal antibodies in cancer radiotherapy. Pharm Sci Technolo Today. 2000;3:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Mäcke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci USA. 2006;103:16436-16441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 376] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 24. | Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1081] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 25. | Aggarwal BB, Danda D, Gupta S, Gehlot P. Models for prevention and treatment of cancer: problems vs promises. Biochem Pharmacol. 2009;78:1083-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Hornick JL, Sharifi J, Khawli LA, Hu P, Biela BH, Mizokami MM, Yun A, Taylor CR, Epstein AL. A new chemically modified chimeric TNT-3 monoclonal antibody directed against DNA for the radioimmunotherapy of solid tumors. Cancer Biother Radiopharm. 1998;13:255-268. [PubMed] |
| 27. | Epstein AL, Chen FM, Taylor CR. A novel method for the detection of necrotic lesions in human cancers. Cancer Res. 1988;48:5842-5848. [PubMed] |
| 28. | Charbit A, Malaise EP, Tubiana M. Relation between the pathological nature and the growth rate of human tumors. Eur J Cancer. 1971;7:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 164] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Malaise EP, Chavaudra N, Tubiana M. The relationship between growth rate, labelling index and histological type of human solid tumours. Eur J Cancer. 1973;9:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Miller GK, Naeve GS, Gaffar SA, Epstein AL. Immunologic and biochemical analysis of TNT-1 and TNT-2 monoclonal antibody binding to histones. Hybridoma. 1993;12:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Chen FM, Taylor CR, Epstein AL. Tumor necrosis treatment of ME-180 human cervical carcinoma model with 131I-labeled TNT-1 monoclonal antibody. Cancer Res. 1989;49:4578-4585. [PubMed] |
| 32. | Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Di Corcia MG, Lucchese A, Dini L. Cell death: apoptosis versus necrosis (review). Int J Oncol. 2002;21:165-170. [PubMed] |
| 33. | Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2262] [Cited by in RCA: 2079] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 34. | Walker NI, Harmon BV, Gobé GC, Kerr JF. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18-54. [PubMed] |
| 35. | Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 824] [Cited by in RCA: 886] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 36. | Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2396] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 38. | Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 964] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 39. | Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2010] [Cited by in RCA: 2008] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 40. | Nanji AA, Hiller-Sturmhöfel S. Apoptosis and necrosis: two types of cell death in alcoholic liver disease. Alcohol Health Res World. 1997;21:325-330. [PubMed] |
| 41. | Ziegler U, Groscurth P. Morphological features of cell death. News Physiol Sci. 2004;19:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Available from: http: //www.humpath.com/spip.php?article4548. |
| 43. | Street HH, Goris ML, Fisher GA, Wessels BW, Cho C, Hernandez C, Zhu HJ, Zhang Y, Nangiana JS, Shan JS. Phase I study of 131I-chimeric(ch) TNT-1/B monoclonal antibody for the treatment of advanced colon cancer. Cancer Biother Radiopharm. 2006;21:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Hdeib A, Sloan AE. Convection-enhanced delivery of 131I-chTNT-1/B mAB for treatment of high-grade adult gliomas. Expert Opin Biol Ther. 2011;11:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Chen S, Yu L, Jiang C, Zhao Y, Sun D, Li S, Liao G, Chen Y, Fu Q, Tao Q. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Khawli LA, Alauddin MM, Hu P, Epstein AL. Tumor targeting properties of indium-111 labeled genetically engineered Fab’ and F(ab’)2 constructs of chimeric tumor necrosis treatment (chTNT)-3 antibody. Cancer Biother Radiopharm. 2003;18:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Anderson PM, Wiseman GA, Lewis BD, Charboneau W, Dunn WL, Carpenter SP, Chew T. A phase I safety and imaging study using radiofrequency ablation (RFA) followed by 131I-chTNT-1/B radioimmunotherapy adjuvant treatment of hepatic metastases. Cancer Ther. 2003;1:283-291. |
| 48. | Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 743] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 49. | Chaplin DJ, Hill SA. The development of combretastatin A4 phosphate as a vascular targeting agent. Int J Radiat Oncol Biol Phys. 2002;54:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Chaplin DJ, Dougherty GJ. Tumour vasculature as a target for cancer therapy. Br J Cancer. 1999;80 Suppl 1:57-64. [PubMed] |
| 51. | Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, Marmé D, Lorusso PM. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res. 2005;11:416-420. [PubMed] |
| 52. | Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11:562-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 53. | Wolfender JL, Queiroz EF, Hostettmann K. Photochemistry. In: Birkhäuser Verlag. St. John's Wort and its Active Principles in Depression and Anxiety. Basel 2005; 5-19. |
| 54. | Dewick PM. Aromatic Polyketides. Structural Modifications: Anthraquinones. Medicinal natural products: a biosynthetic approach, 2nd ed. West Sussex 2002; 63-71. |
| 55. | Leonhartsberger JG, Falk H. The protonation and deprotonation equilibria of hypericin revisited. Monatsh Chem. 2002;133:167–172. [DOI] [Full Text] |
| 56. | Etzlstorfer C, Falk H, Müller N, Schmitzberger W, Wagner UG. Tautomerism and stereochemistry of hypericin: Force field, NMR, and X-ray crystallographic investigations. Monatsh Chemie. 1993;124:751-761. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Gutman I, Marković Z, Solujić S, Sukdolak S. On the tautomers of hypericin. chemistry and materials science. Monatshefte fur Chemie. 1998;129:481-486. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 58. | Kapinus EI, Falk H, Tran HTN. Spectroscopic investigation of the molecular structure of hypericin and its salts. Monatshefte fuÈr Chemie. 1999;130:623-635. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Bánó G, Staničová J, Jancura D, Marek J, Bánó M, Uličný J, Strejčková A, Miškovský P. On the diffusion of hypericin in dimethylsulfoxide/water mixtures-the effect of aggregation. J Phys Chem B. 2011;115:2417-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Buriankova L, Buzova D, Chorvat D, Sureau F, Brault D, Miskovský P, Jancura D. Kinetics of hypericin association with low-density lipoproteins. Photochem Photobiol. 2011;87:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Sosa S, Pace R, Bornancin A, Morazzoni P, Riva A, Tubaro A, Della Loggia R. Topical anti-inflammatory activity of extracts and compounds from Hypericum perforatum L. J Pharm Pharmacol. 2007;59:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Meruelo D, Lavie G, Lavie D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci USA. 1988;85:5230-5234. [PubMed] |
| 63. | Avato P, Raffo F, Guglielmi G, Vitali C, Rosato A. Extracts from St John’s Wort and their antimicrobial activity. Phytother Res. 2004;18:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Blank M, Mandel M, Hazan S, Keisari Y, Lavie G. Anti-cancer activities of hypericin in the dark. Photochem Photobiol. 2001;74:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression--an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 526] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 66. | Ni Y, Bormans G, Chen F, Verbruggen A, Marchal G. Necrosis avid contrast agents: functional similarity versus structural diversity. Invest Radiol. 2005;40:526-535. [PubMed] |
| 67. | Miskovsky P, Hritz J, Sanchez-Cortes S, Fabriciova G, Ulicny J, Chinsky L. Interaction of hypericin with serum albumins: surface-enhanced Raman spectroscopy, resonance Raman spectroscopy and molecular modeling study. Photochem Photobiol. 2001;74:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Song S, Xiong C, Zhou M, Lu W, Huang Q, Ku G, Zhao J, Flores LG, Ni Y, Li C. Small-animal PET of tumor damage induced by photothermal ablation with 64Cu-bis-DOTA-hypericin. J Nucl Med. 2011;52:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Loke KS, Padhy AK, Ng DC, Goh AS, Divgi C. Dosimetric considerations in radioimmunotherapy and systemic radionuclide therapies: a review. World J Nucl Med. 2011;10:122-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Bormans G, Huyghe D, Christiaen A, Verbeke K, de Groot T, Vanbilloen H, de Witte P, Verbruggen A. Preparation, analysis and biodistribution in mice of iodine-123 labeled derivatives of hypericin. Journal of Labeled Compounds and Radiopharmaceuticals. 2004;47:191–198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Sun ZP, inventor . Iodogen method for preparation of radioiodinated Hypericin. Available from: http://www.google.com/patents/CN101475461B?cl=zh. |
| 72. | Sihver W, Bier D, Holschbach MH, Schulze A, Wutz W, Olsson RA, Coenen HH. Binding of tritiated and radioiodinated ZM241,385 to brain A2A adenosine receptors. Nucl Med Biol. 2004;31:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3074] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 74. | Li J, Cona MM, Chen F, Feng Y, Zhou L, Yu J, Nuyts J, de Witte P, Zhang J, Himmelreich U. Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics. 2012;2:1010-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Cona MM, Li J, Bauwens M, Feng Y, Sun Z, Zhang J, Chen F, Alpizar Y, Talavera K, de Witte P. Radioiodinated Hypericin: Its Biodistribution, Necrosis Avidity and Therapeutic Efficacy are Influenced by Formulation. Pharm Res. 2013;Aug 9; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Laurent A, Mottu F, Chapot R, Zhang JQ, Jordan O, Rüfenacht DA, Doelker E, Merland JJ. Cardiovascular effects of selected water-miscible solvents for pharmaceutical injections and embolization materials: a comparative hemodynamic study using a sheep model. PDA J Pharm Sci Technol. 2007;61:64-74. [PubMed] |
| 77. | Reed KW, Yalkowsky SH. Lysis of human red blood cells in the presence of various cosolvents. III. The relationship between hemolytic potential and structure. J Parenter Sci Technol. 1987;41:37-39. [PubMed] |
| 78. | Conte G, Di Blasi R, Giglio E, Parretta A, Pavel NV. Nuclear magnetic resonance and x-ray studies on micellar aggregates of sodium deoxycholate. J Phys Chem. 1984;88:5720–5724. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Miranda Cona M, Feng YB, Jian Zhang, Yue Li, Verbruggen A, Oyen R, Ni Y. Sodium cholate, a solubilizing agent for the necrosis avid radioiodinated hypericin in rabbits with acute myocardial infarction. Drug Delivery. 2014;(in press). [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Miranda Cona M, Koole M, Feng YB, Liu YW, Verbruggen A, Oyen R and Ni Y. Biodistribution and radiation dosimetry of radioiodinated hypericin as a cancer therapeutic. International Journal of Oncology. 2014;44 (in press). [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Seo Y, Gustafson WC, Dannoon SF, Nekritz EA, Lee CL, Murphy ST, VanBrocklin HF, Hernandez-Pampaloni M, Haas-Kogan DA, Weiss WA. Tumor dosimetry using [124I]m-iodobenzylguanidine microPET/CT for [131I]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model. Mol Imaging Biol. 2012;14:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Koppe MJ, Bleichrodt RP, Soede AC, Verhofstad AA, Goldenberg DM, Oyen WJ, Boerman OC. Biodistribution and therapeutic efficacy of (125/131)I-, (186)Re-, (88/90)Y-, or (177)Lu-labeled monoclonal antibody MN-14 to carcinoembryonic antigen in mice with small peritoneal metastases of colorectal origin. J Nucl Med. 2004;45:1224-1232. [PubMed] |
| 83. | Kaminski MS, Estes J, Zasadny KR, Francis IR, Ross CW, Tuck M, Regan D, Fisher S, Gutierrez J, Kroll S. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259-1266. [PubMed] |
| 84. | Persson M, Rasmussen P, Madsen J, Ploug M, Kjaer A. New peptide receptor radionuclide therapy of invasive cancer cells: in vivo studies using 177Lu-DOTA-AE105 targeting uPAR in human colorectal cancer xenografts. Nucl Med Biol. 2012;39:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Persson M, Gedda L, Lundqvist H, Tolmachev V, Nordgren H, Malmström PU, Carlsson J. [177Lu]pertuzumab: experimental therapy of HER-2-expressing xenografts. Cancer Res. 2007;67:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Argyrou M, Valassi A, Andreou M, Lyra M. Dosimetry and therapeutic ratios for rhenium-186 HEDP. ISRN Molecular Imaging. 2013;2013:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Rizvi SN, Visser OJ, Vosjan MJ, van Lingen A, Hoekstra OS, Zijlstra JM, Huijgens PC, van Dongen GA, Lubberink M. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging. 2012;39:512-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in Peptide radionuclide receptor therapy: a review. J Nucl Med. 2006;47:1467-1475. [PubMed] |
| 89. | Li J, Cona MM, Chen F, Feng Y, Zhou L, Zhang G, Nuyts J, de Witte P, Zhang J, Yu J. Sequential systemic administrations of combretastatin A4 Phosphate and radioiodinated hypericin exert synergistic targeted theranostic effects with prolonged survival on SCID mice carrying bifocal tumor xenografts. Theranostics. 2013;3:127-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 90. | Cona MM, Li J, Feng Y, Chen F, Verbruggen A, de Witte P, Oyen R, Ni Y. Targetability and Biodistribution of Radioiodinated Hypericin: Comparison between microdosing and carrier-added preparations. Anticancer Agents Med Chem. 2013;Aug 28; Epub ahead of print. [PubMed] |
| 91. | Hasani A, Leighl N. Classification and toxicities of vascular disrupting agents. Clin Lung Cancer. 2011;12:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Felici A, Verweij J, Sparreboom A. Dosing strategies for anticancer drugs: the good, the bad and body-surface area. Eur J Cancer. 2002;38:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3894] [Cited by in RCA: 4777] [Article Influence: 265.4] [Reference Citation Analysis (0)] |
| 94. | Ng QS, Mandeville H, Goh V, Alonzi R, Milner J, Carnell D, Meer K, Padhani AR, Saunders MI, Hoskin PJ. Phase Ib trial of radiotherapy in combination with combretastatin-A4-phosphate in patients with non-small-cell lung cancer, prostate adenocarcinoma, and squamous cell carcinoma of the head and neck. Ann Oncol. 2012;23:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Burke JM, Miller JE. Evaluation of multiple low doses of copper oxide wire particles compared with levamisole for control of Haemonchus contortus in lambs. Vet Parasitol. 2006;139:145-149. [PubMed] |
| 96. | Subbiah IM, Lenihan DJ, Tsimberidou AM. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist. 2011;16:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Zweifel M, Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol. 2011;22:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 98. | Gould S, Westwood FR, Curwen JO, Ashton SE, Roberts DW, Lovick SC, Ryan AJ. Effect of pretreatment with atenolol and nifedipine on ZD6126-induced cardiac toxicity in rats. J Natl Cancer Inst. 2007;99:1724-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, Gumbrell L, Price PM. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol. 2003;21:2815-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 100. | Fox E, Murphy RF, McCully CL, Adamson PC. Plasma pharmacokinetics and cerebrospinal fluid penetration of hypericin in nonhuman primates. Cancer Chemother Pharmacol. 2001;47:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Gulick RM, McAuliffe V, Holden-Wiltse J, Crumpacker C, Liebes L, Stein DS, Meehan P, Hussey S, Forcht J, Valentine FT. Phase I studies of hypericin, the active compound in St. John’s Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann Intern Med. 1999;130:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Ritz R, Daniels R, Noell S, Feigl GC, Schmidt V, Bornemann A, Ramina K, Mayer D, Dietz K, Strauss WS. Hypericin for visualization of high grade gliomas: first clinical experience. Eur J Surg Oncol. 2012;38:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 103. | Shapiro B. Optimization of radioiodine therapy of thyrotoxicosis: what have we learned after 50 years? J Nucl Med. 1993;34:1638-1641. [PubMed] |
| 104. | Cosgriff PS. Thyroid blocking associated with I123- and I131 MIBG scans. Available from: http: //www.nuclearmedicine.org.uk/thyroid/thyweb.pdf. |
| 105. | Li JJ, Cona MM, Feng YB, Chen F, Zhang GZ, Fu XB, Himmelreich U, Oyen R, Verbruggen A, Ni YC. A single-dose toxicity study on non-radioactive iodinated hypericin for a targeted anticancer therapy in mice. Acta Pharmacol Sin. 2012;33:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Van Landeghem L, Blue RE, Dehmer JJ, Henning SJ, Helmrath MA, Lund PK. Localized intestinal radiation and liquid diet enhance survival and permit evaluation of long-term intestinal responses to high dose radiation in mice. PLoS One. 2012;7:e51310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Cona MM, Feng Y, Verbruggen A, Oyen R, Ni Y. Improved clearance of radioiodinated hypericin as a targeted anticancer agent by using a duodenal drainage catheter in rats. Exp Biol Med (Maywood). 2013;Oct 21; Epub ahead of print. [PubMed] |
| 108. | Cona MM, Li J, Chen F, Feng Y, Alpizar YA, Vanstapel F, Talavera K, de Witte P, Verbruggen A, Sun Z. A safety study on single intravenous dose of tetrachloro-diphenyl glycoluril [iodogen] dissolved in dimethyl sulphoxide (DMSO). Xenobiotica. 2013;43:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Ell PJ, Langford R, Pearce P, Lui D, Elliott AT, Woolf N, Williams ES. 99mTc-imidodiphosphonate: a superior radio-pharmaceutical for in vivo positive myocardial infarct imaging. I: Experimental data. Br Heart J. 1978;40:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Obrador D, Ballester M, Carrió I, Augé JM, López CM, Bosch I, Martí V, Bordes R. Active myocardial damage without attending inflammatory response in dilated cardiomyopathy. J Am Coll Cardiol. 1993;21:1667-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Dec GW, Palacios I, Yasuda T, Fallon JT, Khaw BA, Strauss HW, Haber E. Antimyosin antibody cardiac imaging: its role in the diagnosis of myocarditis. J Am Coll Cardiol. 1990;16:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Frist W, Yasuda T, Segall G, Khaw BA, Strauss HW, Gold H, Stinson E, Oyer P, Baldwin J, Billingham M. Noninvasive detection of human cardiac transplant rejection with indium-111 antimyosin (Fab) imaging. Circulation. 1987;76:V81-V85. [PubMed] |
| 113. | Buja LM, Tofe AJ, Kulkarni PV, Mukherjee A, Parkey RW, Francis MD, Bonte FJ, Willerson JT. Sites and mechanisms of localization of technetium-99m phosphorus radiopharmaceuticals in acute myocardial infarcts and other tissues. J Clin Invest. 1977;60:724-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Khaw BA, Nakazawa A, O’Donnell SM, Pak KY, Narula J. Avidity of technetium 99m glucarate for the necrotic myocardium: in vivo and in vitro assessment. J Nucl Cardiol. 1997;4:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Hiroe M, Ohta Y, Fujita N, Nagata M, Toyozaki T, Kusakabe K, Sekiguchi M, Marumo F. Myocardial uptake of 111In monoclonal antimyosin Fab in detecting doxorubicin cardiotoxicity in rats. Morphological and hemodynamic findings. Circulation. 1992;86:1965-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Bianco JA, Kemper AJ, Taylor A, Lazewatsky J, Tow DE, Khuri SF. Technetium-99m(Sn2+)pyrophosphate in ischemic and infarcted dog myocardium in early stages of acute coronary occlusion: histochemical and tissue-counting comparisons. J Nucl Med. 1983;24:485-491. [PubMed] |
| 117. | Khaw BA, Strauss HW, Moore R, Fallon JT, Yasuda T, Gold HK, Haber E. Myocardial damage delineated by indium-111 antimyosin Fab and technetium-99m pyrophosphate. J Nucl Med. 1987;28:76-82. [PubMed] |
| 118. | Fonge H, Vunckx K, Wang H, Feng Y, Mortelmans L, Nuyts J, Bormans G, Verbruggen A, Ni Y. Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin microSPECT. Eur Heart J. 2008;29:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Cona MM, Feng Y, Li Y, Chen F, Vunckx K, Zhou L, Van Slambrouck K, Rezaei A, Gheysens O, Nuyts J. Comparative study of iodine-123-labeled hypericin and 99mTc-labeled hexakis [2-methoxy isobutyl isonitrile] in a rabbit model of myocardial infarction. J Cardiovasc Pharmacol. 2013;62:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Vorvick LJ, Longstreth GF, Zieve D. Acute cholecystitis. MedlinePlus. 2011; Available from: http: //www.nlm.nih.gov/medlineplus/ency/article/000264.htm. |
| 121. | Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 122. | Pañella H, Rius C, Caylà JA. Transmission of hepatitis C virus during computed tomography scanning with contrast. Emerg Infect Dis. 2008;14:333-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Chen KT, Chen CJ, Chang PY, Morse DL. A nosocomial outbreak of malaria associated with contaminated catheters and contrast medium of a computed tomographic scanner. Infect Control Hosp Epidemiol. 1999;20:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Cantin V, Labadie R, Rhainds M, Simard FC. Intravenous contrast medium administration in computerized axial tomography at the CHUQ Medical Imaging Department. Centre hospitalier universitaire de Québec. Medicinal natural products: a biosynthetic approach, 2nd ed. West Sussex 2007; Available from: http://www.chuq.qc.ca/NR/rdonlyres/03BC97EF-5F04-4375-BC48-3852965A4EDD/0/R%C3%89SUM%C3%89substancedecontrasteVArevised_VF.pdf.Accessed%20Nov%2029,%202010. |
| 125. | Cona MM, Bauwens M, Zheng Y, Coudyzer W, Li J, Feng Y, Wang H, Chen F, Verbruggen A, Oyen R. Study on the microbial safety of an infusion set for contrast-enhanced imaging. Invest Radiol. 2012;47:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |