Published online Nov 20, 2022. doi: 10.5662/wjm.v12.i6.465
Peer-review started: April 11, 2022
First decision: June 27, 2022
Revised: July 14, 2022
Accepted: October 5, 2022
Article in press: October 5, 2022
Published online: November 20, 2022
Processing time: 219 Days and 7 Hours
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can produce a wide range of clinical manifestations from asymptomatic to life-threatening. Various researchers have worked to elucidate the pathogenic mechanisms underlying these variable presentations. Differences in individual responses to systemic inflammation and coagulopathy appear to be modulated by several factors, including sex steroid hormones. Transgender men or non-binary indi
To investigate the potential role of GAHT in the development of COVID-19 infections and complications.
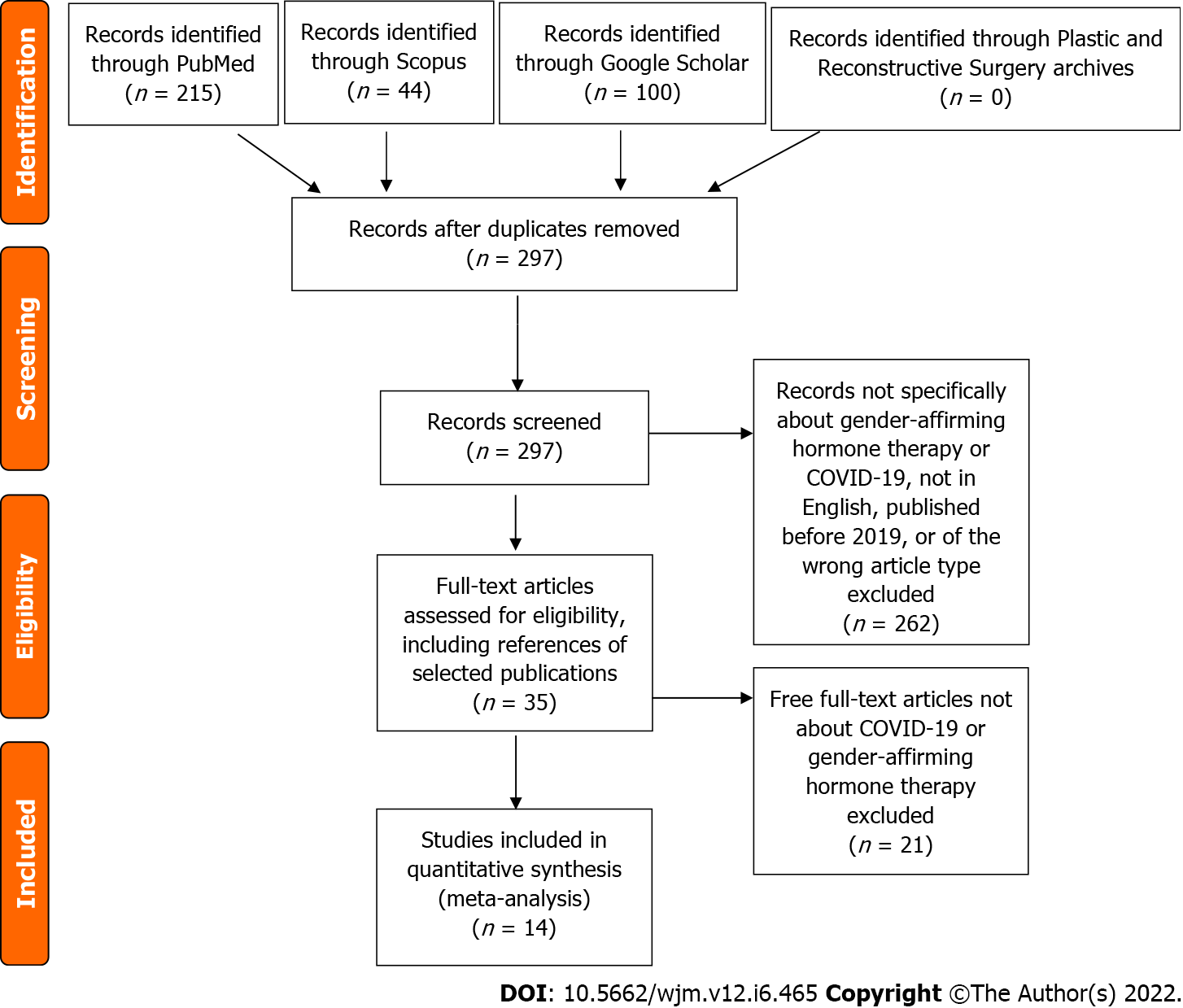
This systematic review implemented an algorithmic approach using PRISMA guidelines. PubMed, Scopus, Google Scholar top 100 results, and archives of Plastic and Reconstructive Surgery was on January 12, 2022 using the key words “gender” AND “hormone” AND “therapy” AND “COVID-19” as well as associated terms. Non-English articles, articles published prior to 2019 (prior to COVID-19), and manuscripts in the form of reviews, commentaries, or letters were excluded. References of the selected publications were screened as well.
The database search resulted in the final inclusion of 14 studies related to GAHT COVID-19. Of the included studies, only two studies directly involved and reported on COVID-19 in transgender patients. Several clinical trials looked at the relationship between testosterone, estrogen, and progesterone in COVID-19 infected cis-gender men and women. It has been proposed that androgens may facilitate initial COVID-19 infection, however, once this occurs, testosterone may have a protective effect. Multiple clinical studies have shown that low baseline testosterone levels in men with COVID-19 are associated with worsening outcomes. The role of female sex hormones, including estrogen and progesterone have also been proposed as potential protective factors in COVID-19 infection. This was exemplified in multiple studies investigating different outcomes in pre- and post-menopausal women as well as those taking hormone replacement therapy. Two studies related specifically to transgender patients and GAHT found that estrogen and progesterone could help protect men against COVID-19, and that testosterone hormone therapy may increase the risk of contracting COVID-19.
Few studies were found related to the role of GAHT in COVID-19 infections. Additional research is necessary to enhance our understanding of this relationship and provide better care for transgender patients.
Core Tip: Severe acute respiratory syndrome coronavirus 2 can produce a wide range of clinical manifestations from asymptomatic to life-threatening. Differences in individual responses to systemic inflammation and coagulopathy appear to be modulated by several factors, including sex steroid hormones. Androgens may facilitate initial coronavirus disease 2019 (COVID-19) infection, however, once this occurs, testosterone may have a protective effect. The role of estrogen and progesterone has also been proposed as potential protective factors in COVID-19 infection. Few studies have investigated the role of gender-affirming hormone therapy in COVID-19 infections. Additional research is necessary to enhance our understanding of this relationship and provide better care for transgender patients.
- Citation: Ferraro JJ, Reynolds A, Edoigiawerie S, Seu MY, Horen SR, Aminzada A, Hamidian Jahromi A. Impact of gender-affirming hormone therapy on the development of COVID-19 infections and associated complications: A systematic review. World J Methodol 2022; 12(6): 465-475
- URL: https://www.wjgnet.com/2222-0682/full/v12/i6/465.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i6.465
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent behind the coronavirus disease 2019 (COVID-19) global pandemic, has a wide array of clinical manifestations ranging from asymptomatic to life-threatening disease[1]. Various researchers have worked to elucidate the pathogenic mechanisms underlying these highly variable presentations, with many agreeing that the critical role of the immunological hyper-response (characterized by widespread endothelial damage, complement-induced blood clotting, and systemic microangiopathy) facilitates inflammation and disease progression[2]. Differences in individual responses to systemic inflammation and coagulopathy appear to be modulated by several factors, including sex steroid hormones[2].
Older age and male sex are known risk factors for more severe manifestations of the COVID-19 disease[3-5]. Even after controlling for other risk factors commonly found among men, such as a hypertension, smoking, and cardiovascular disease, the mortality rate of COVID-19 has been shown to be higher in cis-gender males compared with cis-gender females[6]. The molecular basis of the observation can be attributed to transcription of transmembrane protease serine 2 (TMPRSS2), a protease that processes SARS-CoV-2 spike proteins that bind angiotensin converting enzyme 2 (ACE2) receptors and mediate entry of the virus into host cells[7]. The expression of both TMPRSS2 and ACE2 appears to be androgen-mediated[8,9]. For this reason, androgens like testosterone, and other important sex hormones like estrogen and progesterone, have been investigated for their potential role in the age and sex-specific severity of COVID-19[10-12].
Given the risks associated with male sex hormones, patients with gender dysphoria (transgender men or non-binary individuals) who undergo gender-affirming hormone therapy (GAHT) have become another population of interest for exploring the androgen-mediated COVID-19 hypothesis[13]. Through GAHT, transgender women are prescribed natural or synthetic estrogens[14], while transgender men take exogenous testosterone titrated to physiological female range estradiol levels and male-range serum testosterone levels, respectively[15]. For the latter, there is a paucity of data on the safety and health risks associated with long-term testosterone administration in transgender men[16]. As the search for reliable and effective COVID-19 treatments continues, understanding the risks and benefits of GAHT (especially masculinizing treatments) may mitigate COVID-19 related morbidity and mortality in a unique and vulnerable patient population.
The purpose of this study was to perform a systematic review and meta-analysis of the literature pertaining to potential role of GAHT in the development of COVID-19 infections and associated complications.
The current systematic review implemented an algorithmic approach to review all of the available English medical literature on the impact of GAHT on the development of COVID-19 infections using the preferred reporting items for systematic reviews and metanalysis (PRISMA) principles (Figure 1). A comprehensive search of the medical literature in the “PubMed,” “Scopus,” “Reference Citation Analysis (RCA),” “Google Scholar” top 100 results, and previous issues of Plastic and Reconstructive Surgery was performed by two authors (A.R. and S.E.) on January 12, 2022 using the key words “gender” AND “hormone” AND “therapy” AND “COVID-19” as well as associated terms.
The search string was generated, and records that were not specific about GAHT or COVID-19 were excluded. Foreign language (non-English) articles were not eligible for inclusion. Articles published prior to 2019 were excluded as being prior to the COVID-19 pandemic and therefore not relevant to complications associated with COVID-19 infection. Titles and abstracts were screened by two authors (A.R. and S.E.) after which full-text articles were assessed for eligibility and inclusion. On initial and secondary searches, papers in review, commentaries, letters, or those without accessible full-text articles were excluded. References of the selected publications were additionally screened with the aforementioned inclusion criteria.
In total, 14 studies were included in this review per the inclusion/exclusion criteria (Figure 1). Two studies were laboratory-based research (Table 1), while the remaining were clinical studies, including one randomized-control trial (Table 2). Only two studies directly involved and reported on COVID-19 in transgender patients (Table 3).
| Ref. | Study type | Focus | Results |
| Youn et al[37] | In vitro cell line treatment | Investigated potential protective effects of estrogen on endothelial cells against oxidative stress induced by IL-6 and by SARS-COV-2 spike protein (S protein) | 17B-Estradiol reversed S protein induced activation of NADPH oxidase isoform 2 (NOX2) and ACE-2 dependent ROS production, as well as ACE2 upregulation and induction of pro-inflammatory gene monocyte chemoattractant protein-1 (MCP-1) in endothelial cells, effectively attenuating endothelial dysfunction completely |
| Implications: Estrogen inhibits initial viral response and attenuation of cytokine storm induced endothelial dysfunction, especially in men and post-menopausal women. Data supports hypothesis that estrogen may be used to alleviate viral infection and cytokine storm-induced endothelial dysfunction, a critical mediator of ARDS/multi-organ failure. Thus, attenuating disease progression, severity and mortality | |||
| Samuel et al[42] | In vitro stem cell lines and high throughput drug screens | Established a screening strategy to identify drugs that reduce ACE2 levels in human embryonic stem cell (hESC)-derived cardiac cells and lung organoids. Target analysis of hit compounds revealed androgen signaling as a key modulator of ACE2 levels | Inhibitors of 5-alpha reductase, which dampen androgen signaling reduced ACE2 levels in target cells; Treatment with antiandrogenic drugs reduced ACE2 expression and protected hESC-derived lung organoids against COVID-19 infection; Study also found that clinical data on COVID-19 patients with prostate cancer, which is associated with elevated androgen levels, are significant risk factors and that genetic variants that are associated with higher androgen levels are associated with higher diseases severity |
| Ref. | Focus | Predictors or conditions | Sample Population | Outcomes/Findings |
| Ghandehari et al[36] | Los Angeles, California; Effect of progesterone therapy in men with moderate to severe COVID-19 | Randomized control trial | 42 hospitalized men with confirmed moderate to severe COVID-19 | There was a 1.5 point overall improvement in median clinical status score on a seven-point ordinal scale from baseline to day 7 in the progesterone group (n = 18) compared with the control group (n = 22) |
| Experimental Cohort: re received 100 mg of progesterone subcutaneously twice a day for 5 d while hospitalized | ||||
| This study shows that the use of progesterone may help to lower the length of hospital stay, use of supplemental O2 and need for mechanical ventilation | ||||
| Control Cohort: Standard of care | ||||
| Dhindsa et al[12] | Association of concentration of serum sex hormones with COVID-19 Severity | Prospective cohort study | 152 consecutive patients (59% men and 40.8% women) presenting with COVID-19 to the hospital were recruited. Of the participants, 143 (94.1%) were hospitalized. The mean age of participants was 63 yr | Lower testosterone concentrations and increased estradiol to testosterone ratios during hospitalization are associated with disease severity, inflammation, and mortality in men with COVID-19. Men with severe COVID-19 had 65%-85% lower testosterone concentrations compared with men with milder disease course, and was independent of other known risk factors associated with COVID-19 severity |
| van Zeggeren et al[25] | Assess the association between androgen levels and mortality in patients with severe COVID-19 | Observational Case-control study | 16 postmenopausal women (age > 55), and 24 age matched men | Total and free testosterone were lower in deceased men than in survivors. Significantly lower SHBG levels were associated with in both deceased men and women compared with survivors |
| Low SHBG levels were associated with mortality rate in patients with COVID-19 and low total and free testosterone levels were associated with mortality in men. However, whether these hormone levels influence the disease severity, or are a marker of disease severity needs elucidation | ||||
| Seeland et al[31] | Evidence for treatment with estradiol for women with SARS-COV2 infection | Retrospective cohort study | Electronic health record for a large, 68,466 case international COVID-19 cohort | Incidence of SARS-CoV-2 infection is ≥ 15% higher in women than men, but fatality rate is higher is 50% higher in men. Age stratification showed, that while preadolescent men and women had same risk of infection and fatality rate, compared with men of the same age, premenopausal women had a higher risk of infection, but peri and post-menopausal infection rates were similar to men of the same age; -fatality risk for women > 50 yr receiving hormone therapy with estradiol was reduced by > 50% (OR 0.33, HR 0.29) compared with women not receiving HRT. For younger women, (15–49 yr of age) risk of COVID-19 fatality was the same irrespective of estradiol treatment |
| Infante et al[24] | Asses testosterone levels at time of admission with inflammatory state and in-hospital mortality rate | Retrospective cohort study | 40 symptomatic men with confirmed COVID-19 infections admitted to hospital. Patients were divided into two groups, survivors (n = 20), and non-survivors (n = 39) | Low total testosterone levels and elevated E2/T ratios (a marker of aromatase activity) were associated with a hyperinflammatory state. Low testosterone was an independent risk factor for in-hospital mortality |
| Rambhatla et al[27] | Assessed the outcomes of COVID-19 infection in men on testosterone replacement therapy | Retrospective case control study | 32 men diagnosed with COVID-19 on testosterone replacement therapy (TRT) were matched to 63 men with COVID-19 diagnosis but not on TRT | No statistically significant difference in outcome endpoints (hospitalization, ICU admission, ventilator utilizations, thrombotic event, death) between two groups. Results suggest that no statistically significant difference in outcomes for men treated with TRT than men not on TRT. |
| Salonia et al[26] | Evaluated testosterone levels in men with COVID-19 compared with healthy men | Retrospective case control study | 286 symptomatic men with COVID-19 requiring hospital admission Control group: 281 healthy men | Men with COVID-19 had significantly lower serum testosterone levels than healthy men. Lower testosterone levels were independently associated with COVID-19 infection status, and lower levels of testosterone predicted more severe clinical outcomes |
| Ding et al[30] | Examined how menstrual status and sex hormones affect the progression and outcomes of COVID-19 | Retrospective cohort study | All confirmed hospitalized COVID-19 patients from three hospitals (n = 1902). Cohort 1: Sex differences and disease severity (n = 1902); Cohort 2:Women with menstrual status (n = 509)Cohort 3:Serum hormone levels (n = 78), Cytokines levels (n = 263) | Non-menopausal (NM) women had milder severity and better outcomes compared with age match males. Menopausal(M) patients had longer hospitalization times compared with NM patients. -Anti Mullerian hormone (AMH) and estradiol (E2) negatively correlated with infection severity. Menopause is an independent risk factor for female COVID-19 patients, AMH and E2 inversely corelated with COVID-19 severity. Thought to offer protective benefits, E2 specifically through regulation of cytokines related to immune inflammatory response |
| Lee et al[43] | Assessed the effects of female sex hormones on clinical outcomes of COVID-19 using national claims data | Retrospective cohort study | Adult patients with COVID-19 infection (n = 5061). Subgroup analyses using aged matched case-control data | There was no significant difference in mortality rate between males and females, and HRT was not associated with improved clinical outcomes |
| Ref. | Study type | Findings |
| Masterson et al[38] | Prospective Case study | TW patients treated with E+P as part of feminizing GAHT showed reduced testicular ACE-2 R expression in testicular tissue. In comparison to control group (cis-gender males with no hormone therapy) and the TW cohort treated with E only, O+E cohort also had higher degree of tissue fibrosis. Significance: Support the possibility that short course of E+P or P alone could help protect men against COVID-19 infection through downregulation of ACE-2 Receptor |
| Durcan et al[39] | Single center, cross-sectional web-based survey | Of 238 participants (179 FTM, 59 FTM) with GD receiving hormone therapy, the risk of contracting COVID-19 was 3.46x higher in FTM receiving testosterone therapy, compared with FTM patients receiving estrogen and anti-androgen therapies. Furthermore, among the FTM cohort, longer treatment periods with testosterone was associated with increased risk of contracting COVID-19; Significance: TM receiving Testosterone as part of GAHT are at an increased risk for contracting COVID-19 |
The sexual dimorphism seen in COVID-19 morbidity and mortality outcomes has contributed to the hypothesis that the male sex hormone, testosterone, may be an independent risk factor associated with COVID-19 infection and severity, while female sex hormones, estrogen and/or progesterone may endow a protective effect[17,18].
It has been proposed that androgens are needed for initial entry of SARS-CoV-2 into the cell via the activation of TMPRSS2, whose expression is increased by testosterone[8,9]. This gave rise to the theory that higher androgen levels in cis-gender men may account for the higher rates of infection and worse outcomes compared with their cis-gender women counterparts. Likewise, based on this logic, clinical trials have begun to look at the use of anti-androgens and TMPRSS2 inhibitors as prophylactic agents in the setting of SARS-CoV-2 infections[19]. However, once the initial infection occurs, testosterone is hypothesized to have a protective effect by limiting the collection of free radicals in cells and reducing the risk of a cytokine storm and subsequent development of acute respiratory distress syndrome (ARDS)[20]. Taken together, these two androgen dependent theories suggest that while low testosterone may reduce the risk of initial infection, testosterone later protects against more severe forms of disease and may prevent detrimental outcomes in individuals with COVID-19 infections. Further complicating the role of male sex hormones in gender outcome differences, testosterone levels are highly variable among men, with lower testosterone levels seen in men of older age, as well as men with other comorbidities that concurrently increase the risk of COVID-19 severity and morbidity, i.e. type 2 diabetes, chronic lung disease, obesity, and renal insufficiency[20-22]. While the above-mentioned arguments consider endogenous testosterone as a potential factor impacting the risk of SARS-CoV-2 infection, severity, and morbidity associated with COVID-19, whether exogenous hormone consu
Multiple clinical studies have shown that low baseline testosterone levels in men with COVID-19 at the time of admission are associated with worsening outcomes. A recent prospective study by Dhidsa et al[12] found that lower testosterone concentrations and increased estradiol to testosterone (E2/T) ratios (a marker of aromatase inhibitor activity) during hospitalization are associated with disease severity, inflammation, and mortality in cis-gender men with COVID-19. The authors did not specify if there were transgender individuals in their studied population. Men with severe COVID-19 had 65%-85% lower testosterone concentrations compared with men with a milder disease course. Similarly, a retrospective cohort study by Infante et al[24] evaluated men who were admitted with COVID-19 and found that compared with the survivor cohort, non-survivors had a significantly lower testosterone level at time of admission, which was inversely correlated with E2/T ratios and inflammatory marker levels. The study found that low testosterone levels at time of admission were an independent risk factor for in-hospital mortality and may serve as a surrogate marker for disease severity in male patients[24]. In addition, an observational cohort study in the Netherlands found that lower sex hormone binding globulin (SHBG) levels were associated with a higher mortality rate in both men and women, but low testosterone levels were only associated with mortality in men and not women.
The association of low testosterone and worse outcomes in male patients in these studies supports the theory that low testosterone levels may lead to an increase in proinflammatory cytokine markers, facilitating the development of a cytokine storm and subsequent disease severity and morbidity in men with COVID-19. The findings are consistent with a larger case-control study that found lower serum testosterone in men infected with SARS-CoV-2 at time of admission compared with the unaffected controls, and that the level of testosterone on admission was associated with worse outcomes. Interestingly, this study found that in as many as 85% of cases, sex hormone levels were suggestive of secondary hypogonadism[26].
Despite the repeated observed association between low testosterone levels and COVID-19 disease severity in males, it is not clear if low testosterone in males predisposes individuals to COVID-19 infection and increases the chance of higher severity of the disease, or if low testosterone is simply a marker of illness severity. Further studies looking at testosterone levels prior to infection are required to clarify this relationship. Adding to the possible immune role of testosterone levels in COVID-19 infection and disease course, a retrospective case-control study examining the outcomes of COVID-19 infection in men on testosterone replacement therapy (TRT) (n = 32), found no statistically significant difference in outcomes compared with men not on TRT[27]. However, considering the limited number of cases evaluated and the considerable number of potential confounding factors, the study was not powered enough to draw strong and valid conclusions.
Another possible explanation for the dimorphism in outcomes between cis-gender males and females may be that regardless of testosterone levels in men, female sex hormones provide a much greater protection. Thus the higher levels of female sex hormones in cis-gender women may account for the disparity in outcomes between the two sexes.
The role of female sex hormones, including estrogen and progesterone, have also been proposed as potential protective factors contributing to the dimorphism in COVID-19 infection between cis-gender men and cis-gender women. Earlier studies have shown that estrogen plays an important modulatory role in both cellular and humoral immune responses, including causing a reduction in T-cell exhaustion and suppression of inflammatory cytokines[29,30].
In line with the hypothesis of female sex hormones playing a significant protective role, a retro
Researchers investigating the potential protective effects of estrogen on endothelial cells against oxidative stress induced by interleukin (IL)-6 and by SARS-COV-2 spike protein (S protein) demonstrated that in response to S protein or IL-6 exposure of endothelial cells, estrogen inhibits initial viral response and alleviates cytokine storm-induced endothelial dysfunction, a critical mediator in ARDS/multi-organ failure, ultimately attenuating disease progression, severity, and mortality. This lab based research supports the notion that estrogen provides significant protection against COVID-19 in cis-gender females and underlines the potential utility of estrogen administration as a treatment option for COVID-19 to reduce disease severity and improve survival. While not reviewed in this paper, several clinical studies are currently taking place to study the utility of estrogen treatment in infected cis-gender males and females[38].
While several studies have looked at the interplay of hormone and innate hormone levels on cis-gender male and females, less is known about the impact of COVID-19 on individuals undergoing GAHT. Similar to studies that have looked at the protective effects of progesterone and estrogen in cis-gender females, the mechanism of estrogen and progesterone in relation to COVID-19 infection and susceptibility can also be readily studied in the transgender population. A recent study by Masterson and colleagues has been one of the first to examine the impact of feminizing GAHT in transgender individuals being treated for gender dysphoria GD[39]. Transgender women (TW) are routinely treated with estrogen (E) or estrogen plus progesterone (E+P) as part of feminization GAHT. Compared with orchiectomy samples of cis-gender men and TW on E alone, the TW cohort receiving E+P therapy prior to gender-affirming orchiectomy surgery had fewer Leydig cells and less ACE-2 expression when examined with immunohistochemistry. Their findings suggest that P appears to significantly diminish ACE-2 expression in the testes. This reduction in ACE-2 expression helps to support the hypothesis that a short course of exogenous P or E+P therapy may downregulate ACE-2 expression and help offer protection against COVID-19 infection and limit disease severity in cis-gender men and TW undergoing GAHT. While this study demonstrated the differences in ACE-2 expression in gonadal tissues when exposed to P+E therapy, it is unclear if the lower rate of expression in the studied group confers a lower risk of COVID-19 infection and severity, nor is it broadly applicable to the cis-gender population at large. The findings support prior work published by Montopoliet al[39], which showed that men undergoing prostate cancer treatment who received androgen deprivation therapy (ADT) were four times less likely to be diagnosed with COVID-19 compared with those who did not receive ADT. In contrast, a more recent prospective cohort study consisting of 1779 men with prostate cancer found a higher rate of COVID infection in the ADT group (17.1% ADT group vs 5.7% no ADT group), but upon further multivariable analysis did not indicate a difference in infection rate for men treated with ADT compared with no ADT once confounding variables were accounted for (OR 0.93 95%CI: 0.54–1.61, P = 0.8)[40].
In addition to histochemical and laboratory studies on the mechanism of sex hormones, clinical studies looking at the infection rates and outcomes among transgender individuals treated with hormone therapy help to further deepen our understanding of the role of sex hormones. A recent web-based survey evaluation by Durcan et al[13] found that the risk of COVID-19 infection was 3.46 times higher in transgender men (TM), who were receiving testosterone therapy compared with TW, who received estrogen and anti-androgen therapy. In addition, the TM cohort who contracted COVID-19 had a longer androgen therapy treatment history compared with TM patients who did not contract the virus. While these findings suggest that TM individuals who receive androgen therapy as part of GAHT are at greater risk of COVID-19 infection, the study was limited by the small cohort size and retrospective design. Additionally, while the study stated that most patients who contracted COVID-19 did not require hospital admission, further studies looking at the severity of COVID-19 infection and need for ICU admission among transgender individuals undergoing GAHT would further help to demonstrate the risk of COVID-19 and its relationship with the COVID severity and morbidities in patients receiving supplemental androgen therapy (testosterone).
Despite the interest in the use of exogenous hormone therapies to help reduce COVID-19 infection and severity, still, very little research on the impact of COVID-19 on the transgender community and transgender individuals underdoing GAHT is available, as that cohort has been largely overlooked in demographic data, research studies and public health surveillance data collection[41]. While the currently available literature suggests that GAHT has a role in COVID-19 infected individuals, the current small sample sizes and limited understanding make generalizing to the overall transgender community (TM, TW, and non-binary individuals) or cis-gender individuals receiving sex hormone supplement difficult. As of now, the current hypothesis and available data on transgender-identifying individuals suggests that those undergoing MTF HRT (transgender women) may be more protected from becoming infected or suffering from severe COVID-19. In contrast, those who undergo FTM GAHT (transgender men), including androgens, may carry a higher risk. While the above current literature broadly supports this hypothesis, the impact of other biological and behavioral factors, including genetic differences in biological men and women, and higher rates of comorbidities, including smoking and other chronic illnesses in transgender individuals.
Despite the unique opportunity to study hormone therapy and its impact on COVID-19 in this population, only two studies to date have reported on this subject. The studies are generally retro
Transgender care and use of GAHT within this population represents a unique opportunity to study the implications of these treatments, as currently used, in relation to COVID-19 and biological sex. While several clinical trials looking at the use of E+P in COVID-19 infected cis-gender men, the understanding of their role in transgender care is limited. While clinical trials investigating the utility of hormone therapy in COVID-19 may prove useful, studying the effects of GAHT in transgender individuals already taking these medications may prove a more efficient route to understanding the role of hormone therapy in the treatment of COVID-19. Not only would studying transgender individuals in COVID-19 studies help to further broaden our understanding of the role of biologic sex and hormone treatment in disease susceptibility and course, but it would also serve to benefit those in the transgender community, who are often a vulnerable and underserved population within healthcare.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can produce a wide range of clinical manifestations from asymptomatic to life-threatening. Differences in individual responses to systemic inflammation and coagulopathy appear to be modulated by several factors including sex steroid hormones. Androgens may facilitate initial coronavirus disease 2019 (COVID-19) infection. however, once that occurs, testosterone may have a protective effect. Few studies have investigated the role of GAHT in COVID-19 infections. Additional research is necessary to enhance our understanding of this relationship and provide better care for transgender patients.
The role of estrogen and progesterone has also been proposed as potential protective factors in COVID-19 infection.
To investigate the potential role of GAHT in the development of COVID-19 infections and complications.
The current systematic review implemented an algorithmic approach using PRISMA guidelines. PubMed, Scopus, Google Scholar top 100 results, and archives of Plastic and Reconstructive Surgery was on January 12, 2022 using the key words of “gender” AND “hormone” AND “therapy” AND “COVID-19” as well as associated terms.
The database search resulted in the final inclusion of 14 studies related to GAHT COVID-19. Of the included studies, only two studies directly involved and reported on COVID-19 in transgender patients. Several clinical trials looked at the relationship between testosterone, estrogen, and progesterone in COVID-19 infected cis-gender men and women. It has been proposed that androgens facilitate initial COVID-19 infection, however, once that occurs, testosterone may have a protective effect. A number of clinical studies have shown that low baseline testosterone levels in men with COVID-19 are associated with worsening outcomes. The role of female sex hormones, including estrogen and progesterone have also been proposed as potential protective factors in COVID-19 infection. This is exemplified in multiple studies investigating different outcomes in pre- and post-menopausal women as well as those taking hormone replacement therapy. Two studies related specifically to transgender patients and GAHT found that estrogen and progesterone could help protect men against COVID-19, and that testosterone hormone therapy may increase the risk of contracting COVID-19.
Few studies were found related to the role of GAHT in COVID-19 infections. Additional research is necessary to enhance our understanding of this relationship and provide better care for transgender patients.
SARS-CoV-2 can produce a wide range of clinical manifestations from asymptomatic to life-threatening. Differences in individual responses to systemic inflammation and coagulopathy appear to be modulated by several factors, including sex steroid hormones. Androgens may facilitate initial COVID-19 infection, however, once that occurs, testosterone may have a protective effect. The role of estrogen and progesterone has also been proposed as potential protective factors in COVID-19 infection. Few studies have investigated the role of GAHT in COVID-19 infections. Additional research is necessary to enhance our understanding of this relationship and provide better care for transgender patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chavan RP, India; El Sayed S, Egypt S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Lotfi R, Kalmarzi RN, Roghani SA. A review on the immune responses against novel emerging coronavirus (SARS-CoV-2). Immunol Res. 2021;69:213-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 387] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 3. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 4. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2124] [Article Influence: 424.8] [Reference Citation Analysis (0)] |
| 5. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 6. | Sharifi N, Ryan CJ. Androgen hazards with COVID-19. EndocrRelat Cancer. 2020;27:E1-E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83:308-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 8. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14273] [Article Influence: 2854.6] [Reference Citation Analysis (0)] |
| 9. | Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 671] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 10. | Bravaccini S, Fonzi E, Tebaldi M, Angeli D, Martinelli G, Nicolini F, Parrella P, Mazza M. Estrogen and Androgen Receptor Inhibitors: Unexpected Allies in the Fight Against COVID-19. Cell Transplant. 2021;30:963689721991477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Cattrini C, Bersanelli M, Latocca MM, Conte B, Vallome G, Boccardo F. Sex Hormones and Hormone Therapy during COVID-19 Pandemic: Implications for Patients with Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, Mani K, Randolph GJ, Edwards JR, Mudd PA, Diwan A. Association of Circulating Sex Hormones With Inflammation and Disease Severity in Patients With COVID-19. JAMA Netw Open. 2021;4:e2111398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | Durcan E, Turan S, Bircan BE, Yaylamaz S, Demirel O, Demir AN, Sulu C, Kara Z, Sahin S, Taze SS, MefkureOzkaya H, Kadioglu P. TransCOVID: Does Gender-Affirming Hormone Therapy Play a Role in Contracting COVID-19? J Sex Marital Ther. 2022;48:415-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Randolph JF Jr. Gender-Affirming Hormone Therapy for Transgender Females. Clin ObstetGynecol. 2018;61:705-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Moravek MB. Gender-Affirming Hormone Therapy for Transgender Men. Clin ObstetGynecol. 2018;61:687-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Wadman M. Sex hormones signal why virus hits men harder. Science. 2020;368:1038-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Khan N. Possible protective role of 17β-estradiol against COVID-19. J Allergy Infect Dis. 2020;1:38-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | McCoy J, Goren A, Cadegiani FA, Vaño-Galván S, Kovacevic M, Situm M, Shapiro J, Sinclair R, Tosti A, Stanimirovic A, Fonseca D, Dorner E, Onety DC, Zimerman RA, Wambier CG. Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial. Front Med (Lausanne). 2021;8:668698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Pozzilli P, Lenzi A. Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108:154252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 21. | Dhindsa S, Ghanim H, Batra M, Dandona P. Hypogonadotropic Hypogonadism in Men With Diabesity. Diabetes Care. 2018;41:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Dhindsa S, Reddy A, Karam JS, Bilkis S, Chaurasia A, Mehta A, Raja KP, Batra M, Dandona P. Prevalence of subnormal testosterone concentrations in men with type 2 diabetes and chronic kidney disease. Eur J Endocrinol. 2015;173:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. CurrOpinPulm Med. 2012;18:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Infante M, Pieri M, Lupisella S, D'Amore L, Bernardini S, Fabbri A, Iannetta M, Andreoni M, Morello M. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. Eur Rev Med Pharmacol Sci. 2021;25:5889-5903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | van Zeggeren IE, Boelen A, van de Beek D, Heijboer AC, Vlaar APJ, Brouwer MC; Amsterdam UMC COVID-19 Biobank. Sex steroid hormones are associated with mortality in COVID-19 patients: Level of sex hormones in severe COVID-19. Medicine (Baltimore). 2021;100:e27072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AM, Cazzaniga W, Rowe I, Ramirez GA, Tresoldi C, Mushtaq J, Locatelli M, Santoleri L, Castagna A, Zangrillo A, De Cobelli F, Tresoldi M, Landoni G, Rovere-Querini P, Ciceri F, Montorsi F. Severely low testosterone in males with COVID-19: A case-control study. Andrology. 2021;9:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Rambhatla A, Bronkema CJ, Corsi N, Keeley J, Sood A, Affas Z, Dabaja AA, Rogers CG, Liroff SA, Abdollah F. COVID-19 Infection in Men on Testosterone Replacement Therapy. J Sex Med. 2021;18:215-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 616] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 29. | Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 30. | Ding T, Zhang J, Wang T, Cui P, Chen Z, Jiang J, Zhou S, Dai J, Wang B, Yuan S, Ma W, Ma L, Rong Y, Chang J, Miao X, Ma X, Wang S. Potential Influence of Menstrual Status and Sex Hormones on Female Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Cross-sectional Multicenter Study in Wuhan, China. Clin Infect Dis. 2021;72:e240-e248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 31. | Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, Preissner R, Preissner S. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 32. | Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128-133. [PubMed] |
| 33. | Buyon JP, Korchak HM, Rutherford LE, Ganguly M, Weissmann G. Female hormones reduce neutrophil responsiveness in vitro. Arthritis Rheum. 1984;27:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3418] [Article Influence: 683.6] [Reference Citation Analysis (0)] |
| 35. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1960] [Article Influence: 392.0] [Reference Citation Analysis (0)] |
| 36. | Ghandehari S, Matusov Y, Pepkowitz S, Stein D, Kaderi T, Narayanan D, Hwang J, Chang S, Goodman R, Ghandehari H, Mirocha J, Bresee C, Tapson V, Lewis M. Progesterone in Addition to Standard of Care vs Standard of Care Alone in the Treatment of Men Hospitalized With Moderate to Severe COVID-19: A Randomized, Controlled Pilot Trial. Chest. 2021;160:74-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Youn JY, Zhang Y, Wu Y, Cannesson M, Cai H. Therapeutic application of estrogen for COVID-19: Attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol. 2021;46:102099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Masterson JM, Bui C, Zhang Y, Yuan X, Huynh C, Jawanda H, Hasan W, Tourtellotte W, Luthringer D, Garcia MM. Feminising hormone therapy reduces testicular ACE-2 receptor expression: Implications for treatment or prevention of COVID-19 infection in men. Andrologia. 2021;53:e14186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Klein EA, Li J, Milinovich A, Schold JD, Sharifi N, Kattan MW, Jehi L. Androgen Deprivation Therapy in Men with Prostate Cancer Does Not Affect Risk of Infection with SARS-CoV-2. J Urol. 2021;205:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Wozniak RJ, Nixon DF, Marston JL. Involvement of Cisgender and Transgender Individuals in Studies on the Impact of Hormonal Therapy on COVID-19. AIDS Patient Care STDS. 2020;34:367-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Samuel RM, Majd H, Richter MN, Ghazizadeh Z, Zekavat SM, Navickas A, Ramirez JT, Asgharian H, Simoneau CR, Bonser LR, Koh KD, Garcia-Knight M, Tassetto M, Sunshine S, Farahvashi S, Kalantari A, Liu W, Andino R, Zhao H, Natarajan P, Erle DJ, Ott M, Goodarzi H, Fattahi F. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell. 2020;27:876-889.e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 43. | Lee JH, Kim YC, Cho SH, Lee J, You SC, Song YG, Won YB, Choi YS, Park YS. Effect of sex hormones on coronavirus disease 2019: an analysis of 5,061 laboratory-confirmed cases in South Korea. Menopause. 2020;27:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |