Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.88
Peer-review started: June 29, 2015
First decision: August 10, 2015
Revised: September 24, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 12, 2015
Processing time: 175 Days and 0.9 Hours
AIM: To integrally understand the effects of human vascular endothelial cells (VECs) on the proliferation of vascular smooth muscle cells (VSMCs).
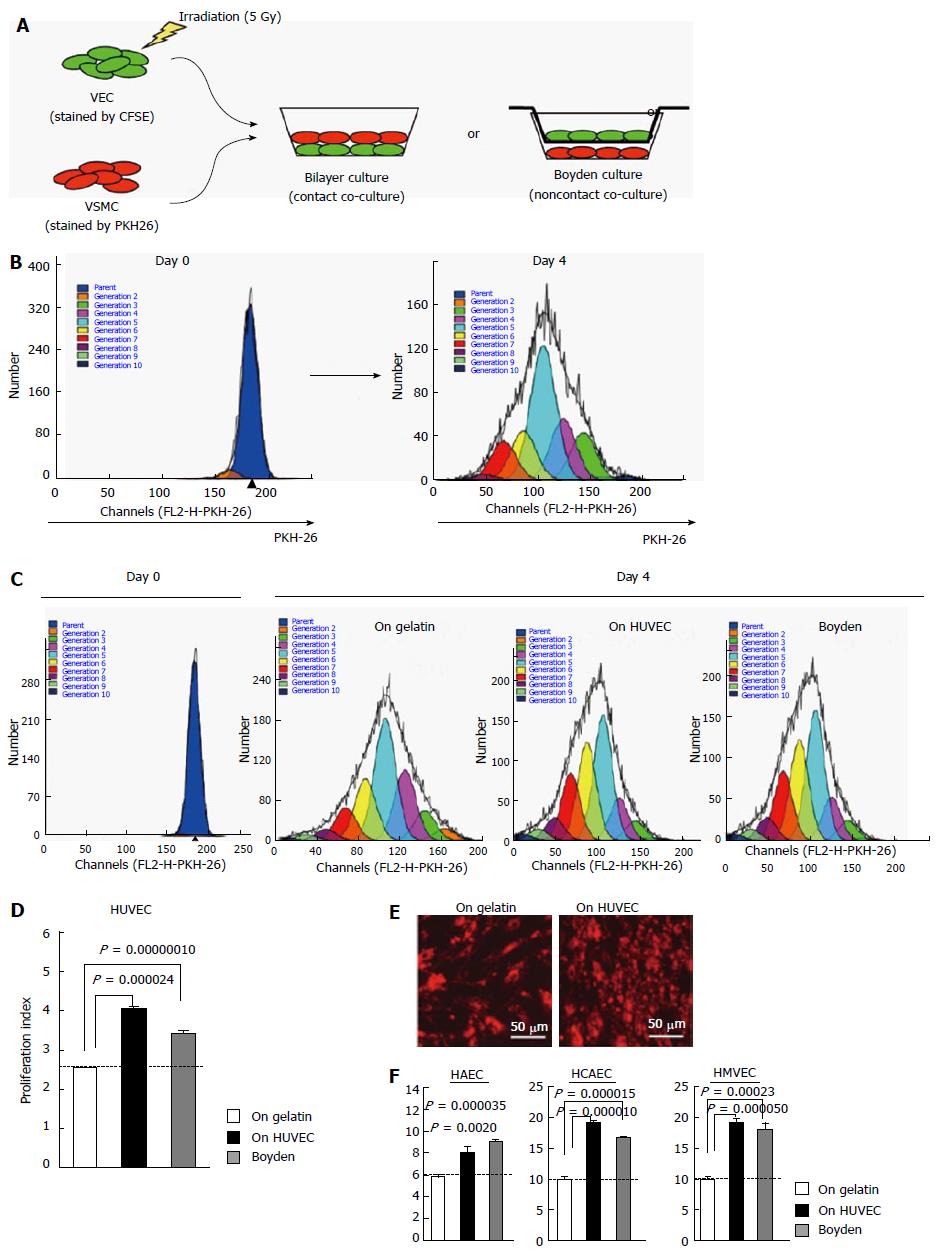
METHODS: Various kinds of human VECs of different origins were co-cultured with human aortic smooth muscle cells, a representative of human VSMCs. To exclude the irrelevant effects due to growth competition between VECs and VSMCs, the proliferation of VECs had previously been arrested via a low-dose gamma ray irradiation. To discriminately analyze the proliferation of VSMCs from that of VECs, the former cells were labeled with red fluorescent dye while the latter cells were labeled with green fluorescent dye before performing co-culture experiments. After 4 d, total cells were harvested and subjected to flow cytometric analyses. Decrements in red fluorescence intensities due to proliferation-mediated dilutions were measured and mathematically processed using a specific software to quantitatively evaluate the proliferation of VSMCs. The findings obtained from the flow cytometry-based analyses were further validated by microscopic observations.
RESULTS: Commercially available primary cultured human VECs exclusively promoted VSMC proliferation regardless of their tissue origins and we termed these pro-proliferative VECs as “type-I”. By contrast, VECs freshly generated from human bone marrow-derived endothelial progenitors cells or human pluripotent stem cells including embryonic stem cells and induced pluripotent stem cells suppressed VSMC proliferation and we termed these anti-proliferative VECs as “type-II”. Repetitive subcultures as well as oxidative stress induced “type-II VECs to type-I” conversion along with an induction of Regulator of G-protein signaling 5 (RGS5). Compatibly, anti-oxidant treatments suppressed both the subculture-dependent “type-II to type-I” conversion and an induction of RGS5 gene. Immunostaining studies of clinical specimens indicated that RGS5 protein expressions in endothelial layers were low in normal arteries but they were up-regulated in pathological arteries including hypertension, atherosclerosis and autoimmune vasculitis in a dose-dependent manner. Overexpression and knockdown of RGS5 caused that “type-II to type-I” and “type-I to type-II” phenotype conversions of VECs, respectively.
CONCLUSION: Human VECs are categorized into two types: pro-proliferative RGS5high VECs (type-I) and anti-proliferative RGS5low VECs (type-II).
Core tip: There is a longstanding controversy over the effects of vascular endothelial cells (VECs) on the proliferation of vascular smooth muscle cells (VSMCs). Since the controversy came from lack of systematic studies, we performed an integrated analysis using various human VECs to quantitatively evaluate their effects on VSMC proliferation. Here we report that: (1) human VECs are classified into two groups: pro-proliferative (type-I) vs“anti-proliferative” (type-II); (2) oxidative stress and ageing induced “type-II to type-I” conversion; and (3) RGS5 is the responsible gene for VEC phenotype determinations. Thus, human VECs are categorized into pro-proliferative RGS5high (type-I) and anti-proliferative RGS5low (type-II) VECs.
- Citation: Nishio M, Nakahara M, Sato C, Saeki K, Akutsu H, Umezawa A, Tobe K, Yasuda K, Yuo A, Saeki K. New categorization of human vascular endothelial cells by pro- vs anti-proliferative phenotypes. World J Transl Med 2015; 4(3): 88-100
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/88.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.88
Ischemic diseases are caused by stenosis of the artery (i.e., arteriostenosis). Although cholesterol medications have produced remarkable results by reducing the rate of atherosclerosis, there still remain problems including medication-resistant patients and cases with restenosis after stent therapies. It is well known that the pathological basis of arteriostenosis is the hyperproliferation of vascular smooth muscle cell (VSMC). However, roles for vascular endothelial cells (VEC) in the development of arteriostenosis remain elusive. Controversial ideas have been raised regarding the effects of VECs on the proliferation of VSMC. From a clinical standpoint, it has been suggested that VECs prevent the proliferation of VSMC because VSMC proliferation is usually observed under circumstances where VECs are lost or dysfunctional. In accordance with this idea, VECs of hypertensive rats, but not those of normal counterparts, reportedly promote VSMC proliferation[1]. On the other hand, in vitro co-culture experiments using fetal human umbilical vein endothelial cells (HUVEC) and bovine aortic smooth muscle cells showed that VECs enhanced VSMC proliferation[2]. Nevertheless, this finding requires detailed validation. First, it must be re-validated by co-culture experiments using the cells of the identical species (e.g., human VSMCs and human VECs). Secondly, it must be re-checked by co-culture experiments using adult samples because dynamic vasculogenesis with active VSMC proliferations occurs during fetal development. In addition, physiological events during the fetal development often resemble to pathological events in the adult life such as fetal gene expression profiles in angiosarcoma[3], coronary artery disease[4], diabetic retinopathy[5] and arteriostenosis[6] in adults. Thirdly, it must be re-validated by co-culture experiments using freshly produced VECs because characters of the cells often change during the process of ex vivo subcultures. For example, the quality of ex vivo-expanded primary cultured human cells becomes considerably lower than that of freshly differentiated human embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC)[7].
To address all those issues, we performed VEC/VSMC co-culture experiments using various kinds of human VECs of diverse origins. Furthermore, we performed microarray analyses to identify the key gene that determines the phenotypes of human VECs.
HUVEC, human neonatal dermal microvascular endothelial cells (HMVEC), human adult aortic endothelial cells (HAEC) and human adult coronary arterial endothelial cells (HCAEC) were purchased from Dainippon Sumitomo Pharma Co., Ltd. (Osaka Japan). Human endothelial progenitors cells (EPCs) were provided as follows: Two lots of human adult bone marrow mononuclear cell-derived endothelial progenitor outgrowth cells (EPOCs) at 4th passage were purchased from BioChain Institute, Inc., Hayward, CA. Two lots of human umbilical cord blood endothelial colony forming cells (ECFC) were purchased from Lonza Group Ltd., Basel, Switzerland, and one lot of human cord blood-derived EPOCs at 4th passage, were purchased from BioChain Institute, Inc. The cells were cultured on 0.1% gelatin-coated plates using EGM®-2 BulletKit (Lonza Group Ltd. Basel, Switzerland). Human aortic smooth muscle cells of different donors were purchased from Lonza Group Ltd. (Basel, Switzerland) and cultured using SmGM™-2 BulletKit™ (Lonza Group Ltd.). Cells were re-seeded at split ratios of 1:3-1:4 twice a week. VECs within 8th passage were used in all experiments. The hESC lines (KhES-1, -3, -5) were established by the Institute for Frontier Medical Science, Kyoto University[8]. SeV-hiPSCs were established from HUVEC[9] and BJ fibroblast[9] by using iPS-Tune™ (ID Pharma Co., Ltd., Ibaraki, Japan). Ret-hiPSC lines were provided as follows: 253G1[10] and 201B7[11] were provided by CiRA at Kyoto University; #25 was provided by National Research Institute for Child Health and Development as used elsewhere[7]. Astaxanthin (A3236, Sigma-Aldrich Co. LLC. St. Louis, MO 63178, United States) was dissolved by DMSO at the concentration of 5 mmol/L. Frozen sections of clinical specimens were purchase from BioChain Institute, Inc.: normal human artery (Cat: T5595-4763 Lot: L11052523C11052523), arteries of hypertension patients (Cat: T1236013Hd-2 Lot: B502175), arteries of systemic lupus erythematosus (Cat: T1236013LUP Lot: A804253), arteries of arteriosclerosis (Cat: T1236013Hd-4 Lot: B502176).
VECs were -irradiated (5 Gy) and stained with carboxyfluorescein diacetate, succinimidyl ester by using CFSE Cell Division Assay Kit (Cayman Chemical Co., Ann Arbor, MI), while VSMCs were stained with PKH26 by using PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich Co. LLC., St. Louis, MO 63178) according to the manufacturer’s guidance. For contact co-culture, irradiated and CFSE-stained VECs were seeded at the density of 2 × 105 cells/well on 0.1% gelatin-coated 24-well culture plates, and on the following day, PKH26-stained VSMCs were seeded at the density of 3.75 × 103 cells/well on VEC layers or gelatin layers as control. After 4 d, total cells were harvested and subjected to flow cytometry analyses by FACSCaliburTM (BD Biosciences, San Jose, CA) and FL1 and FL2 fluorescence intensities were measured by CellQuest™ Pro software (BD Biosciences). FL2 (PKH26) fluorescence intensities were further analyzed mathematically by ModFit LT™ software (Verity Software House Inc., Topsham, ME) to calculate the proliferation index. Regarding experiments on astaxanthin treatments, VECs were stained by PKH26 and VSMCs were treated with CFSE because of red colored-fluorescence interference by astaxanthin.
Total RNAs were isolated by using TRIzoL® Reagent (Life Technologies, Inc., Grand Island, NY) and subjected to GeneChip® Gene 1.0 ST array (Affymetrix, Inc., Santa Clara, CA, United States) by Pharma Frontier Co. Ltd. (Tokyo, Japan). Alterations in gene expressions were estimated as significant if > 2.0-fold increments or < 0.5-fold decrements in signal intensities were detected. Functional analyses were performed using IPA® Ingenuity Pathways Analysis software ver. 14855783 (Ingenuity Systems, Inc. Redwood City, CA) and hierarchical clustering was executed using GeneSpring GX 12.0 software (Agilent Technologies, Santa Clara, CA). All analyses were performed by Chemicals Evaluation and Research Institute (CERI, Tokyo, Japan).
Total RNA was extracted from VECs using TRIzol® Reagent (Life Technologies, Inc.). First strand DNA was synthesized by using SuperScriptTMIII First-Strand Synthesis System kit (Life Technologies, Inc.). PCR was performed using GeneAmp® PCR system 9700 (Life Technologies, Inc) and Ex-Taq (Takara Shuzo Co. Ltd., Shiga, Japan) with a following program: the initial denaturation at 94 °C for 5 min, 24-28 cycles and 20-22 cycles of amplification process for RGS5 and -actin, respectively, renature (55 °C, 30 s), extension (72 °C, 30 s) and denature (94 °C, 30 s) with a final extension (72 °C, 10 min). Primers used for RGS5 were Fw: CTGGATTGCCTGTGAGGATT and Rv: TCAGGGCATGGATTCTTTTC and those for b-actin were Fw: GCAGGAGATGGCCACGGCGGC Rv: TCTCCTTCTGCATCCTGTCAGC. The RT-PCR products were subjected to 1.5% agarose gel electrophoresis and the amplified DNA bands were visualized by ethidium bromide staining.
Total RNA was extracted from VECs using TRIzol® Reagent (Life Technologies, Inc.). Complementary DNA was prepared from 1 g of RNA using SuperScriptTMIII First-Strand Synthesis System kit (Life Technologies, Inc.), and used in quantitative PCR reactions with FAST SYBR® Green Master Mix (Applied Biosystems® from Life Technologies, Inc.). qRT-PCR was performed using the StepOnePlusTM PCR machine (Applied Biosystems® from Life Technologies, Inc.). Primers used for RGS5 were Fw: GGAGGCTCCTAAAGAGGTGA and Rv: GGGAAGGTTCCACCAGGTTC, and primers used for GAPDH were Fw: CCACTCCTCCACCTTTGAC and Rv: ACCCTGTTGCTGTAGCCA.
Cultured cells and clinical specimens were fixed by methanol/acetone (1:1) for 10 min on ice. The 1st antibody reactions were performed by using a 1:200-diluted chicken polyclonal anti-human RGS5 antibody (ab14265, Abcam plc., Cambridge, United States) and/or a 1:50-diluted rabbit polyclonal anti-human PECAM antibody (sc-8306, Santa Cruz Biotechnology Inc.) and the 2nd antibody reactions were performed by using an Alexa Fluor® 594-conjugated goat anti-chicken IgG (A11042, Life Technologies, Inc) and/or Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A11008, Life Technologies, Inc). Photomicrographs were taken by Olympus BX51 Fluorescence Phase contrast Microscope (Olympus Optical Co. Ltd.) equipped with DP-2 TWAIN digital camera system (Olympus Optical Co. Ltd.) and cellSens® standard imaging software (Olympus Optical Co. Ltd.). Regarding clinical specimens, white balance was adjusted so that autofluorescence from lamina elastic became white.
The 1 × 105 VECs were lysed by using 20 μL sample buffer solution [(2ME+) (× 2), (Cat. 196-11022) and (WAKO Pure Chemical Industries, Osaka, Japan)]. The first antibody reaction was performed by using a 1:1000-diluted anti-human RGS5 antibody (ab83230, Abcam, Cambridge, United States) or a 1:1000-diluted anti-human b-tubulin antibody(sc-9104, Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States)and the second antibody reaction was performed by using a 1:2000-diluted anti-rabbit IgG HRP-linked antibody (#7074S) (Cell Signaling Technology, Inc.).
Expression vectors for shRNA against RGS5 were purchased from OriGene Technologies Inc. (Rockville, MD, United States). A Homo sapiens cDNA, FLJ96402, which corresponds to Homo sapiens regulator of G-protein signaling 5 (RGS5), transcript variant 1, mRNA (NM_003617.3), with two nucleotide substitutions, was purchased from National Institute of Technology and Evaluation (Tokyo, Japan), and the two substituted nucleotides were corrected by using KOD-Plus-Mutagenesis Kit (Toyobo Co. Ltd., Osaka, Japan) to become identical to the nucleotide sequences in NM_003617.3. The RGS5 cDNA was inserted into pmaxCloning™ expression vector (Lonza Group Ltd. Basel, Switzerland). Transfection was performed by using a Nucleofector™ (Lonza Group Ltd., Basel, Switzerland) according the manufacturer’s guidance. The Amaxa HUVEC Nucleofector Kit (#VPB-1002, Lonza Group Ltd.) was used for HUEVC and the Amaxa Basic Nucleofector Kit Primary Endothelial Cells (#VPI-1001, Lonza Group Ltd.) was used for EPCdECs, hESdECs and hiPSdECs.
Experiments were performed independent three experiments (n = 3) and the data were analyzed according Student t test. Results were shown as averages (AV) ± SD.
The effects of VECs on the proliferation of VSMCs were quantitatively evaluated by a flow cytometry-based technique (Figure 1A and B). Briefly, “contact” or “non-contact” (i.e., Boyden) co-culture experiments were performed using various kinds of human VECs, whose growths were previously arrested by a low dose gamma ray irradiation, and adult human VSMCs. After 4 d, VSMC proliferation was assessed by mathematically processing the reduction degree of the red fluorescence intensity using ModFit LT™ software.
First, the effects of commercially available primary cultured VECs were examined. As previously reported[2], the proliferation of VSMCs was up-regulated by both “contact” and “non-contact” co-cultures with HUVEC (Figure 1C and D), indicating that HUVEC enhanced VSMC proliferation via a soluble factor(s). These findings were confirmed by microscopic observations (Figure 1E). Similar results were obtained from HAEC, HCAEC and HMVEC (Figure 1F). Thus, commercially available primary cultured human VECs exclusively enhance VSMC proliferation via a soluble factor(s).
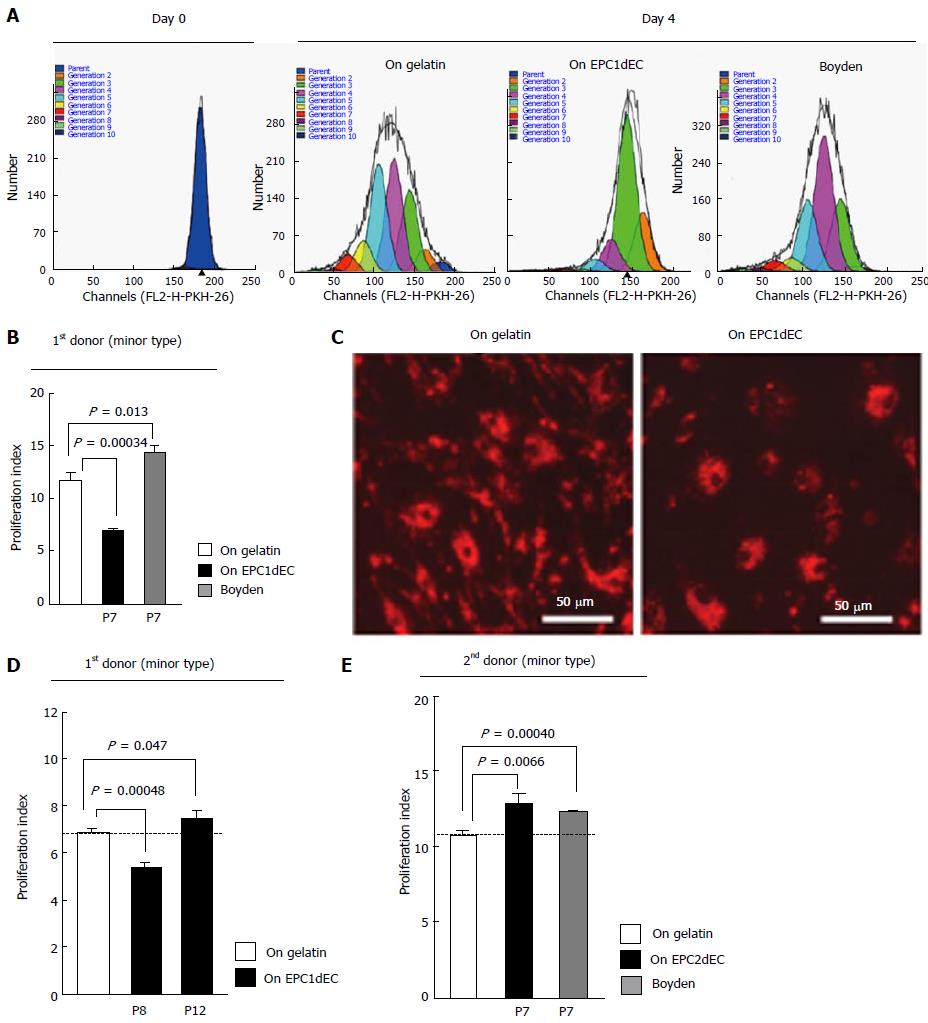
We next evaluated the effects of VECs that were produced from commercially available human (EPCs). Adult bone marrow mononuclear cell-derived EPCs at passage 4 were purchased. Then, EPC-derived mature VECs (EPCdECs) were prepared after additional three passages using a specialized medium (totally at passage 7) and subjected to co-culture experiments. Surprisingly, EPCdECs of the first donor (EPC1dEC) suppressed the proliferation of VSMCs under contact co-culture (Figure 2A and C). By contrast, EPC1dEC enhanced VSMC proliferation under non-contact culture (Figure 2A, right panel; Figure 2B, gray column), indicating that a potent growth-inhibitory activity, which was stronger than the growth-promoting activity of the soluble factor, was transmitted via cell-cell interactions. We confirmed that EPC1dEC purchased as an independent package after a half year provided similar findings (data not shown), guaranteeing the high reproducibility of our assay system. Interestingly, anti-proliferative potentials of EPC1dEC were considerably attenuated at passage 8 (Figure 2D, middle column) and finally nullified at passage 12 (Figure 2D, right column), indicating that the anti-proliferative capacity is susceptible to subculture-dependent stresses. In addition, anti-proliferative potentials could not be detected when fixed VECs were used (data not shown), indicating that living VECs were required for transmitting the anti-proliferative capacity. We termed VECs with anti-proliferative capacities as “type-II”, whereas we termed VECs with pro-proliferative capacities as “type-I”.
We further examined the effects of EPCdECs of different donors. In contrast to EPC1dECs, donor 2 EPC-derived VEC (EPC2dEC) showed type-I phenotype from the earliest phase (i.e., at passage 7) (Figure 2E). Moreover, all the other commercially available EPCdECs were exclusively type-I VECs (data not shown). Thus, EPCs that could produce type-II VECs belonged to a rather rare population among commercially available EPC sources. We also examined the phenotypes of VECs generated from fetal umbilical cord-derived EPCs (UCEPCdECs) and found that they were exclusively “type-I” VECs (data not shown), reflecting dynamic vasculogenesis during fetal development.
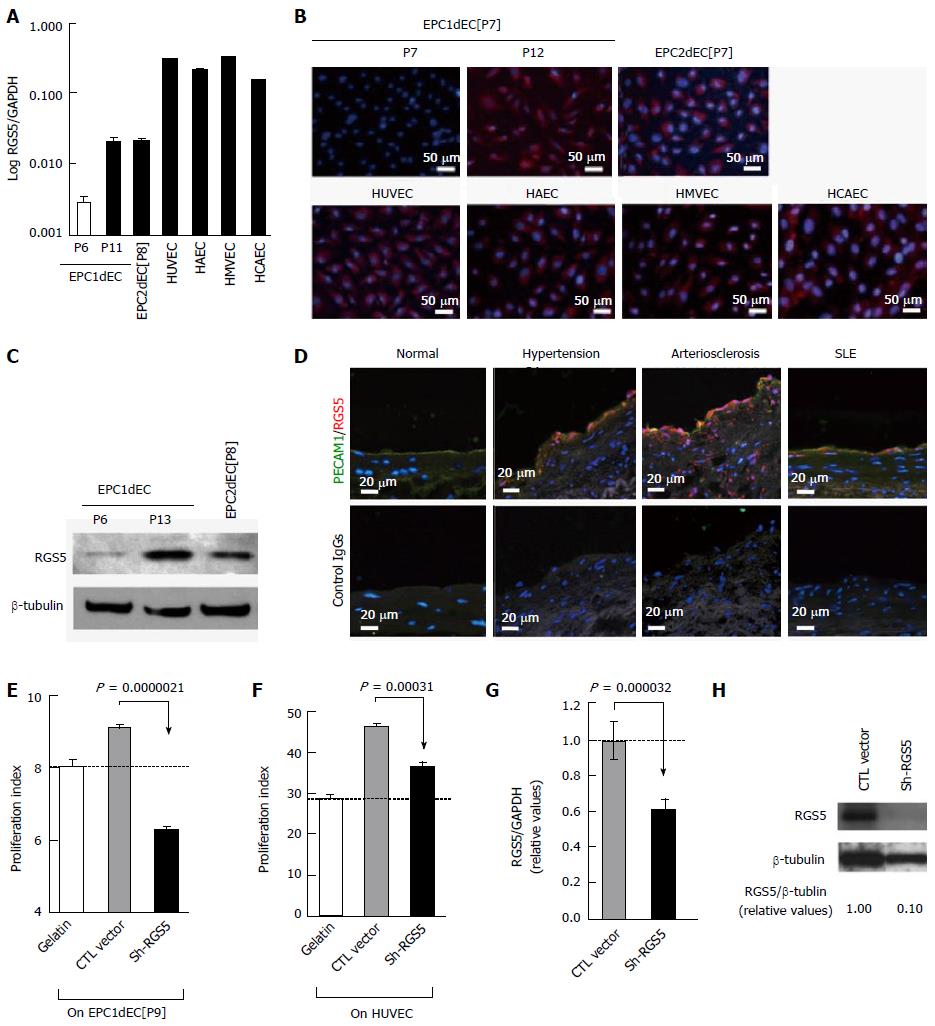
To determine the responsible gene for “type-I vs type-II” phenotyping of human VECs, microarray analyses were performed using “type-II” VECs, which were EPC1dEC[P7] of the two independent packages, and “type-I” VECs, which were HUVEC, HAEC, HMVEC, EPC1dEC[P12], EPC2dEC[P7] and UCEPC1dEC (GEO Accession ID: GSE60999). We found that regulator of G-protein signaling 5 (RGS5) was the only gene that showed a discriminating expression pattern between “type-II” and “type-I” VECs. The results of the microarray were confirmed by quantitative RT-PCR (qRT-PCR): About one-order up-regulations of RGS5 expressions in “type-I” EPC1dEC[P11] and EPC2dEC[P8] and almost two-order up-regulations of RGS5 expression in HUVEC, HAEC and HMVEC compared to EPC1dEC[P6] (Figure 3A). Immunostaining (Figure 3B) and Western blotting (Figure 3C) further confirmed the findings. In addition, clinical relevance of RGS5 induction was evidenced by immunostaining studies using human specimens: Undetectable expression in normal subjects, mild inductions in patients suffering from hypertension or systemic lupus erythematosus and high-level inductions in patients with arteriosclerosis (Figure 3D). In the specimen of arteriosclerosis, RGS5 protein was even detected in neointima especially at perimeters of vasa vasorum, which were recognized as hollow spaces. The distribution pattern of RGS5 was highly analogous to that of oxidative stress-damaged cells[12], suggesting that oxidative stress is one of the major triggers of the “type-II to type-I” conversion of VECs.
The involvement of RGS5 in the phenotype conversion of VECs was verified by gene knockdown studies. As shown in Figure 3E, “type-I” EPC1dEC[P9] was converted into “type-II” VECs by an introduction of shRNA-RGS5 expression vector (Figure 3E). Regarding HUVEC, pro-proliferative capacities were lowered by RGS5 knockdown (Figure 3F). Although a shRNA-RGS5 introduction into HUEVC effectively lowered the levels of RGS5 message expression (Figure 3G) and protein expression (Figure 3H), its effect on phenotype alteration was rather mild probably due to particularly high RGS5 expression in HUVEC (Figure 3A). Nevertheless, the results were reproducible when distinct shRNA-RGS5 with different nucleotide sequences were introduced (data not shown). Thus, RGS5 is the causative gene for the “type-II to type-I” conversion of VECs.
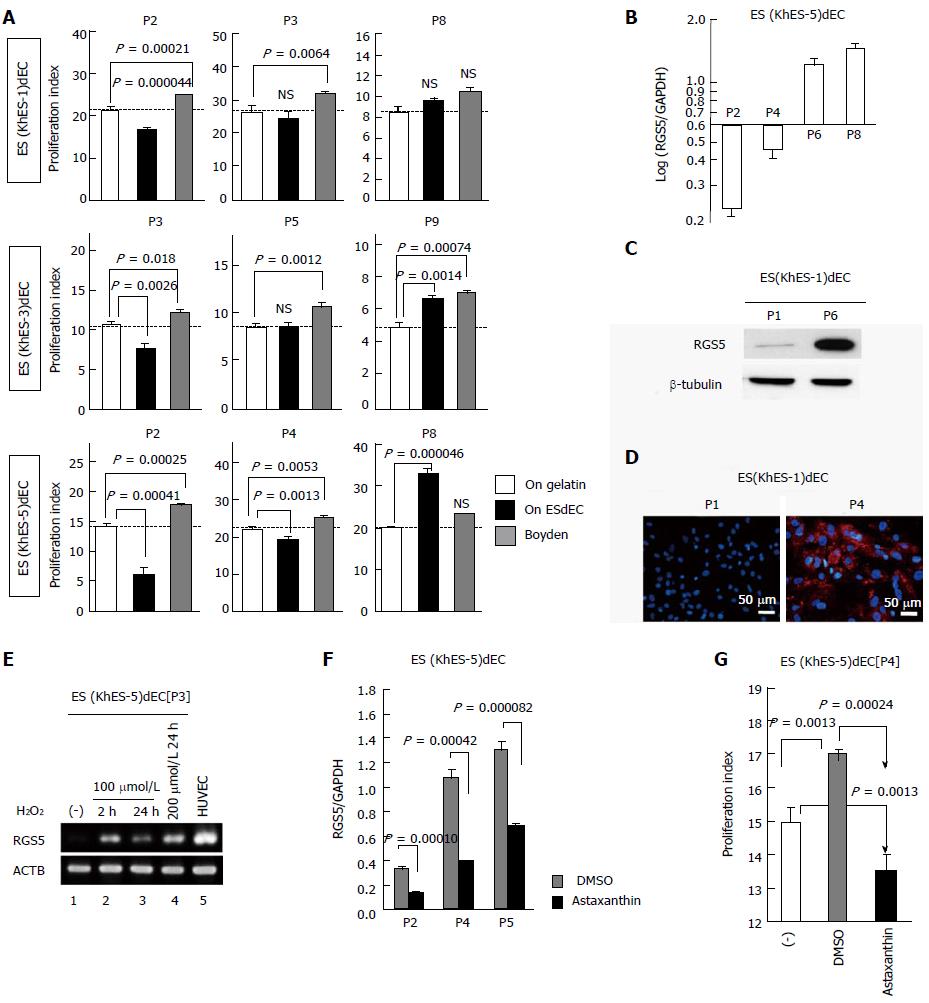
Although we discovered the existence of “type-II” VECs for the first time in the world, their applications were highly limited because EPC1dEC[P7] could not be expanded without losing type-II phenotype and because commercially available EPCdECs of other donors were exclusively type-I VECs. To find an alternative source for the production of type-II VECs, we examined the phenotypes of VECs that were produced from human embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). High-purity subculturable VECs were generated without a contamination by mural cells based on our previously reported method[13,14]. We found that human ESC-derived VECs (ESdECs) showed exclusively “type-II” phenotypes at their early passages; however, they were converted into “type-I” VECs after a few rounds of subcultures (Figure 4A) with up-regulated expressions of RGS5 message (Figure 4B) and protein (Figure 4C and D) although the timing of conversion differed depending on lines. Because an involvement of oxidative stress in RGS5 induction was suggested by the similarity in tissue-distributing profiles between RGS5-positive cells (Figure 3D) and oxidative stress-damaged cells[12], we examined the effect of hydrogen peroxide treatments on RGS5 gene expression. We found that hydrogen peroxide treatments induced RGS5 expressions in type-II ESdECs (Figure 4E) and a treatment with astaxanthin, which is the most potent anti-oxidant whose singlet oxygen-quenching activity[15] and its anti-lipid peroxidation activity are reportedly superior to vitamin E by two-order[16], suppressed RGS5 inductions (Figure 4F). Moreover, astaxanthin treatment significantly delayed the timing of “type-II to type-I” conversion (Figure 4G), suggesting that oxidative stress is one of the major causes of RGS5 induction.
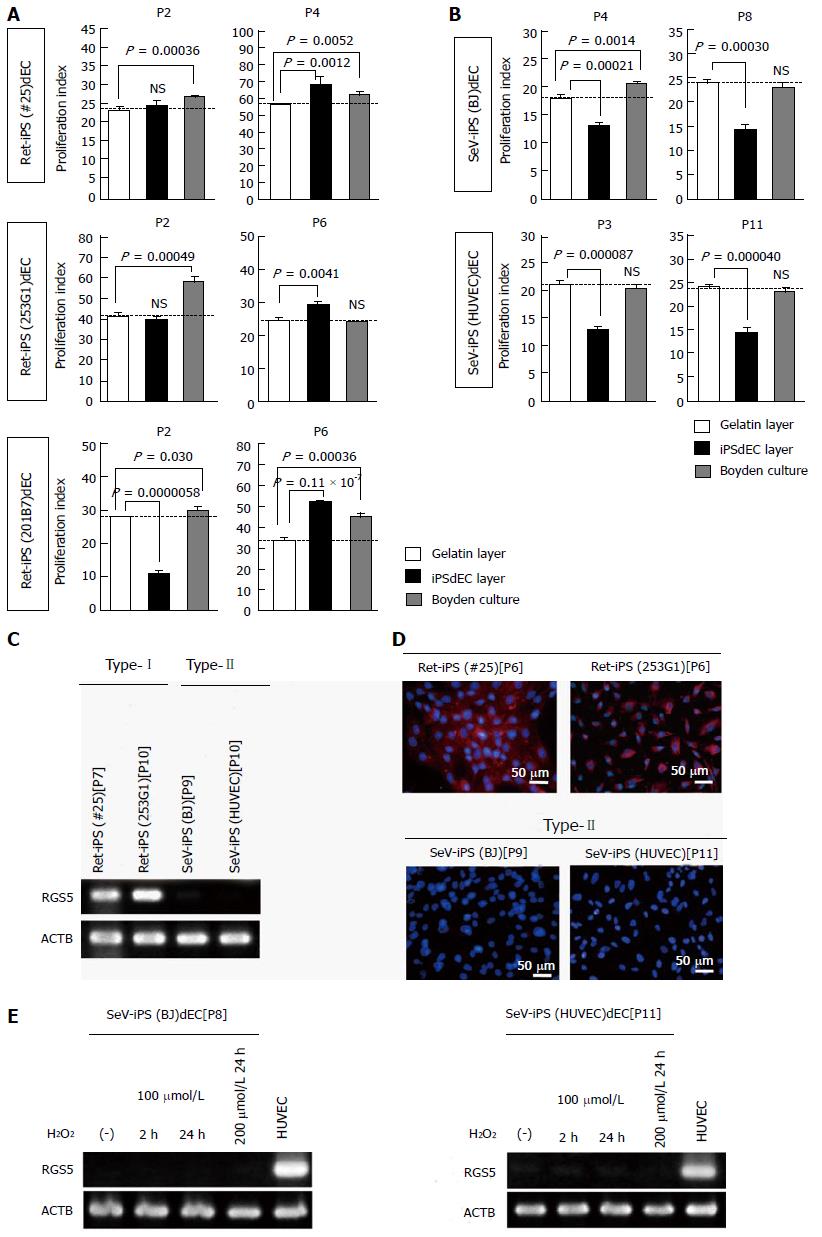
Because astaxanthin treatment could not completely block RGS5 induction in type-II ESdECs after repetitive subcultures (Figure 4F), we searched for still other candidates for type-II VECs. Since the timing of the “type-II to type-I” conversion was latest in the VECs produced from KhES-5, which was established latest among the three lines of human ESCs, we hypothesized that “the more recently human iPSC lines are established, the more stably type-II phenotype will be maintained”. Since we recently established two lines of Sendai virus vector-based iPSCs (SeV-iPSCs) from HUVEC and BJ fibroblast[9], we produced VECs from these SeV-iPSCs (SeV-iPSdECs) and examined their characters. At the same time, we generated VECs from widely distributed conventional retrovirus vector-based iPSCs (Ret-iPSCs) including #25[7,17], 253G1[7,17,18] and 201B7[7,17,19,20] and examined the phenotypes of these Ret-iPSC-derived VECs (Ret-iPSdECs). Regarding #25 and 253G1, anti-proliferative potentials were undetectable even at early passages (Figure 5A, upper and middle); nevertheless, VSMC proliferations were suppressed under contact co-culture conditions compared to non-contact co-culture conditions. Therefore, weak anti-proliferative potentials were transmitted from these Ret-iPSdECs. Regarding 201B7, anti-proliferative potentials were clearly detected at early passages (Figure 5A, lower) as in the case of ESdECs (Figure 4A). In all three lines of Ret-iPSdECs, pro-proliferative capacities were augmented after repetitive subcultures (Figure 5A). Thus, comparable results were obtained from Ret-iPSdECs to ESdECs as a whole. On the other hand, SeV-iPSdECs well preserved anti-proliferative capacities until later passages (Figure 5B) without an induction of RGS5 gene (Figure 5C and D). They also showed high resistance to oxidative stress-induced RGS5 induction (Figure 5E), supporting the idea that the most recently established human pluripotent stem cells provide the most effectual type-II VECs.
Collectively, human pluripotent stem cells provide an excellent source for the production of anti-proliferative type-II VECs.
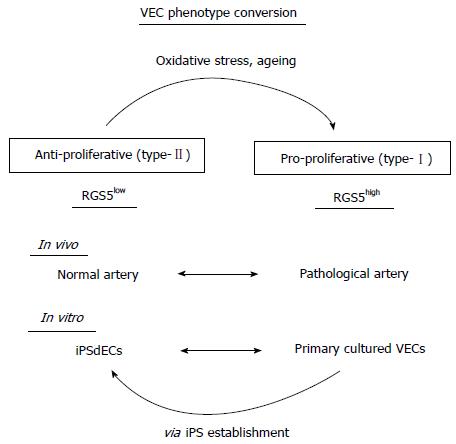
In the current study, we presented a new concept for the categorization of human VECs based on their effects on VSMC proliferation: pro-proliferative RGS5high VEC (type-I) and anti-proliferative RGS5low VECs (type-II) (Figure 6). Since oxidative stresses and subculture-dependent mechanochemical stresses induced “type-II to type-I” conversion along with an induction of RGS5 expression, “type-I” RGS5high VECs may well be regarded as degenerative VECs. It might be shocking that widely used commercially available primary cultured human VECs exclusively belong to type-I VECs. However, there is a rational reason for this idea because primary cultured cells have inevitably received multiple kinds of stresses during the process of their preparations including tissue removal, cell dissociation and cell expansion. It is almost impossible for them to preserve every character that they had in in vivo environments. Indeed, commercially available primary cultured human VECs preserve fundamental functions of VECs such as cord-forming activities and acetylated low density lipoprotein (Ac-LDL)-up-taking capacities; however, they may have possibly lost certain sophisticated functions before they are on the distribution routes. The type-II anti-proliferative capacity may be one of such refined functions.
We have also shown that RGS5 is a causative gene for “type-II to type-I” conversion. Because EPC2dEC at passage 7 (EPC2dEC[P7]) was larger in size and showed longer doubling time than EPC1dEC passage 7 (EPC1dEC[P7]) (data not shown), EPC2dEC might be in ageing states. This idea was supported by the cluster analysis of the microarray data (GEO Accession ID: GSE61000): EPC2dEC[P7] was located closer to EPC1dEC[P12] than EPC1dEC[P7] (data not shown) and the gene function item “senescence” marked the highest value in the matching rate (data not shown). Thus, the “type-II to type-I” phenotype conversion may well be considered as an ageing-associated degeneration. Nevertheless, “type-II to type-I” conversion is not an identical concept to senescence because type-I primary cultured human VECs show no signs of senescence. Rather, it should be considered as a sign of degeneration. A clinical study supported this idea, showing that VECs of the subcutaneous vessels of normal individuals are negative for RGS5 expression while those of scleroderma patients are positive for RGS5 expression along with an induction of interferon alpha gene[21,22]. Similar to the case of human VECs, murine VECs are reportedly negative for RGS5 expression[23]. Thus, RGS5 induction in VECs may provide a useful marker for degenerative vessels.
RGS5 plays beneficial roles depending on the kinds of cells. Lack of RGS5 expression in VECs is advantageous as reported in RGS5-deficient mice, which show an advantageous phenotype with normalization of tumor vasculatures[24]. However, up-regulated expressions of RGS5 in VSMCs reportedly bring about favorable outcomes such as angiogenesis promotion[25] and atherosclerosis improvements[26,27]. It seems that opposite effects are exerted by RGS5 between VECs and VSMCs. Thus, we have to be careful enough in performing RGS5-targetted drug discovery to avoid the side effects due to reduced RGS5 expression in VSMCs.
Although “type-II to type-I” conversion is usually a one-way process, “type-I” primary cultured human VECs can be converted into “type-II” VECs via“iPSC establishment and subsequent VEC differentiation” (Figure 5B). We showed that VECs generated from freshly established SeV-iPSCs bare high resistance to stress-induced “type-II to type-I” conversion (Figure 5E), and thus, they may provide an excellent tool for the transplantation therapy for the treatments of refractory arteriostenosis.
Authors would like to thank Mr. Shinnosuke Suzuki and Mr. Yoshinori Yanagi for technical assistance and Dr. Jiro Takahashi at Fuji Chemical Industry Co., Ltd. (Toyama, Japan) and Dr. Yasuhiro Furuichi at GeneCare Research Institute Co., Ltd. (Kanagawa, Japan) for valuable discussions. Chikako Sato moved to Faculty of Engineering/Graduate School of Science and Engineering, Yamagata University after she had finished her work in National Center for Global Health and Medicine, Tokyo, Japan.
Ischemia leads to the development of life-threatening diseases including ischemic heart disease and stroke. It is caused by narrowing of arteries (i.e., arteriostenosis), whose pathological basis is hyperproliferation of vascular smooth muscle cells. Although roles for vascular smooth muscle cells (VSMCs) and macrophages in the development of arteriostenosis are well understood, those for vascular endothelial cells (VECs) remain controversial. Toward the development of new therapeutics, however, involvements of VECs in the progression of arteriostenosis should be elucidated.
There is a longstanding controversy over the effect of VECs on the proliferation of VSMCs: Clinical observations suggest that VECs prevent the proliferation of VSMCs while in vitro co-culture experiments showed that human umbilical cord VECs enhanced the proliferation of bovine VSMCs.
The controversy came from lack of systematic studies, and thus, the authors performed an integrated analysis to quantitatively evaluate the effects various kinds of adult human VECs on the proliferation of adult human VSMCs. The authors have discovered for the first time that human VECs are categorized into two groups by their effects on VSMC proliferation and expression levels of Regulator of G-protein signaling 5 (RGS5): pro-proliferative RGS5high VECs (type-I) and anti-proliferative RGS5low VECs (type-II). Clinical relevance of our finding was supported by the fact that VECs of pathological arteries with tunica media thickening were RGS5high while VECs of normal arteries were RGS5low.
RGS5 expression in VECs provides a useful indicator for the drug discovery for the treatment of ischemic diseases. Furthermore, human pluripotent stem cell-derived type-II VECs will provide a useful tool for transplantation therapy of arteriostenosis.
Regulator of RGS5 is known as an inhibitory molecules against the signaling from G protein-coupled receptor. It is reportedly involved in the regulation of VEC-VEC interaction via VE-cadherin and VEC-VSMC interaction via N-cadherin.
Authors tried to solve the controversy in human VECs to the proliferation of human vascular smooth muscle cells by characterize the human VECs from various sources in two groups as either pro-proliferative or antiproliferative. The studies presented herein implicate regulator of RGS5 as a modulator of VEC phenotype, where VECs expressing high RGS5 are pro-proliferative and VECs expressing low RGS5 are anti-proliferative. Oxidative stress induces RGS5 expression and shifts VECs into a pro-proliferative phenotype. The studies are novel and the manuscript is well written.
P- Reviewer: Yeligar SM, Zhang L S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Lu MH, Chao CF, Huang CG, Chang LT. Coculture of vascular endothelial cells and smooth muscle cells from spontaneously hypertensive rats. Clin Exp Hypertens. 2003;25:413-425. [PubMed] |
| 2. | Shinoda E, Yui Y, Hattori R, Tanaka M, Inoue R, Aoyama T, Takimoto Y, Mitsui Y, Miyahara K, Shizuta Y. Tissue factor pathway inhibitor-2 is a novel mitogen for vascular smooth muscle cells. J Biol Chem. 1999;274:5379-5384. [PubMed] |
| 3. | Miettinen M, Sarlomo-Rikala M, Lasota J. KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol. 2000;13:536-541. [PubMed] |
| 4. | Baldinger A, Brehm BR, Richter P, Bossert T, Gruen K, Hekmat K, Kosmehl H, Neri D, Figulla HR, Berndt A. Comparative analysis of oncofetal fibronectin and tenascin-C expression in right atrial auricular and left ventricular human cardiac tissue from patients with coronary artery disease and aortic valve stenosis. Histochem Cell Biol. 2011;135:427-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Khan ZA, Cukiernik M, Gonder JR, Chakrabarti S. Oncofetal fibronectin in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:287-295. [PubMed] |
| 6. | Watanabe N, Kurabayashi M, Shimomura Y, Kawai-Kowase K, Hoshino Y, Manabe I, Watanabe M, Aikawa M, Kuro-o M, Suzuki T. BTEB2, a Krüppel-like transcription factor, regulates expression of the SMemb/Nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ Res. 1999;85:182-191. [PubMed] |
| 7. | Nakamura N, Saeki K, Mitsumoto M, Matsuyama S, Nishio M, Saeki K, Hasegawa M, Miyagawa Y, Ohkita H, Kiyokawa N. Feeder-free and serum-free production of hepatocytes, cholangiocytes, and their proliferating progenitors from human pluripotent stem cells: application to liver-specific functional and cytotoxic assays. Cell Reprogram. 2012;14:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926-932. [PubMed] |
| 9. | Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [PubMed] |
| 11. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] |
| 12. | Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927-932. [PubMed] |
| 13. | Saeki K, Yogiashi Y, Nakahara M, Nakamura N, Matsuyama S, Koyanagi A, Yagita H, Koyanagi M, Kondo Y, Yuo A. Highly efficient and feeder-free production of subculturable vascular endothelial cells from primate embryonic stem cells. J Cell Physiol. 2008;217:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Nakahara M, Nakamura N, Matsuyama S, Yogiashi Y, Yasuda K, Kondo Y, Yuo A, Saeki K. High-efficiency production of subculturable vascular endothelial cells from feeder-free human embryonic stem cells without cell-sorting technique. Cloning Stem Cells. 2009;11:509-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141-146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 654] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Nishida Y, Yamashita E, Miki W. Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System. Carot Sci. 2007;11:16-20. |
| 17. | Gokoh M, Nishio M, Nakamura N, Matsuyama S, Nakahara M, Suzuki S, Mitsumoto M, Akutsu H, Umezawa A, Yasuda K. Early senescence is not an inevitable fate of human-induced pluripotent stem-derived cells. Cell Reprogram. 2011;13:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Hayashi R, Ishikawa Y, Ito M, Kageyama T, Takashiba K, Fujioka T, Tsujikawa M, Miyoshi H, Yamato M, Nakamura Y. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7:e45435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Homma K, Sone M, Taura D, Yamahara K, Suzuki Y, Takahashi K, Sonoyama T, Inuzuka M, Fukunaga Y, Tamura N. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells. Atherosclerosis. 2010;212:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108:16825-16830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 21. | Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, Molitor JA, Henstorf G, Lafyatis R, Pritchard DK. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Fleming JN, Nash RA, Mahoney WM, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep. 2009;11:103-110. [PubMed] |
| 23. | Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, Genové G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol. 2008;28:2324-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Gröne HJ, Hämmerling GJ. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Arnold C, Feldner A, Pfisterer L, Hödebeck M, Troidl K, Genové G, Wieland T, Hecker M, Korff T. RGS5 promotes arterial growth during arteriogenesis. EMBO Mol Med. 2014;6:1075-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Gunaje JJ, Bahrami AJ, Schwartz SM, Daum G, Mahoney WM. PDGF-dependent regulation of regulator of G protein signaling-5 expression and vascular smooth muscle cell functionality. Am J Physiol Cell Physiol. 2011;301:C478-C489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4277-4282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |