Published online Jul 6, 2017. doi: 10.5527/wjn.v6.i4.217
Peer-review started: February 8, 2017
First decision: March 31, 2017
Revised: April 12, 2017
Accepted: June 6, 2017
Article in press: June 8, 2017
Published online: July 6, 2017
Processing time: 154 Days and 7.5 Hours
Cyclophosphamide is frequently used to treat cancer, autoimmune and renal diseases, such as rapidly progressive glomerulonephritis. Its side effects are well-known, including bone marrow depression, infections, alopecia, sterility, bladder malignancy and hemorrhagic cystitis. Moreover, in some cases cyclophosphamide use has been related to the onset of hyponatremia, by development of a syndrome of inappropriate antidiuresis. Indeed, severe hyponatremia has been previously reported in patients treated with high-dose or moderate-dose of intravenous cyclophosphamide, while only few cases have been reported in patients treated with low dose. Here, we discuss a case of a syndrome of inappropriate antidiuresis followed to a single low-dose of intravenous cyclophosphamide in a patient with a histological diagnosis of acute glomerulonephritis, presenting as acute kidney injury. After cyclophosphamide administration (500 mg IV), while renal function gradually improved, the patient developed confusion and headache. Laboratory examinations showed serum sodium concentration dropped to 122 mmol per liter associated with an elevated urinary osmolality of 199 mOsm/kg, while common causes of acute hyponatremia were excluded. He was successfully treated with water restriction and hypertonic saline solution infusion with the resolution of the electrolyte disorder. This case, together with the previous ones already reported, highlights that electrolyte profile should be strictly monitored in patients undergoing cyclophosphamide therapy in order to early recognize the potentially life-threatening complications of acute water retention.
Core tip: The syndrome of inappropriate antidiuresis (SIAD) is a disorder of sodium and water balance characterized by hypotonic hyponatremia and impaired urinary dilution in the absence of renal disease or any non-osmotic stimulus known to release anti-diuretic hormone. It may be caused by several conditions including infections, neoplasms and use of some medications, such as antipsychotics, antidepressant and immunosuppressive drugs. Here, we report the clinical course of a case of SIAD attributed to administration of a single low-dose of intravenous cyclophosphamide in a patient with an acute glomerulonephritis.
- Citation: Esposito P, Domenech MV, Serpieri N, Calatroni M, Massa I, Avella A, La Porta E, Estienne L, Caramella E, Rampino T. Severe cyclophosphamide-related hyponatremia in a patient with acute glomerulonephritis. World J Nephrol 2017; 6(4): 217-220
- URL: https://www.wjgnet.com/2220-6124/full/v6/i4/217.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i4.217
Hyponatremia is the most frequent electrolyte disorder, being potentially a marker of different underlying diseases or a cause of morbidity itself.
Clinical manifestations vary from asymptomatic forms to severe neurological alterations, in dependence of hyponatremia severity, progression rate and intra- to extracellular osmotic gradient entity. Differential diagnosis is often challenging, since it includes diseases, which, in view of similar clinical pictures, have very different pathophysiological bases, such as syndrome of inappropriate antidiuresis (SIAD), cerebral/renal salt wasting syndrome (C/RSWS) and endocrine syndromes[1].
SIAD is a disorder of sodium and water balance characterized by hypotonic hyponatremia and impaired urinary dilution in the absence of renal disease or any identifiable non-osmotic stimulus known to release antidiuretic hormone (ADH). SIAD may be itself caused by different conditions, including infections, neoplasms and use of several medications, such as antipsychotics, antidepressant[2] and immunosuppressive drugs[3].
Here, we report the clinical course of a rare case of SIAD attributed to cyclophosphamide (CYC) administration in a patient with a proliferative glomerulonephritis.
A 56-year-old man was referred to our Nephrology Department for rapidly decline of glomerular filtration rate (GFR), proteinuria and haematuria.
He presented a history of hypertension, chronic kidney disease (with a stable serum creatinine of 158 mmol/L, estimated GFR 40 mL/min per 1.73 mq CKD-EPI), Crohn disease, psychiatric disorder in treatment with selective serotonin reuptake inhibitors (SSRIs) and normal pressure hydrocephalus with ventriculo-peritoneal shunt.
At the admission, the patient presented a well-controlled blood pressure (17.3/10 kPa) and significant peripheral oedema. Laboratory examinations showed: serum creatinine 290 mmol/L (corresponding to an estimated GFR of 19 mL/min per 1.73 mq), urea 47 mmol/L, sodium 137 mmol/L, potassium 3.69 mmol/L, serum albumin 30 g/L, cholesterol 3.93 mmol/L and triglycerides 2.19 mmol/L.
Urinalysis showed microhematuria, while quantitative proteinuria was 2.26 g/24 h. Autoimmunity evaluation, which included ANA, ENA, ANCA, C3 and C4, resulted negative.
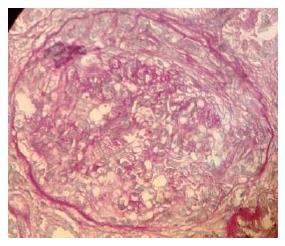
So, in order to better elucidate the causes of renal disorder, we performed a percutaneous renal biopsy. Histological examination showed glomeruli with mesangial expansion and endocapillary hypercellularity due mostly by neutrophils infiltration with some karyorrhectic bodies, fibrinoid necrosis of small arterioles, and fibrocellular crescents (Figure 1). There was also moderate tubular atrophy within massive protein droplets, and moderate interstitial fibrosis. Immunofluorescence analysis did not show immune deposits, while electronic microscopy was not performed. So, considering clinical and histological findings our final diagnosis was rapidly progressive glomerulonephritis secondary to ANCA-negative pauci-immune crescentic glomerulonephritis.
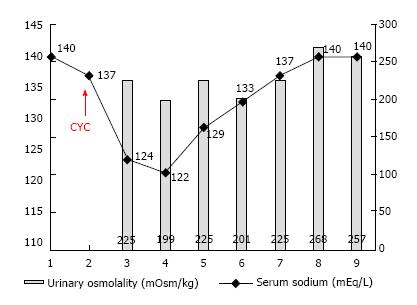
At the time of biopsy pharmacological treatments included intravenous diuretics (furosemide 125 mg/d), antihypertensive drugs and SSRI. After the renal biopsy, also in consideration of the further deterioration of renal function (serum creatinine till 343 mmol/L), intravenous methylprednisolone was administered at the dose of 500 mg for three days followed by oral steroid at the initial dose of 1 mg/kg per day. In addition, the induction therapy was completed with a single dose of 500 mg of intravenous CYC (8 mg/kg). To minimize the risk of haemorrhagic cystitis, saline solution was infused 2 h before the cyclophosphamide administration. In the following days, while renal function gradually improved, patient clinical conditions worsened with development of confusion and headache. Laboratory examinations showed serum sodium 122 mmol/L, serum osmolality 261 mOsm/kg, urinary osmolality 199 mOsm/kg, and serum creatinine 202 mmol/L. Symptomatic hypotonic hyponatremia was further confirmed in the subsequent controls, so that fluid restriction and hypertonic saline solution (at initial concentration of 3%) treatment were initiated, while SSRIs were gradually withdrawn. In the following days, serum sodium progressively improved and we were able to reduce infusive therapy, also to prevent the increase of oedema (Figure 2).
To establish the pathogenesis of the acute hyponatremia we investigated thyroid and adrenal functions, which resulted normal and performed a whole-body CT scan that ruled out pulmonary or cerebral complications. Therefore, in absence of further evidence and considering the temporal relationship between CYC infusion and onset of hyponatremia, the fact that diuretic and psychiatric therapy were unchanged and the presence of hypotonic hyponatremia with impaired urinary dilution we established a diagnosis of CYC-related syndrome of inappropriate anti-diuresis (SIAD)[3]. So, in order to prevent other hyponatremia episodes further cyclophosphamide administration was avoided.
Cyclophosphamide is an alkylating agent used in the treatment of malignant and autoimmune diseases. Its well-known side effects include bone marrow depression, infections, alopecia, sterility, bladder malignancy and haemorrhagic cystitis. Hyponatremia due to SIAD has been infrequently described and it is considered a rare adverse effect related to CYC use. Indeed, until now severe hyponatremia has been reported only in few patients treated with high-dose (30-40 mg/kg)[4,5] and moderate-dose (20-30 mg/kg) of intravenous cyclophosphamide, while even less were the cases of hyponatremia occurred in patients treated with low dose[6-8]. Different underlying mechanisms have been proposed to explain the onset of water retention and SIAD after CYC administration. They can act either stimulating ADH release or accentuating its renal effects, finally causing hyponatremia[9]. Harlow et al[10] demonstrated in a patient who received high dose of cyclophosphamide, the loss of Herrings bodies and degranulation of various hypothalamic neurosecretory organelles, leading to the inappropriate secretion of ADH. It has been also demonstrated a direct effect of an alkylating metabolite of CYC on the kidney resulting in enhanced permeability of the distal tubules to water[11]. Moreover, CYC might cause hyponatremia by up-regulating expression of the ADH receptor V2R and aquaporin 2 (AQ2) through the suppression of interleukin-1 and tumor necrosis factor-α, which generally act as negative regulators of VR2 expression[12]. In addition, recently Kim et al[13] demonstrated in experimental models (rat and inner medullary collecting cell cultures) that CYC could also induce V2R activation and AQP2 expression in the absence of ADH stimulation.
Actually, in our case the patient presented several predisposing factors that could have caused water retention, such as renal failure, the continuative use of SSRI and the normal pressure hydrocephalus; but the temporal association with the administration of CYC makes plausible its role in the development of the severe hyponatremia, also considering that prior to CYC treatment, he had normal serum electrolytes and did not present nausea or vomiting. Moreover, our case has common features with other reports in which hyponatremia was induced by intravenous cyclophosphamide. Hyponatremia usually occurs 12-48 h after the administration of cyclophosphamide, and returns to normal in few days.
In conclusion, we think that our case, together with the previous ones already reported, underlined the need to be aware of the potentially life-threatening complications of water intoxication when intravenous pulse cyclophosphamide is applied, especially in patients with other concomitant risk factors. Therefore, we strongly suggest checking electrolytes before and after CYC administration.
A patient with an acute glomerulonephritis who developed an acute symptomatic hyponatremia after treatment with cyclophosphamide.
Acute Hyponatremia secondary to development of syndrome of inappropriate antidiuresis related to the administration of cyclophosphamide.
Other causes of acute hyponatremia: Cancers, infections, hypovolemia, use of diuretics.
Hypotonic hyponatremia associated with impaired urinary dilution.
CT to exclude cerebral or pulmonary disorders.
Renal biopsy proving the presence of an acute ANCA-negative pauci-immune crescentic glomerulonephritis.
Water restriction, hypertonic saline solution infusion.
Previous cases of hyponatremia in patients treated with different doses of intravenous cyclophosphamide.
It is necessary to carefully monitor electrolyte and water balance before and after cyclophosphamide administration.
This case report is well-written and has interesting information.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Watanabe T S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 565] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 2. | Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Esposito P, Piotti G, Bianzina S, Malul Y, Dal Canton A. The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options. Nephron Clin Pract. 2011;119:c62-c73; discussion c73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Abe T, Takaue Y, Okamoto Y, Yamaue T, Nakagawa R, Makimoto A, Sato J, Kawano Y, Kuroda Y. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) in children undergoing high-dose chemotherapy and autologous peripheral blood stem cell transplantation. Pediatr Hematol Oncol. 1995;12:363-369. [PubMed] |
| 5. | Lazarevic V, Hägg E, Wahlin A. Hiccups and severe hyponatremia associated with high-dose cyclophosphamide in conditioning regimen for allogeneic stem cell transplantation. Am J Hematol. 2007;82:88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Koo TY, Bae SC, Park JS, Lee CH, Park MH, Kang CM, Kim GH. Water intoxication following low-dose intravenous cyclophosphamide. Electrolyte Blood Press. 2007;5:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lee YC, Park JS, Lee CH, Bae SC, Kim IS, Kang CM, Kim GH. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25:1520-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Gilbar PJ, Richmond J, Wood J, Sullivan A. Syndrome of inappropriate antidiuretic hormone secretion induced by a single dose of oral cyclophosphamide. Ann Pharmacother. 2012;46:e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000;13:673-675. [PubMed] |
| 10. | Harlow PJ, DeClerck YA, Shore NA, Ortega JA, Carranza A, Heuser E. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44:896-898. [PubMed] |
| 11. | DeFronzo RA, Braine H, Colvin M, Davis PJ. Water intoxication in man after cyclophosphamide therapy. Time course and relation to drug activation. Ann Intern Med. 1973;78:861-869. [PubMed] |
| 12. | Park SJ, Kim JH, Shin JI. Insight on mechanism of hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25:3453; author reply 3453-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kim S, Choi HJ, Jo CH, Park JS, Kwon TH, Kim GH. Cyclophosphamide-induced vasopressin-independent activation of aquaporin-2 in the rat kidney. Am J Physiol Renal Physiol. 2015;309:F474-F483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |