Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.538
Peer-review started: April 25, 2016
First decision: July 5, 2016
Revised: August 14, 2016
Accepted: October 5, 2016
Article in press: October 9, 2016
Published online: November 6, 2016
Processing time: 192 Days and 16.5 Hours
To analyse current literature focusing on pathogenesis and therapeutic aspects of urolithiasis with inflammatory bowel disease (IBD) and following bariatric surgery.
A systematic literature search was performed using PubMed, supplemented with additional references. Studies assessing the association of IBD or bariatric surgery with renal stones in both paediatric and adulthood were included.
Certain types of stones are seen more frequently with IBD. Hyperoxaluria and hypocitraturia are the main metabolic changes responsible for urolithiasis. The incidence of renal stones in malabsorptive types of bariatric surgery such as gastric bypass is high; this is not as common in modern restrictive surgical methods. Preventative methods and urine alkalinisation have been shown to be beneficial.
Both conditions are associated with renal stones. Patients’ counselling and prevention strategies are the mainstay of urolithiasis management in these patients.
Core tip: Urolithiasis continues to be a complication associated with inflammatory bowel disease and post bariatric surgery. Lowered urinary levels of anti-lithogenic substances (magnesium and citrate) have been suggested to be important in calculi development. Prevention is best achieved through dietary changes and targeted medical therapy.
- Citation: Gkentzis A, Kimuli M, Cartledge J, Traxer O, Biyani CS. Urolithiasis in inflammatory bowel disease and bariatric surgery. World J Nephrol 2016; 5(6): 538-546
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/538.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.538
Inflammatory bowel disease (IBD) is a condition which affects patients of all age groups and ethnicities. The two main entities are Crohn’s disease (CD) and ulcerative colitis (UC). It is estimated that the incidence of each of these diseases is approximately 3-14/100000 patients/year respectively in the United States[1]. The association of IBD with complications in the urinary tract has been reported in studies almost 40 years ago, with urolithiasis being a common manifestation[2].
In recent years, the use of bariatric surgery for treatment of morbid obesity and its medical and functional consequences is rapidly rising up in the world’s medical organisations agenda, as a consequence of social and lifestyle choices. In the United Kingdom almost 18000 operations have been done for this purpose in 2011-2013[3] whereas in the United States, the incidence of bariatric surgery is estimated at 113000 cases per year[4]. Although the metabolic syndrome (and obesity) is a known risk factor for urolithiasis[5], those patients subjected to bariatric surgery appear to have an even higher risk of developing renal stone disease[6].
As the exact association of urolithiasis with the above clinical entities has not been fully established, we sought to review the published data on this topic.
Systematic search of the literature in PubMed using the combination of the words: “renal stones” or “kidney stones” or “urolithiasis” or “nephrolithiasis” and “inflammatory bowel disease” or “Crohn’s” or “ulcerative colitis” or “short bowel” or “bariatric surgery”.
Search was limited to English-language papers. Further references were identified from the reference list of retrieved articles.
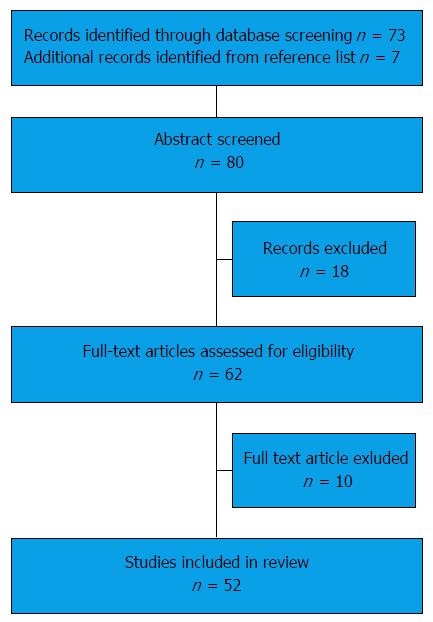
Studies assessing the association of IBD or bariatric surgery with renal stones in both paediatric and adulthood were included. Case reports or studies including small number of patients (< 5) were excluded. Figure 1 summarises the search process. Out of the 62 studies assessed for eligibility, 10 were excluded: 3 were case reports, 5 included small number of patients, 1 referred to ankylosing spondylitis only rather than IBD and 1 referred to other urinary complications of IBD rather than renal stones.
The retrieved published studies were mainly one-arm, uncontrolled and observational. We separated the studies into those referring to IBD or bariatric surgery respectively.
Incidence and clinical evaluation: No large epidemiological studies assessing the prevalence of IBD and urolithiasis co-existence were identified.
Small and historical studies initially reported this association along with other urogenital IBD complications (such as fistulation, intrinsic renal disease and obstructive uropathy)[2,7]. From smaller studies it has been estimated that 9%-18% of adult IBD patients may be diagnosed with renal stones at some time in their life[8,9]. In cases when the patients with IBD (Crohn’s) had resection of the terminal ileum, the percentage of associated urolithiasis is much higher (reported up to 28%)[9]; this is attributed to metabolic disturbances due to steatorrhoea and bile salt malabsorption. Interestingly, in patients with short bowel syndrome post several resections for complicated Crohn’s, preservation of colon has been associated with higher incidence of renal stones compared with those who had a completion colectomy (24% vs 0% in a study of 52 patients)[10]. In a study of UC patients who had a panproctocolectomy, the risk of renal stone disease was compared between conventional ileostomy and ileal J-pouch; the risks of forming uric acid stones were high for both ileostomy and J-pouch patients, but J-pouch reduced the risk of renal stones containing calcium (the relative probability of calcium stone formation was 0.58 in ileostomy group vs 0.18 in the J pouch group)[11]. Another study on patients with ileal J-pouch post panproctocolectomy for UC showed that the presence of several extra-intestinal manifestations, no use of antibiotics and low serum bicarbonate level were the most important risk factors for the presence of concurrent urolithiasis; the overall incidence of the latter was 37% in this group[12].
A study from Japan assessed the risk factors for developing urolithiasis in patients with CD. Renal stones were more frequent in men. The mean time from Crohn’s diagnosis to diagnosis of calculi was 8.8 years (range 0 to 22 years). The commonest type of stone was calcium oxalate. The probability of developing calculi was approximately eight times higher for patients with a urine pH of ≤ 6.0 than for those with a urine pH of ≥ 6.5[13].
Renal stone disease appears to be less common in the paediatric population with IBD. A report from a national IBD registry in Italy estimated its incidence to be at 0.37%[14].
In a study when computerised tomography was used to diagnose asymptomatic extra-intestinal manifestations of CD, 4% of patients were found to have concomitant urolithiasis[15].
With regards to patients’ awareness, only 12% of those included in a cross-sectional survey in Canada reported that they had been aware of the IBD-nephrolithiasis association. They had obtained their information from their gastroenterologist or the internet and they had much better knowledge on other potential extra-intestinal IBD manifestations such as risk of colon cancer (75%), arthritis (77%) and dermatological manifestations (49%)[16].
An interesting observation on patients with IBD and urolithiasis was made in a large study (14352 patients) from emergency departments in the United States. IBD patients with urolithiasis presented with infections, sepsis and renal failure more frequently than non-IBD patients [infections (10.4% vs 9.1%; P < 0.001), sepsis (0.6% vs 0.2%; P < 0.001), and end-organ failure (6.3% vs 1.6%; P < 0.001) respectively]. This was due to the increased occurrence and severity of infected urolithiasis in this group as well as the fact that the urolithiasis was noted to occur more commonly in older patients (with IBD) compared with non-IBD controls[17]. In another study it was noted that recurrent urolithiasis and surgical interventions for its treatment, along with bowel resections are the two independent risk factors for the development of chronic kidney disease in IBD patients; this is generally a rare phenomenon particularly in UC[18].
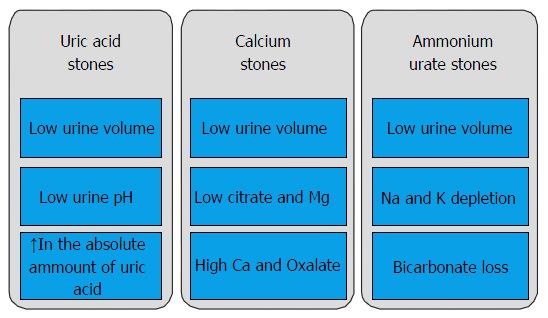
Pathogenesis: CD and UC are characterized by recurrent inflammatory involvement of different intestinal segments resulting in malabsorption of bile salts and fatty acids[19]. This causes increased oxalate absorption by increasing oxalate solubility in the intestinal lumen and permeability of the colonic mucosa[20]; this secondary hyperoxaluria is associated with oxalate renal stones[9,21]. In addition to that, the loss of bicarbonate in the liquid stool and the dehydration cause acidosis and subsequent reduced citrate excretion. This can lead to uric acid or mixed stones[21]. Factors such as decreased urinary excretion of other inhibitors of crystallisation (magnesium), dietary parameters and medications (such as aminosalicylates)[22], can also play a role in stone formation in these patients[23] (Figure 2).
In a study including both paediatric and adult patients with CD, the patients’ 24 h urine oxalate levels and intestinal oxalate absorption were assessed vs the relevant parameters in healthy individuals. The intestinal absorption of oxalate was significantly higher in CD patients; (0.92 ± 0.57 mmol/1.73 m2 per 24 h) compared with those without the disease (0.53 ± 0.13 mmol/1.73 m2 per 24 h) respectively (P < 0.05); this also correlated with hyperoxaluria and risk of developing urolithiasis[24].
Kumar et al[25] examined the association of intestinal oxalate degrading bacteria Oxalobacter formigenes with the development of hyperoxaluria in IBD. The investigators studied stool samples of IBD patients and controls respectively for the presence of O. formigenes using polymerase chain reaction. Only 10.4% of patients with IBD compared to 56% of controls had positive for O. formigenes stool samples. Patients without O. formigenes had higher urinary oxalate than those with it. They were also more likely to develop renal stones. In this study there was no significant difference in the excretion of citrate. However, in those patients who had hypermagnesiuria (another stone inhibitor) the formation of stones was not common, irrespective of the IBD status. A recent study suggested that oxalate is not only absorbed by the gastrointestinal (GI) tract in different concentrations depending on its permeability, but can also be secreted by the small intestine[26]. This is an interesting observation which provides some evidence that perhaps diseased GI tract can influence the oxalate metabolism and result in hyperoxaluria and subsequent urolithiasis.
Contrary to the above, a study suggested that the formation of renal stones in IBD patients is more likely related to low urinary concentration of magnesium and citrate relative to calcium than the hyperoxaluria[27]. However, only 40 patients with IBD and 17 controls were included in the study with 2 IBD patients developing renal stones.
Evan et al[28] analysed renal biopsies of IBD patients with urolithiasis. The histopathological abnormalities seen, included moderate glomerular sclerosis, tubular atrophy, and interstitial fibrosis. Randall’s plaque was abundant, with calcium oxalate stone overgrowth; as the authors suggested this was similar to that seen in idiopathic calcium oxalate stone formers, and primary hyperparathyroidism. Abundant plaque was compatible with low urine volume and acidic pH.
Interestingly a recent study provided early evidence of genetic background as an independent risk factor for urolithiasis in IBD patients alongside known mechanisms such as malabsorption and medication[29].
Another risk factor for urolithiasis in IBD patients with intestine failure (short bowel) is the use of vitamin C in parenteral nutrition. This has been shown to increase urinary oxalate excretion and subsequently increase the risk of urinary stone formation[30].
Ileostomy: The formation of ileostomy post bowel resection is an independent risk factor for the formation of renal stones[13]. The relative dehydration from liquid stool, and the acidic PH from bicarbonate loss are responsible for this. In this acidic environment, the urinary citrate level excretion reduces. The stones most commonly seen in these patients contain uric acid or are of mixed composition[21]. In addition, the risk of calcium containing stones also increases with ileostomy. In a retrospective study comparing IBD patients with ileostomy vs IBD patients with J-pouch vs controls, the relative risk of calcium containing stones formation was significantly higher (0.58 vs 0.18) in those with ileostomy[11].
Treatment: In our review, no study directly assessing the in vivo effect of a treatment to reduce urolithiasis in IBD patients was identified.
In a study using computer modelling, simulated urine situations based on reported composition values of IBD patients were compared with the relevant ones from normal individuals. In this model, supplementation of calcium would reduce the urinary calcium oxalate supersaturation in IBD patients as their hyperoxaluria is much more prominent compared with non-IBD stone formers. The authors suggested that calcium supplements can help reduce stone risk in those patients, but initial efforts should be directed towards reducing urinary oxalate by reducing dietary oxalate. Citrate therapy that increases both urine pH and urinary citrate could also provide an additional therapeutic benefit[31].
Several authors have advocated preventative strategies. These include close monitoring of IBD patients with urinalysis and imaging of the upper tracts at regular intervals[13] as well as early surgical intervention even in cases of small renal stone burden[17]. Also the avoidance of low urinary citrate and magnesium levels could prevent the stone formation in these patients[25,27,32]. In UC patients who had panproctocolectomy and formation of ileoanal pouch, close monitoring for renal stone formation and administration of prophylactic oral bicarbonate has been suggested[12]. Urinary alkalinisation along with increased hydration is also advocated in IBD patients receiving aminosalicylates[22].
Incidence: Bariatric operations have traditionally been divided into three groups: (1) restrictive, i.e., procedures that produce weight loss solely by limiting intake (gastric banding, gastric sleeve); (2) malabsorptive, i.e., operations that induce weight loss totally by interference with digestion and absorption (intestinal bypass); and (3) mixed, i.e., procedures that limit intake and produce malabsorption (gastric bypass, duodenal switch)[33].
The most commonly performed bariatric operation is Roux-en-Y gastric bypass (RYGB)[3]. It belongs to the “mixed group” in the bariatric operations classification. Nelson et al[34] first described the association of gastric bypass surgery with the formation of calcium oxalate renal stones. Matlaga et al[35] reviewed the insurance claims and records of 4639 patients who had RYGB for obesity and compared them with the relevant ones from other 4639 obese patients over a four-year period. Urolithiasis was diagnosed in 7.65% (355 of 4639) of RYGB patients compared to 4.63% (215 of 4639) of patients in the control group (P < 0.0001). The patients in the treatment group more commonly underwent shock wave lithotripsy and ureteroscopy, making the RYGB surgery a significant predictor of diagnosis and requirement for treatment of urolithiasis in the post-operative period. In a retrospective study of 972 patients who underwent RYGB, 3.2% developed de-novo urolithiasis post-operatively (mean FU was 2.8 years); in those individuals who had renal stones pre-operatively (8.8%), the recurrence of the disease was even more common (32%)[36]. The authors concluded that although the RYGB had a significant influence on the development of stones, the combination of pre-operative stone history with this type of bariatric surgery is the most important risk factor for recurrent urolithiasis. Other reviewers have come to the same conclusions[6]. In a large, prospective study of 151 patients undergoing laparoscopic RYGB, Valezi et al[37] reported de novo stone incidence of 8% at 12 mo post-surgery. The relative risk for development of stones increased by approximately 70%[37]. In another study of patients who had RYGB for gastric cancer, the investigators assessed the incidence of renal stones post-operatively. Computed tomography scans from gastric cancer patients who had either distal gastrectomy with Bilroth I anastomosis/RYGB vs total gastrectomy with RYGB reconstruction were reviewed. Patients with total gastrectomy were more likely to have renal stones (25%) than patients with some remaining stomach (7%). The authors hypothesised that total gastrectomy may lead to more fat malabsorption than partial gastrectomy, perhaps exacerbating hyperoxaluria[38]. Although these procedures were not directly done for the purpose of weight loss, the study adds some evidence that the malabsorptive procedures are associated with increased incidence of urolithiasis; in particular, in this study the extent of resection, and not just the bypass itself, was an independent factor for the development of renal stones.
A relative historical bariatric operation, jejuno-ileal bypass, has also been implicated with significant risk of post-operative urolithiasis. In a retrospective study of 56 patients who had undergone this surgery because of morbid obesity, twenty-two (39.3%) were found to have renal calculi in a 16-year mean follow-up[39]. Interestingly, patients who had surgery for reversal of this bypass continued to have high incidence of renal stones despite the decrease in their 24-h urinary oxalate levels[40]. The authors of this study concluded that the persistence of low citrate in the urine is implicated on this phenomenon.
Sleeve gastrectomy and gastric banding are the most commonly done bariatric operations of the restrictive type. Patients who undergo these procedures generally appear to have less likelihood of developing renal stones post-operatively. Chen et al[41] reported an approximate stone incidence rate of 1% in a retrospective review of 332 cases of gastric banding and 85 cases of sleeve gastrectomy respectively; they estimated a person-time incidence rate of 3.40 stone diagnoses per 1000 person-years for gastric banding and 5.25 stone diagnoses per 1000 person-years for sleeve gastrectomy respectively[41]. Semins et al[42] reported no association of gastric banding surgery with increased risk of urolithiasis or renal stone intervention post-operatively. In their case-control study, the authors retrospectively reviewed the insurance claims of 201 morbidly obese patients who had gastric banding surgery and 201 morbidly obese controls through a national database. The incidence of new renal stones was 1.5% in the operated individuals and 6% in the controls; this was the first study which provided evidence that the outcome of restrictive bariatric surgery on the development of urolithiasis is different compared to malabsorptive or mixed procedures.
Pathophysiology: Bariatric surgery has been associated with several metabolic and urinary changes first described in the 1980s[43]. The most common one is the development of hyperoxaluria, first seen post jejuno-ileal bypass surgery.
In a prospective study of 21 patients undergoing RYGB, 24-h urine specimens were analysed pre- and post-operatively[44]; urinary oxalate excretion increased significantly after RYGB (33 ± 9 mg/d pre-operatively vs 63 ± 29 mg/d post-operatively). De novo hyperoxaluria developed in 11 (52%) patients. There was also significant increase of patients with hypocitraturia (from 10% at baseline to 48%) at 2 years. Wu et al[45] performed a similar study; 38 patients undergoing RYGB had serum and urine chemistry assessed preoperatively and 6 mo post-operatively. Urinary changes known to increase the risk of urolithiasis were found: Decrease in total urine volume (from 2 L/d to 1.5 L/d respectively), increase in calcium (from 139 mg/d to 182 mg/d respectively), and increase in oxalate (from 38 mg/d to 48 mg/d respectively); all these changes were statistically significant. Their conclusion was that this study provided evidence that renal stones post RYGB is caused by many biochemical factors, with hyperoxaluria being the main one[45].
Similar conclusions were drawn by Patel et al[46] in their case-control study of 58 patients who underwent RYGB (52) or duodenal switch (6) procedures. In this study 67.2% of patients who had bariatric surgery developed hyperoxaluria (in their 24-h urine specimens 6 mo post-operatively) compared to almost 34.1% of non-stone formers (controls) and 37% of stone formers with no history of bariatric surgery respectively. Hyperoxaluria and hypocitraturia were also demonstrated in post RYGB patients in a cross-sectional study by Maalouf et al[47]. They also pointed out that in their study the urinary calcium excretion decreased which may be counteracting the effects of the above significant risk factors. Asplin et al[48] provided evidence that hyperoxaluria is a common metabolic finding in patients post RYGB with calcium oxalate being the main driving force for the development of kidney stones; the levels of urinary oxalate were not as high as they found in the jejunoileal bypass cohort[48].
The hyperoxaluria in post bariatric surgery patients is believed to be caused by increased intestinal oxalate absorption due to the relative fat malabsorption, particularly in the bypass operations. As fat is malabsorbed, fat-soluble vitamins and calcium ions are saponified by intraluminal free fatty acids, leading to steatorrhea. The calcium is less available in the intestinal lumen, which subsequently decreases binding with oxalate. When oxalate is free, it gets absorbed by the intestine and then majority is excreted in the kidney. It may then precipitate with urinary calcium to form insoluble crystals and eventually kidney stones. In addition to the above, permeability of the intestine to oxalate is increased by exposure to unconjugated bile salts and long chain fatty acids, both of which are increased in the GI tract of RYGB patients[49,50].
A study by Froeder et al[51] used the oxalate load test as a direct assessment of intestinal oxalate absorption in patients post gastric bypass surgery. The mean oxaluric response to this load was markedly elevated in post-bariatric surgery patients, suggesting that increased intestinal absorption of dietary oxalate is a predisposing mechanism for enteric hyperoxaluria[51].
In the restrictive group of bariatric operations (such as gastric banding and sleeve gastrectomy) the metabolic changes are not as common as they are in the malabsorption group. Studies comparing the metabolic profile of patients’ groups who had one procedure from each group concluded that urinary oxalate excretion of the “restrictive” cohorts was significantly less than the “RYGB” cohorts and not significantly different from that of the normal subjects and routine stone-formers[52-55]. This difference is likely related to the lower supersaturation of calcium oxalate, predominantly due to higher urinary volume and lower urinary calcium excretion in restrictive procedures compared to RYGB[56].
Treatment: Patients submitted to bariatric surgery are at risk of nephrolithiasis and nephropathy. Accurate stone screening, careful monitoring of renal function and diet counselling are strongly encouraged in these patients[6,45]. Close monitoring with renal tract imaging/screening is advocated mainly for patients who undergo gastric bypass surgery and have a previous history of urolithiasis[36].
Strategies to prevent stones after bariatric surgery are similar to those recommended to all stone formers. They include increased daily fluid intake to achieve urine volumes higher than 2 L/d and low oxalate (< 100 mg/d) intake. Additionally, low sodium and animal protein intake are indicated[49]. Bariatric stone formers should particularly reduce daily fat intake to minimise enteric oxalate absorption and consider calcium supplementation[56]. Citrate salts, like potassium citrate, can be used to correct metabolic acidosis and hypocitraturia[35,44].
A phase II randomised study has assessed the use of potassium calcium citrate (PCC) in post RYGB patients[57]. PCC significantly increased citrate, and pH. The urinary saturation of uric acid decreased significantly. Also calcium oxalate agglomeration was significantly inhibited by PCC. As it has not yet been tested in phase III trials it is not available for clinical use.
The prevalence of symptomatic nephrolithiasis is higher in patients with IBD and after bariatric surgery. It is prudent to understand the pathophysiology of this condition as well as the basics of prevention. IBD commonly results in impaired intestinal function and in a malabsorptive state. Similarly, bariatric surgery reforms the normal GI track with subsequent metabolic disturbances. Both these entities have been associated with the development of urolithiasis. The malabsorption state may result in increased absorption of oxalate or decreased absorption of citrate, magnesium, bicarbonate, water, potassium leading to increase in supersaturation state.
Urate stones form due to loss of intestinal fluid and bicarbonate which lead to concentrated acidic urine. This increases the precipitation of urate. In addition, inhibitors of crystallization (e.g., citrate) are lost with diarrhoea. Management of uric acid stones includes treatment of diarrhoea, alkalinising the urine and increasing fluid intake. If stones recur allopurinol is of great help but it interacts with azathioprine.
Calcium oxalate stones are related to increased urinary oxalate excretion caused by increased intestinal absorption known as enteric hyperoxaluria. Enteric hyperoxaluria is firstly related to bile salt malabsorption in the diseased or resected distal ileum. Malabsorbed fats bind intraluminal calcium, decreasing the amount of calcium available for oxalate resulting in increased oxalate absorption. Increased colonic permeability to oxalate caused by the malabsorbed bile salts and fatty acids can also cause hyperoxaluria. Hyperoxaluria may be seen in patients on long-term parenteral nutrition and with minimal oral intake. Risk of calcium oxalate stones can be reduced by managing enteric hyperoxaluria, increasing oral fluid intake, a low fat and oxalate diet, calcium supplementation, oxalate synthesis can be reduced by cholestyramine and pyridoxine (B6). In case of recurrence, alkalinisation of urine, citrate and Mg supplementation can be used (Tables 1 and 2).
| IBD and stones treatment | |
| All patients | Counselling about IBD association with renal stones |
| Increased hydration | |
| Monitor with regular urinalysis and imaging of upper tracts | |
| Early surgical intervention even in small renal stone burden | |
| Established hyperoxaluria or calcium oxalate stones | Reduction in dietary oxalate |
| Calcium supplements | |
| Uric acid stones | Urinary alkalinisation |
| Citrate supplementation | |
| Panproctocolectomy | Consideration for oral bicarbonate supplementation |
| Bariatric surgery and stones treatment | |
| All patients | Counselling about bariatric surgery association with renal stones |
| Regular upper tract imaging (in gastric bypass) | |
| Increased hydration | |
| Focused dietary advice | |
| Low oxalate | |
| Low sodium | |
| Low animal protein | |
| Low fat | |
| Citrate supplementation if evidence of hypocitraturia |
This review highlights the lack of good quality studies reported in the literature. Majority of studies are retrospective and with small numbers therefore a prospective database is required to capture all information and develop a better understanding of urolithiasis in this group of patients. We suggest a complete blood and urine metabolic work up according to European guidelines at baseline should be considered and 2 yearly thereafter along with routine tests to monitor IBD. This will allow us to gather evidence to recognise lithogenic factors. In addition, a renal USS at diagnosis and 2 yearly thereafter should be considered for early diagnosis. Furthermore, early diagnosis may help to establish a proper correlation between severity of IBD and urinary metabolic parameter.
IBD and bariatric surgery are closely associated with the development of urolithiasis. The mechanism of this relation is multifactorial but has mainly related to the increased absorption of oxalate in the intestine which is usually increased in those conditions and also other metabolic disturbances found in these entities. The patients should be informed of this association and follow the appropriate preventative strategies to reduce the risk of renal stones.
The intestinal malabsorption from inflammatory bowel disease (IBD) and bariatric surgery commonly leads to urolithiasis. In this review, the known evidence in pathogenesis and prevention/treatment of this entity is analysed. The increased absorption of oxalate or reduced absorption of citrate, magnesium, bicarbonate, water and potassium result in supersaturation and formation of renal stones. Counselling about IBD/bariatric surgery association with renal stones, increased hydration, monitoring with regular urinalysis and imaging of upper tracts as well as early surgical intervention even in small renal stone burden are required to reduce the morbidity of urolithiasis in these patients. A summary of lithogenic risk factors and recommendations for prevention is included.
Potential future studies could focus more on IBD/bariatric surgery association with urolithiasis epidemiology with better data capture and analysis. There is also a field to explore the most efficient investigations’ protocol for diagnosis and monitoring in these patients’ groups.
This review emphasizes the lack of good quality studies in the field of urolithiasis in patients with IBD or following bariatric surgery. It will assist the readers to understand its pathogenesis and also the ways to prevent it. It will also increase the awareness of this association in clinicians (such as General Practitioners, Physicians, Gastroenterologists and Gastrointestinal Surgeons) as well as the patients with IBD or following bariatric surgery.
The authors suggest a complete blood and urine metabolic work up (according to European guidelines) at baseline. Repeated tests should be considered 2 yearly thereafter along with routine tests to monitor IBD. In addition, a renal USS at diagnosis of IBD or following bariatric surgery and 2 yearly thereafter should also be considered for early diagnosis and appropriate monitoring respectively.
Crohn’s disease is a chronic or long lasting disease that causes inflammation in the gastrointestinal tract. Most commonly, Crohn’s affects the small intestine and the beginning of the large intestine. Duodenal switch procedure involves a partial gastrectomy (reducing stomach along greater curvature) effectively restricting its capacity while maintaining its normal function. Gastric banding is an adjustable gastric band, frequently called a lap-band (when done laparoscopically). A band is an inflatable silicone device placed around the top portion of the stomach to treat obesity, intended to slow utilisation of food and thus reduce the amount of food consumed. Gastric bypass surgery is a surgical procedure in which the stomach is divided into a small upper pouch and a much larger lower remnant pouch and then the small intestine is repositioned to connect to both. Jejunoileal bypass surgery is the most effective surgical intervention for achieving and maintaining weight loss. Typically, 35 centimeters of proximal jejunum is anastomosed, end-to-side or end-to-end, to the terminal 10 centimeters of ileum. Ileostomy a surgical operation in which a damaged part is removed from the ileum and the cut end diverted to an artificial opening in the abdominal wall. Ileal J pouch, the ileal pouch-anal anastomosis is a surgical procedure that is used to restore gastrointestinal continuity after surgical removal of the colon and rectum. A J pouch or an internal pouch, the procedure involves the creation of a pouch of small intestine to recreate the removed rectum. Panproctocolectomy is the removal of the entire colon, rectum and anal canal. This type of stoma is permanent. Roux-en-Y gastric bypass is a surgical procedure which may be done for severe obesity. The procedure involves cutting the stomach in two to create a pouch out of the smaller proximal portion of the stomach, attaching it to the small intestine, bypassing a large part of the stomach and all of the duodenum. Ulcerative colitis is a long-term inflammatory bowel disease. It causes swelling and ulcerations of the colon and rectum.
This is an excellent paper.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Friedman EA, Nechifor G S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2150] [Article Influence: 102.4] [Reference Citation Analysis (1)] |
| 2. | Sigel A, Botticher R, Wilhelm E. Urological complications in chronic inflammatory diseases of the bowel. Eur Urol. 1977;3:7-10. [PubMed] |
| 3. | British Obesity and Metabolic Surgery Society. The UK National Bariatric Surgery Registry Report, 2014. |
| 4. | Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200:378-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Besiroglu H, Otunctemur A, Ozbek E. The metabolic syndrome and urolithiasis: a systematic review and meta-analysis. Ren Fail. 2015;37:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Tasca A. Metabolic syndrome and bariatric surgery in stone disease etiology. Curr Opin Urol. 2011;21:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kane S. Urogenital complications of Crohn’s disease. Am J Gastroenterol. 2006;101:S640-S643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Torio M, Ishimura M, Ohga S, Doi T, Utsunomiya R, Ohkubo K, Suga N, Tatsugami K, Matsumoto T, Takada H. Nephrolithiasis as an extra-intestinal presentation of pediatric inflammatory bowel disease unclassified. J Crohns Colitis. 2010;4:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Andersson H, Bosaeus I, Fasth S, Hellberg R, Hultén L. Cholelithiasis and urolithiasis in Crohn’s disease. Scand J Gastroenterol. 1987;22:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 203] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Christie PM, Knight GS, Hill GL. Comparison of relative risks of urinary stone formation after surgery for ulcerative colitis: conventional ileostomy vs. J-pouch. A comparative study. Dis Colon Rectum. 1996;39:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Mukewar S, Hall P, Lashner BA, Lopez R, Kiran RP, Shen B. Risk factors for nephrolithiasis in patients with ileal pouches. J Crohns Colitis. 2013;7:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Ishii G, Nakajima K, Tanaka N, Hara H, Kato M, Ishii N. Clinical evaluation of urolithiasis in Crohn’s disease. Int J Urol. 2009;16:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Castro M, Papadatou B, Baldassare M, Balli F, Barabino A, Barbera C, Barca S, Barera G, Bascietto F, Berni Canani R. Inflammatory bowel disease in children and adolescents in Italy: data from the pediatric national IBD register (1996-2003). Inflamm Bowel Dis. 2008;14:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Paparo F, Bacigalupo L, Garello I, Biscaldi E, Cimmino MA, Marinaro E, Rollandi GA. Crohn’s disease: prevalence of intestinal and extraintestinal manifestations detected by computed tomography enterography with water enema. Abdom Imaging. 2012;37:326-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Huang V, Mishra R, Thanabalan R, Nguyen GC. Patient awareness of extraintestinal manifestations of inflammatory bowel disease. J Crohns Colitis. 2013;7:e318-e324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Varda BK, McNabb-Baltar J, Sood A, Ghani KR, Kibel AS, Letendre J, Menon M, Sammon JD, Schmid M, Sun M. Urolithiasis and urinary tract infection among patients with inflammatory bowel disease: a review of US emergency department visits between 2006 and 2009. Urology. 2015;85:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Primas C, Novacek G, Schweiger K, Mayer A, Eser A, Papay P, Gratzer C, Angelberger S, Dejaco C, Reinisch W. Renal insufficiency in IBD--prevalence and possible pathogenetic aspects. J Crohns Colitis. 2013;7:e630-e634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Andersson H, Bosaeus I. Hyperoxaluria in malabsorptive states. Urol Int. 1981;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chadwick VS, Modha K, Dowling RH. Mechanism for hyperoxaluria in patients with ileal dysfunction. N Engl J Med. 1973;289:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 154] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Trinchieri A, Lizzano R, Castelnuovo C, Zanetti G, Pisani E. Urinary patterns of patients with renal stones associated with chronic inflammatory bowel disease. Arch Ital Urol Androl. 2002;74:61-64. [PubMed] |
| 22. | Russinko PJ, Agarwal S, Choi MJ, Kelty PJ. Obstructive nephropathy secondary to sulfasalazine calculi. Urology. 2003;62:748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Rudman D, Dedonis JL, Fountain MT, Chandler JB, Gerron GG, Fleming GA, Kutner MH. Hypocitraturia in patients with gastrointestinal malabsorption. N Engl J Med. 1980;303:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 97] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, Hoppe B. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol. 2012;27:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Kumar R, Ghoshal UC, Singh G, Mittal RD. Infrequency of colonization with Oxalobacter formigenes in inflammatory bowel disease: possible role in renal stone formation. J Gastroenterol Hepatol. 2004;19:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Reddy TG, Knight J, Holmes RP, Harvey LM, Mitchem AL, Wilcox CM, Monkemuller KE, Assimos DG. Oxalate Concentrations in Human Gastrointestinal Fluid. J Endourol. 2016;30 Suppl 1:S8-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | McConnell N, Campbell S, Gillanders I, Rolton H, Danesh B. Risk factors for developing renal stones in inflammatory bowel disease. BJU Int. 2002;89:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Evan AP, Lingeman JE, Worcester EM, Bledsoe SB, Sommer AJ, Williams JC, Krambeck AE, Philips CL, Coe FL. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int. 2010;78:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | van Sommeren S, Janse M, Karjalainen J, Fehrmann R, Franke L, Fu J, Weersma RK. Extraintestinal manifestations and complications in inflammatory bowel disease: from shared genetics to shared biological pathways. Inflamm Bowel Dis. 2014;20:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Peña de la Vega L, Lieske JC, Milliner D, Gonyea J, Kelly DG. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. JPEN J Parenter Enteral Nutr. 2004;28:435-438. [PubMed] |
| 31. | Rodgers AL, Allie-Hamdulay S, Jackson GE, Sutton RA. Enteric hyperoxaluria secondary to small bowel resection: use of computer simulation to characterize urinary risk factors for stone formation and assess potential treatment protocols. J Endourol. 2014;28:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Worcester EM. Stones from bowel disease. Endocrinol Metab Clin North Am. 2002;31:979-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181:2573-2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Durrani O, Morrisroe S, Jackman S, Averch T. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20:749-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Valezi AC, Fuganti PE, Junior JM, Delfino VD. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg. 2013;23:1575-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Shimizu H, Ichikawa D, Tamagaki K, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Ota N, Otsuji E. Evaluation of postoperative nephrolithiasis and renal dysfunction in gastric cancer patients. Gastric Cancer. 2013;16:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Annuk M, Backman U, Holmgren K, Vessby B. Urinary calculi and jejunoileal bypass operation. A long-term follow-up. Scand J Urol Nephrol. 1998;32:177-180. [PubMed] |
| 40. | Dhar NB, Grundfest S, Jones JS, Streem SB. Jejunoileal bypass reversal: effect on renal function, metabolic parameters and stone formation. J Urol. 2005;174:1844-1846; discussion 1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Chen T, Godebu E, Horgan S, Mirheydar HS, Sur RL. The effect of restrictive bariatric surgery on urolithiasis. J Endourol. 2013;27:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Semins MJ, Matlaga BR, Shore AD, Steele K, Magnuson T, Johns R, Makary MA. The effect of gastric banding on kidney stone disease. Urology. 2009;74:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Nordenvall B, Backman L, Larsson L. Oxalate metabolism after intestinal bypass operations. Scand J Gastroenterol. 1981;16:395-399. [PubMed] |
| 44. | Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, Kellogg T, Stessman M, Moeding A, Monga M. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Wu JN, Craig J, Chamie K, Asplin J, Ali MR, Low RK. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2013;9:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Patel BN, Passman CM, Fernandez A, Asplin JR, Coe FL, Kim SC, Lingeman JE, Assimos DG. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis. 2014;10:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, Bergstralh EJ, Li X. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Froeder L, Arasaki CH, Malheiros CA, Baxmann AC, Heilberg IP. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol. 2012;7:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Semins MJ, Asplin JR, Steele K, Assimos DG, Lingeman JE, Donahue S, Magnuson T, Schweitzer M, Matlaga BR. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology. 2010;76:826-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Penniston KL, Kaplon DM, Gould JC, Nakada SY. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol. 2009;182:2340-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Hutchinson R, Parker AS, Arnold M, Bowers SP, Haley WE, Diehl N, Stone R, Lynch SA, Smith CD, Thiel DD. Prospective evaluation of 24-hour urine profiles following bariatric surgery in a modern comprehensive care bariatric clinic. Clin Nephrol. 2014;81:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Gonzalez RD, Canales BK. Kidney stone risk following modern bariatric surgery. Curr Urol Rep. 2014;15:401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Whitson JM, Stackhouse GB, Stoller ML. Hyperoxaluria after modern bariatric surgery: case series and literature review. Int Urol Nephrol. 2010;42:369-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Sakhaee K, Griffith C, Pak CY. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis. 2012;8:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |