Published online Nov 6, 2014. doi: 10.5527/wjn.v3.i4.169
Revised: August 6, 2014
Accepted: September 4, 2014
Published online: November 6, 2014
Processing time: 231 Days and 20.7 Hours
Proteinuria is a frequently detected symptom, found in 20% of pregnancies. A common reason for proteinuria in pregnancy is preeclampsia. To diagnose preeclampsia clinically and to get new insights into the pathophysiology of the disease it is at first essential to be familiar with conditions in normal pregnancy. Animal models and biomarkers can help to learn more about disease conditions and to find new treatment strategies. In this article we review the changes in kidney function during normal pregnancy and the differential diagnosis of proteinuria in pregnancy. We summarize different pathophysiological theories of preeclampsia with a special focus on the renal facets of the disease. We describe the current animal models and give a broad overview of different biomarkers that were reported to predict preeclampsia or have a prognostic value in preeclampsia cases. We end with a summary of treatment options for preeclampsia related symptoms including the use of plasmapheresis as a rescue therapy for so far refractory preeclampsia. Most of these novel biomarkers for preeclampsia are not yet implemented in clinical use. Therefore, we recommend using proteinuria (measured by UPC ratio) as a screening parameter for preeclampsia. Delivery is the only curative treatment for preeclampsia. In early preeclampsia the primary therapy goal is to prolong pregnancy until a state were the child has an acceptable chance of survival after delivery.
Core tip: This review summarises different pathophysiological theories of preeclampsia with a special focus on the renal facets of the disease. In this context current animal models are presented. The reader gets a broad overview about different biomarkers for preeclampsia. Furthermore, the article discusses treatment options for preeclampsia related symptoms including the use of plasmapheresis as a rescue therapy for so far refractory preeclampsia.
- Citation: Müller-Deile J, Schiffer M. Preeclampsia from a renal point of view: Insides into disease models, biomarkers and therapy. World J Nephrol 2014; 3(4): 169-181
- URL: https://www.wjgnet.com/2220-6124/full/v3/i4/169.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i4.169
There are many physiological changes in the function of different organs during normal pregnancy. Changes in kidney function and low grade proteinuria are common findings in pregnancy. However, new onset of proteinuria is also one of the primary symptoms for the clinical diagnosis of preeclampsia[1]. To understand pathological conditions in pregnancy it is important to know the normal changes and also typical complications that may occur during pregnancy. In pregnancy the physician always treats two patients. What is required for treatment of the mother is not always beneficial for the child. Many drugs are contraindicated in pregnancy or there is no data on their safety. We review the development of kidney function and proteinuria in pregnancy in general and then discuss preeclampsia in particular. This is likely the first review where all prevailing animal models for preeclampsia and all currently suggested markers for early detection of the disease are presented with special focus on the kidney. Further more, we give treatment strategies for preeclampsia and discuss controversial new methods for therapy refractory preeclampsia.
During a normal pregnancy kidneys increase in size and the kidney volume can enlarge up to 30%[2]. In pregnant women the ureter and the renal pelvis are frequently dilated which can lead to an increased risk for urinary tract infections and pyelonephritis. There are no renal histological changes due to pregnancy but higher urinary frequency, nocturia, dysuria, urgency and stress incontinence are common[3]. The glomerular filtration rate (GFR) rises by approximately 40% to 50% above baseline levels in pregnancy. Thus, a normal serum creatinine can actually reflect significant renal insufficiency in a pregnant woman. Equations like Modification of Diet in Renal Disease (MDRD) formula, Cockcroft-Gault formula and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula are usually used to estimate GFR but are not accurate during pregnancy. Smith et al[4] compared eGFR calculated using the MDRD formula with inulin clearance in 24 healthy women during and after pregnancy. They found that MDRD formula underestimated GFR by more than 40 mL/min during pregnancy. The Cockcroft-Gault formula, the MDRD formula and the CKD-EPI formulas were evaluated for their accuracy in preeclampsia by Alper et al[5] All of these equations were inaccurate in predicting GFR in preeclamptic women compared with creatinine clearance obtained from 24-h urine collections.
Cystatin-C does not seem to be a useful marker in pregnancy because it increases in the third trimester. This is thought to be due to changes in size and charge selectivity of the glomerular filtration barrier leading to decreased filtration of cystatin-C [6]. Increased placental production of cystatin-C may also play a role in the increased levels observed in late pregnancy[7]. In line with this Saxena et al[8] found that serum cystatin-C did not correlate with inulin clearance during pregnancy or postpartum. Therefore, the best method to determine the GFR in pregnancy is the mean of urea and creatinine clearance obtained from collected urine.
There are several methods used clinically to measure proteinuria. A dipstick test is the easiest method to quantify proteinuria, however, in a recent study the dipstick test in spot-urine over and underestimated proteinuria[9]. Another alternative to estimate proteinuria is the urine protein creatinine (UPC) ratio. A UPC-ratio above 700 mg/g creatinine predicts significant proteinuria while a UPC ratio of less than 150 mg/g creatinine is normal. Nevertheless, the UPC ratio has several disadvantages. It cannot detect changes in proteinuria over the course of the day or take into account orthostatic changes that can potentially cause relevant changes in proteinuria. Moreover, likely day-to-day biological variation of the UPC-ratio has to be considered and only relatively large changes indicate a reliable change in disease status[10]. Proteinuria is known to have a circadian rhythm so when samples for the calculation of UPC ratio are collected at a fixed time of the day UPC ratio can be an acceptable alternative for 24-h urine collections, especially in an outpatient setting[11]. UPC ratio was a reasonable “rule-out” test for detecting proteinuria of 0.3 g/d or more in hypertensive pregnancy[12], but normal UPC ratio cannot rule out mild preeclampsia[13].
Different cut-off values for UPC ratio have been suggested with different results. In one study a cut-off value of 220 mg/g creatinine predicted significant proteinuria with 87% sensitivity and 92.6% specificity[14]. According to another study random UPC ratio is helpful primarily when it is below 150 mg/g creatinine, in that proteinuria of more than 300 mg is unlikely below this threshold. The accuracy of this UPC ratio in predicting 300 mg of protein in 24-h urine collection in pregnant patients with suspected preeclampsia had a sensitivity ranged from 90%-99% and specificity ranged from 33%-65%[15]. In contrast to that UPC ratio and 24-h urine total protein level showed a poor correlation with negative predictive value of 47.5% and specificity of 55.8% in a study of 220 women by Durnwald et al[16] Nevertheless he same authors admit that UPD ratio can predict severe preeclampsia and thus can be used for rapid diagnosis of severe preeclampsia as the correlation of UPD ratio and 24-h proteinuria increases with the amount of proteinuria.
Therefore the gold standard for proteinuria is the 24-h urine collection. However, the 24-h urine collection is inconvenient for pregnant women, expensive and may be inaccurate due to insufficient collection. To address this problem and for validation of the results quantification of the urine creatinine excretion should be done. Urinary creatinine excretion should be between 15 and 20 mg/kg body weight if the collection was adequate.
Urinary protein excretion increases due to both increased glomerular filtration and increased permeability of the glomerular filtration barrier during normal pregnancy. Urinary protein excretion rises to about 180 to 200 mg/d in the third trimester of the pregnancy. In women with pre-existing proteinuria the rise in proteinuria is often higher and cannot be explained by increased GFR alone.
The majority of women with pre-existing glomerular disease have increased proteinuria during the course of their pregnancy and can develop nephrotic range proteinuria in the third trimester. Nevertheless, the presence of nephrotic syndrome due to renal disease, in the absence of significant renal insufficiency or significant hypertension, does not seem to affect the natural course of renal disease or foetal survival[17]. De novo renal disease like lupus nephritis or renal diseases secondary to diabetes or hypertension are other possible causes of increased proteinuria in pregnant women. In addition a symptomatic urinary tract dilatation may also be associated with proteinuria in pregnancy[18]. Thus, the underlying reason for proteinuria in pregnancy is often clinically uncertain. Sometimes a definitive cause of renal disease can only be found histologically. The published evidence for the benefit of a kidney biopsy during pregnancy is heterogeneous and there are only a few reports of renal biopsies during pregnancy which were performed to determine the definite diagnosis of renal disease.
Packham et al[19] reported 111 renal biopsies performed before the 29th week of gestation where complications of the procedure were similar to those in the non-pregnant population. Day et al[20] showed that pregnancy itself does not increase the risk associated with a renal biopsy. In contrast to that, other investigators reported a significantly higher risk of complications for kidney biopsies in pregnancy, with a peak at around the 25th gestational week[21]. Some clinicians prescribe empirical therapy with steroids in nephrotic syndrome in pregnancy. However, diabetic nephropathy or amyloidosis may be exacerbated by steroid therapy. Lupus nephritis during pregnancy follows a variable course and the type and extent of renal lesions can only be assessed histologically. Patients with a biopsy-proven diagnosis of mesangial-proliferative lupus nephritis usually have a favourable prognosis. Diffuse proliferative lupus nephritis typically results in a decreased glomerular filtration rate, a poor prognosis and requires aggressive therapy. Renal biopsy for the diagnosis of glomerulonephritis or preeclampsia led to therapeutic changes in 66% of cases[21]. In general we would recommend waiting until postpartum before performing a renal biopsy unless an unexplained rapidly progressive loss of renal function or unexplained nephrotic range proteinuria occurs. Therapeutic options in pregnancy are given below.
A common reason for increased proteinuria in pregnancy is preeclampsia. Preeclampsia affects 2%-8% of pregnancies and is defined as the combination of pregnancy induced hypertension and proteinuria[22]. Recently the American College of Obstetricians and Gynecologists removed proteinuria as an essential criterion for diagnosis of preeclampsia in 2013[23]. Therefore, it is possible that in recent studies 10% of women with clinical and/or histological manifestations of preeclampsia had no proteinuria[24].
It has been hypothesized that preeclampsia results from a reduction in uteroplacental perfusion which leads to uteroplacental ischemia. In the preeclamptic placenta trophoblasts do not develop normally and are unable to invade the myometrium effectively[25]. Specifically the placental tissue but not the foetus is involved in the development of preeclampsia, since preeclampsia also occurs in women with a hyaditiform mole[26-29]. Risk factors for preeclampsia include family history of preeclampsia, multiple gestation, nulliparity, obesity, older maternal age, molar pregnancies, diabetes mellitus, pre-existing hypertension, chronic renal disease and thrombotic vascular disease[30-33]. Paradoxically, smoking during pregnancy is associated with a reduced risk of preeclampsia[34,35]. Nicotine inhibition of thromboxane A2 production might explain this. However, it must be stated that smoking in general and especially during pregnancy has an increased health risk and is absolutely contraindicated.
Preeclampsia can cause small-for-gestational-age infancy, preterm delivery, hypoxic neurologic injury and foetal death. Perinatal mortality is approximately 10% and maternal mortality even occurs in 10% to 15%[36]. Maternal complications of preeclampsia include renal failure, eclampsia, HELLP syndrome (haemolysis, elevated liver enzymes, and thrombocytopenia), seizures, liver failure and stroke. In contrast to normal pregnancy where blood urea nitrogen (BUN) and creatinine decrease, preeclamptic women have BUN and creatinine levels similar to non-pregnant women due to reduced GFR and RPF.
Clinical signs of preeclampsia generally resolve spontaneously within 12 wk after delivery whereas proteinuria due to other renal disease does not. New-onset proteinuria after 20 wk of gestation together with new-onset hypertension is a strong indicator of preeclampsia. The severity of proteinuria does not correlate with the severity of preeclampsia and can even be absent in 10% of the cases[1,37,38]. However, a high UPC ratio in preeclamptic women is associated with a highly increased likelihood of adverse maternal outcomes[39].
In cases where information on the presence or absence of proteinuria in early pregnancy is lacking, the distinction between an underlying primary renal disease and preeclampsia can be very difficult. If thrombotic thrombocytopenic purpura occurs for the first time during pregnancy, it may mimic severe preeclampsia.
The timing of preeclampsia can also be atypical with onset before the 20th week of gestation or up to 4 wk postpartum. Thus, in some cases, the distinction between preeclampsia and other renal diseases in pregnancy can only be made in retrospect.
Postpartum preeclampsia is the occurrence of hypertension and proteinuria after delivery. Late postpartum eclampsia is an atypical form of eclampsia beginning between 48 h to 4 wk after delivery[40]. The incidence of postpartum preeclampsia is dependent on the population included in the study. In one 10-year retrospective case series it was 5.7%[41]. In the same analysis 15.9% of hypertensive or preeclamptic women in the postpartum period develop eclampsia.
Most patients with postpartum preeclampsia have no evidence of preeclampsia during pregnancy[42]. Hypertension is a common but not universal finding in postpartum preeclampsia. In postpartum preeclampsia proteinuria may occur less often than in preeclampsia during pregnancy[43]. Seizures are often more severe and refractory to treatment.
Persistence of trophoblasts is associated with the development of preeclampsia in gestational trophoblastic disease[28,29]. Even though found only on the microscopic level, trophoblastic, tissue was found in patients with postpartum preeclampsia and suggests that it causes the disease. Epstein et al[44] demonstrated that women with preeclampsia develop hypertension more often than their non-preeclamptic siblings.
A number of animal models have been proposed for preeclampsia but have some limitations. Mice have shallow trophoblast invasion and three trophoblast layers verses a single layer of trophoblasts of the human placenta in pregnancy. Therefore mice models are less useful for studying trophoblast invasion processes. In order to model reduced uterine perfusion pregnant rats undergo clipping of the aorta above the iliac bifurcation at day 14 of gestation[45]. There are also some mouse models of preeclampsia that employ manipulation of sFlt-1, VEGF 121[46], endothelin, endothelial nitric oxide synthase[47] or the renin-angiotensin system[48].
From a renal point of view the sFLT model is one of the most promising because it is the only one that shows glomerular endotheliosis as well as hypertension and proteinuria[49]. Karumanchi et al[50] created a rat model of preeclampsia by administration of a sFLT1-expressing adeno-virus. The administration of the sFLT1 by this vector resulted in a dose-dependent hypertension, proteinuria, and glomerular endotheliosis in pregnant rats[50]. As preeclampsia only occurs spontaneously in pregnant women, no animal model can completely mimic the entire pathogenesis of human preeclampsia and all animal models only reflect some limited aspects of the underlying disease. Thus, the definitive studies on preeclampsia must be clinical.
In the last few years many potentially useful biochemical markers have been proposed for the prediction and outcome of preeclampsia. The timeframe of diagnostic usefulness of these biomarkers to distinguish women at risk for preeclampsia from healthy pregnant women will be reviewed below.
Gant et al[51] identified hypersensitivity to infused Angiotensin II in preeclamptic patients. However, circulating levels of Angiotensin II are not increased in preeclampsia[52]. Instead, immunoglobulins from preeclamptic women increased the beating rate of neonatal rat cardiomyocytes. These immunoglobulins contained Angiotensin II type 1 (AT1) autoantibodies that stimulate the Angiotensin-receptor. The increased heartbeat rate could be blocked by treatment with losartan and it could be demonstrated that the autoantibodies bind to the second extracellular loop of the AT1 receptor[53]. AT1 agonistic autoantibodies are not only found in preeclampsia but also in antibody mediated kidney transplant rejection[54]. In kidney-transplant recipients who had severe allograft dysfunction without anti-HLA antibodies but detection of AT1 agonistic autoantibodies rejection was accompanied by accelerated hypertension and convulsions[55]. It is proposed that similar mechanisms might be involved in preeclampsia and refractory allograft rejection and it was found that one rejecting kidney-transplant recipient had had preeclampsia 16 years earlier[55].
Pregnancy is associated with high concentrations of adrenomedullin in maternal and foetal blood and in the amniotic fluid[56]. Adrenomedullin has a potent and long-lasting hypotensive effect when injected intravenously in anaesthetised rats. Hata et al[57] measured circulating adrenomedullin concentrations in preeclampsia and normotensive pregnant women and showed that adrenomedullin concentrations are significantly lower in preeclamptic women.
Renal involvement in preeclampsia can be at least partly explained by impaired podocyte function. Podocytes are the major source of VEGF in the glomerulus[58]. Podocyte-derived VEGF has paracrine functions on endothelial cells as well as autocrine functions on the podocytes themselves[58-60]. New data suggest that detection of podocyturia might serve as an early diagnostic marker for preeclampsia prior to the development of proteinuria and hypertension. Garovic et al[61] showed that podocyturia is present at delivery in women with preeclampsia. Podocyturia also had a significantly greater sensitivity and specificity for the subsequent diagnosis of preeclampsia than any single angiogenic marker or a combination thereof in the second trimester[62]. A strong correlation was found by Aita et al[63] between the number of podocytes lost in urine and blood pressure, but no correlation with proteinuria. Several markers have been used in different studies to detect podocyturia. Nevertheless, it is important to keep in mind that the expression of marker proteins does not allow a definite allocation of the involved glomerular cell types. De- or transdifferentiation and detachment of cells as well as changes in the urine milieu have a direct effect on marker protein expression. According to Skoberne et al[64], the urine markers most reliable for assessing disease activity of certain glomerular diseases are PDX- or CD68-positive cells.
Recently, quantitative polymerase chain reaction for podocyte-specific markers was found to be a rapid method to detect preeclampsia. Significantly elevated mRNA levels of nephrin, podocin, and VEGF were detected in preeclamptic women compared with healthy controls[65].
Placental protein 13 (PP13) is a member of the galectin super family and is important for differentiation and proliferation. Than et al[66] found reduced PP13 mRNA levels in placentas obtained from patients with preeclampsia and HELLP syndrome in the first trimester compared to controls. Blood levels of PP13 mRNA were also significantly lower in preeclampsia compared to controls[67].
Pregnancy associated plasma protein-A (PAPP-A) is mainly produced by the placental trophoblasts. PAPP-A and PP13 serum levels were significantly lower in the first and second trimesters in women who developed preeclampsia[68]. First-trimester PAPP-A provided a prediction for preeclamspia when combined with uterine artery pulsatility measured by Doppler velocimetry[69].
During the first trimester of pregnancy, the foeto-placental unit is the main source of circulating activin A and inhibin A. Activin A enhances Follicle-stimulating hormone (FSH) biosynthesis and secretion and is involved in the control of trophoblast cell differentiation in the first trimester. Inhibin A down regulates FSH synthesis and inhibits FSH secretion. Activin A seems to be a sensitive marker for the risk of later development of preeclampsia at 21-25 wk of gestation[70]. Inhibin A is thought to be more sensitive than activin A in predicting cases of early-onset preeclampsia at 15-19 wk of gestation[70].
P-selectin belongs to the group of cell adhesion molecules. It is expressed in granules of platelets and the Weibel-Palade bodies of endothelial cells and is involved in leukocyte-endothelial interactions. The P-selectin concentration was found to have a negative predictive value of almost 99% for preeclampsia. Mean plasma P-selectin concentrations were significantly elevated at 10-14 wk of gestation in women who later developed preeclampsia[71]. Wang et al[72] suggested that the increase in neutrophil-endothelial adhesion and activation seen in preeclampsia is at least in part due to up-regulation of P-selectin. This would be in line with the theory that preeclampsia reflects an excessive maternal inflammatory response to pregnancy[73].
Another inflammatory molecule involved in preeclampsia is Pentraxin 3. It is expressed in response to inflammatory stimuli by endothelial cells, monocytes, macrophages and fibroblasts. Elevated maternal plasma levels of pentraxin 3 in preeclamptic in comparison to normal pregnancies could represent altered endothelial function[74,75]. The increase in maternal plasma develops from 11th to 13th week of gestation in women with subsequent preeclampsia[76].
Maternal plasma fibronectin levels of patients with preeclampsia were significantly higher than those of healthy pregnant women[77]. Significant elevations in fibronectin levels with an extra type III domain occurred in the first trimester before clinical evidence of preeclampsia. Fibronectin plays a major role in embryonic development, cell adhesion, growth, migration and differentiation.
Heat-shock proteins (Hsps) are highly conserved molecules that have chaperone functions. Circulating Hsps may also be cytoprotective, as exogenous Hsp70 increases the survival and protects from apoptosis in stressed arterial smooth muscle cells[78]. Fukushima et al[79] reported significantly higher Hsp70 serum levels in preeclampsia. Higher serum levels of Hsp70 were also found in patients with early onset of severe preeclampsia[80,81]. The difference in serum Hsp70 concentration between preeclamptic patients and the control group was statistically significant in each gestational age. Thus, Hsp70 might not only be a marker but also play a role in the pathogenesis of preeclampsia.
Gene expression profile studies identified the regulation of soluble fms-like tyrosine kinase 1 (sFlt-1) in preeclampsia. SFlt-1 binds and antagonises vascular endothelial growth factor (VEGF) and placental growth factor (PlGF).
The described functions of VEGF include induction of matrix metalloproteinases, regulation of angiogenesis, lymphangiogenesis and hematopoiesis and cell signalling. Serum concentration of sFlt-1 decreases from 8-12 wk to 16-20 wk of gestation, gradually increases at 26-30 wk of gestation and rapidly elevates at 35-39 wk of gestation in normal pregnancy[82].
SFlt1 concentrations increased gradually throughout pregnancy in women with preeclampsia and was significantly higher between 25 and 28 wk of gestation in women with preeclampsia than in women with normal pregnancies or isolated hypertension[83]. Of note, sFlt1 are high 5-6 wk prior to the onset of preeclampsia and correlate with the severity of disease[83-85]. In rats sFlt-1 infusion increased vascular and placental oxidative stress, decreased maternal circulating VEGF and NO and reduced foetal weight[85].
Serum concentration of PlGF increases gradually from 8 wk until 29-32 wk of gestation and then decreases at 33-40 wk of gestation in normal pregnancy[79]. PlGF levels in women who later developed preeclampsia were significant lower than those of controls from 13-16 wk of gestation until delivery[85]. As the change of PlGF occurs earlier than that of sFlt-1, it might be the better angiogenic factor for predicting preeclampsia. Serum sFlt-1 to PlGF ratio (sFLT-1/PIGF) was also suggested as screening parameter. An adenovirus-expressing sFlt-1 in rodents caused a clinical syndrome with glomerular endotheliosis, proteinuria, and hypertension[86]. Glomerular capillary endotheliosis is another typical lesion in preeclampsia.
Serum levels of sEng in normal pregnancy are quite stable and slightly increase by 33-42 wk of gestation[87]. Placental endoglin is up-regulated in preeclampsia and released in the circulation. Rising levels of circulating soluble endoglin (sEng) herald the onset of preeclampsia. Women with higher sEng levels at 21 through 32 wk of gestation had an increased risk of preterm preeclampsia and an increased risk for a small-for-gestational-age infant[87].
Cellfree fetal DNA (cffDNA) is increased at 11-13 wk of gestation in pregnancies that experience preeclampsia[88]. Hypoxia within the intervillous space of the placenta leads to tissue oxidative stress and increases placental apoptosis and necrosis. This might be the cause of increased levels of cffDNA. Elevated cffDNA is not specific for preeclampsia and is also seen in other conditions associated with placental pathology[17].
Uric acid is the end product of purine metabolism in the liver. In normal pregnancy uric acid concentrations initially fall 25%-35% due to estrogens, expanded blood volume and increased glomerular filtration rate[89]. By term concentrations slowly rise to those observed in non-pregnant women. In contrast to that, uric acid levels increase at 10 wk of gestation and continue to rise until 48 h postpartum in preeclamptic women[90]. The increase in uric acid precedes the reduction in plasma volume in preeclampsia[91]. Uric acid may be protective during preeclampsia as an antioxidant, but is at the same time proinflammatory and contributes to endothelial dysfunction[92]. In a recent study the concentration of serum uric acid in preeclamptic women was associated with disease severity[93].
Nevertheless, lowering uric acid with probenacid had no effect on the degree of hypertension in preeclamptic women[94]. Another study with allopurinol showed no significant effects on the outcome of pregnancy in humans[95].
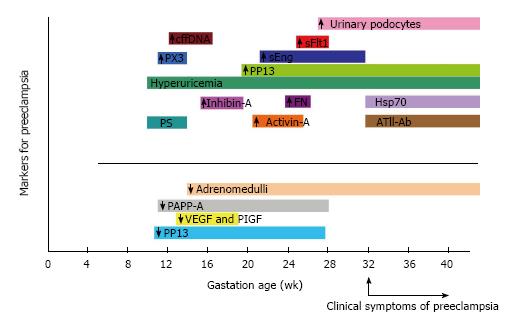
A summary of timed expression of these biomarkers in preeclampsia is given in Figure 1.
The optimal management of a pregnant woman with preeclampsia depends on gestational age and disease severity. Delivery is the only curative treatment for preeclampsia. Indicators for delivery in preeclampsia are given in Table 1 (modified from[22]).
| Maternal | Foetal |
| Eclampsia | Severe foetal growth retardation |
| Shortness of breath, pulse oximetry of < 94% on room air, pulmonary oedema | severe oligohydramnios |
| AST or ALT > 2 times above normal | Foetal death |
| Uncontrolled severe hypertension | Repetitive late or variable foetal heart rate decelerations |
| Oliguria , serum creatinine level of ≥ 1.5 mg/d | Umbilical artery doppler imaging with reverse diastolic blood flow |
| Suspected abruptio placentae | |
| Persistent platelet count < 100000 /mm3 |
The severity of the disease must always be weighed against the risks of infant prematurity. A mild preeclampsia at or beyond 37 wk should be delivered. In severe preeclampsia, induction of delivery should be considered after 34 wk of gestation. Prior to induction corticosteroids should be given to accelerate lung maturity. The prevention of seizures and adequate control of maternal blood pressure should also be of high priority. Maternal evaluation includes monitoring of blood pressure, urine output, cerebral status, epigastric status, tenderness or vaginal bleeding. Platelet count, liver enzymes and serum creatinine should be controlled closely. The target of blood pressure is between 140-160 mmHg systolic and 90-105 mmHg diastolic. The blood pressure should not be lowered under 140/90 mmHg to prevent insufficient utero-placental blood flow and reduced birth weight[96,97]. Foetal evaluation includes foetal heart rate monitoring, a biophysical profile, ultrasonographic assessment of foetal growth, amniotic fluid status and umbilical artery doppler velocimetry.
Methyldopa, nifedipine, labetalol and hydralazine are the antihypertensives of choice for preeclampsia. Oral Methyldopa is suggested for mild to moderate hypertension in an outpatient with preeclampsia. Oral nifedipine is used for treatment of moderate or severe pregnancy hypertension in a dose of 10-20 mg every 4-6 h[98]. As a calcium channel blocker nifedipine acts on arteriolar smooth muscle cells and induces vasodilatation by blocking calcium entry into the cells. The side effects of nifedipine include tachycardia, palpitations and headaches. The calcium channel blockade with isradipine lowered the maternal mean arterial blood pressure in women with hypertension but not in women with proteinuria[99].
In hospital settings intravenous hydralazine (5-10 mg every 15-30 min) is commonly administered for hypertensive emergencies associated with pregnancies. Hydralazine is a direct peripheral arteriolar vasodilator. The most common adverse effect of hydralazine is the unpredictable hypotension. Other side effects are headache, nausea, maternal hypotension and vomiting. Labetalol is a selective alpha blocker and a nonselective beta blocker. The side effects of labetalol are dizziness, nausea and headaches. When the medications mentioned above have failed to lower blood pressure sodium nitroprusside may be given. Nitroprusside causes vasodilatation by the release of nitric oxide. Severe rebound hypertension may result. Therefore, nitroprusside should be reserved for use in postpartum care or just before the delivery because cyanide poisoning of the foetus is also a possible side effect.
Despite peripheral oedema, the intravascular volume is depleted in patients with preeclampsia. In contrast, pulmonary oedema can occur 48-72 h postpartum due to mobilization of extravascular fluid.
As preeclampsia is characterized by a reduction in circulating plasma volume diuretics are not generally recommended in preeclampsia. There are significant warnings against the use of thiazides during pregnancy like metabolic risks to the mother and fetus including hyponatremia, hypokalemia, thrombocytopenia, hyperglycemia.
Furthermore they may result in inhibition of labor and decrease placental perfusion in pregnany. Therefore we do not recommend the general use of diuretics in preeclampsia.
Nevertheless, the use of thiazide diuretics or loop diuretics is occasionally indicated for severe intractable or pulmonary oedema. The reduction of excessive oedema should be done slowly and under close supervision. There are no recommendations for dating, dosage or time for diuretics in this situation as it would be an individual symptom dependent and symptom guided therapy. We recommend to get written approval for the use of diuretics from the mother after detailed education on risks and side effects during pregnancy. Measures to respond to blood pressure drops must always be available and vital signs of the mother and foetus must be controlled continuously under diuretic treatment. This includes continuous electronic foetal heart rate monitoring and cardiovascular monitoring of the mother.
Magnesium sulfate is the drug of choice for the treatment and prevention of eclampcia. Magnesium sulphate more than halves the risk of eclampsia[100]. The Magpie-Trial compared magnesium sulphate with placebo for women with preeclampsia and found a preventive effect[101,102]. Therefore, prophylactic treatment with magnesium sulfate is indicated for all patients with severe preeclampsia. There is no consensus if patients with mild preeclampsia need magnesium prophylaxis. However, active seizures should be treated with an intravenous loading dose of 4 g magnesium sulphate over 5-10 min followed by an infusion of 1 g/h for 24 h. Seizures that are refractory to magnesium sulfate may be treated with lorazepam and phenytoin.
There is an imbalance between thromboxane and prostacyclin production in preeclampsia. Thus, the use of low-dose aspirin in preeclampsia seems to be reasonable. Wallenburg et al[103] conducted the first prospective double-blind controlled trial using 60 mg aspirin per day for the treatment of women at risk for preeclampsia. Only two of the 23 treated women versus 12 of the 23 controls became preeclamptic. Supplementation of Aspirin at or before the 16th week of pregnancy reduced preterm preeclampsia without any effect on term preeclampsia[104]. In other studies low-dose aspirin had no significant effect on the incidence of preeclampsia in the low-risk groups but was more beneficial in high-risk groups[105,106].
The experience and safety of plasmapheresis (PE) in pregnancy is limited to case reports. In 1986 a successful use of plasmapheresis during pregnancy was reported in a patient with unusually fulminant, antibody-negative myasthenia gravis[107].
Another case report has suggested that PE may be a successful treatment for pregnant women with antiphospholipid syndrome[108]. PE was also successfully used in pregnant patients with acute fatty liver of pregnancy[109,110]. Additionally two cases of hypertriglyceridemia-induced acute pancreatitis during pregnancy and a case of a pregnant woman with Pemphigus vulgaris were successfully treated by PE[111,112]. Thrombocytopenia associated with microangiopathic disease in severe preeclampsia generally resolves within 3 to 4 d after delivery. It was suggested to use PE when thrombocytopenia persists beyond this time[113].
In preeclampsia a potentially useful approach of PE would be to subtract circulating autoantibodies from maternal circulation. In 1986 fourteen cases of PE with fresh frozen plasma for maternal indications in selected cases of preeclampsia and eclampsia were reviewed with promising results[114]. In contrast, PE did not prolong pregnancy in preterm preeclampsia in a report by Martin et al[115]. In another report, plasma exchange was commenced at 23, 26 and 29 wk of gestation in preeclamptic women and continued until delivery. Here preeclamptic signs regressed and renal function stabilised. One baby with severe hyaline membrane disease died but the others were delivered in good health[116].
Like PE with fresh frozen plasma heparin-mediated extracorporeal low-density lipoprotein precipitation has been attempted in preeclampsia[117]. Pregnancies were prolonged for 3 to 49 d in 9 very preterm preeclamptic women by the use of this apheresis.
Thadhani et al[118] hypothesized that a selective adsorption column would create a concentration gradient and augment the removal of sFlt1. They treated 5 women with very preterm preeclampsia with dextran sulfate cellulose apheresis. This treatment reduced circulating sFlt-1 levels and proteinuria in a dose-dependent manner and stabilized blood pressure without apparent adverse events. Dextran sulfate cellulose apheresis was able to reduce sFlt-1 plasma levels by 20%-30%. Pregnancy was continued for 15 d with the use of two apheresis and for 23 d with four apherese sessions[118].
Maternal blood pressure was stable and was not markedly decreased after apheresis. However, antihypertensive medications were stopped before treatments and saline infusions were given during treatments. Three patients with postpartum HELLP syndrome and persistent thrombocytopenia were treated with PE with prompt resolution of their diseas[113]. There is no animal model for plasmapheresis at the meantime. Therefore the only way to get more experience on this filed is the clinical use in selected patients.
Taken together single cases indicate that PE seems to be a treatment with low risk during pregnancy and could be a promising treatment option for otherwise refractory preeclampsia. We recommend only use this therapy in specialized centers with first class experience on the filed and with written consent of the mother after detailed education about the risks and experimental status of this therapy.
Serum albumin levels of preeclamptic women are often even below 10 g/L. Thus, one can suggest albumin substitution in preeclampsia. Albumin infusions increased serum albumin and colloid osmotic pressure values in preeclampsia[119].
However, daily albumin infusions did not lower blood pressure and was unable to stabilise renal function. Albumin substitution was also associated with higher foetal mortality[120]. Therefore, we do not recommend using albumin substitution in preeclampsia.
Uterine curettage immediately after delivery accelerates the recovery of severe preeclampsia[121]. This operation was also successfully used in single cases of postpartum preeclampsia[122,123]. Due to the fact that even microscopic level of trophoplastic tissue can perpetuate preeclampsia uterine curettage should be done after delivery.
We reviewed the literature with a focus on proteinuria during pregnancy and preeclampsia. Several new diagnostic markers for preeclampsia were presented. Most of these are not yet implemented in clinical use and several are only used in studies or experimental conditions. Therefore, we recommend using proteinuria as a screening parameter for preeclampsia. The first screening should be UPC ratio. If the UPC ratio is below 150 mg/g total proteinuria is unlikely to be more than 300 mg/d and needs no further investigation at that time. For a UPC ratio greater than 150 mg/g we suggest performing a full 24-h urine protein collection as a second diagnostic tool for confirming accurate results. If a new onset of proteinuria greater than 300 mg/d together with hypertension and/or general oedema occurs after the 20th week of gestation the diagnosis of preeclampsia can be made. Atypical presentation must always be kept in mind. We presented different therapeutic options for preeclampsia.
Delivery is the only curative treatment for preeclampsia. In early preeclampsia the primary therapy goal is to prolong pregnancy until a state were the child has an acceptable chance of survival after delivery. In the mean time close maternal and foetal monitoring and evaluation is necessary. We presented therapeutic options to treat hypertention, oedema and seizures during this period.
Plasmapheresis is not a common treatment strategy in preeclampsia but could be considered as rescue therapy in otherwise therapy refractory cases.
When performing PE in preeclampsia measures to respond to blood pressure drops must always be available and vital signs must be controlled during and after the entire session. This includes continuous electronic foetal heart rate monitoring and cardiovascular monitoring of the mother. Eventually, antihypertensives must be paused before and/or after PE. In general after the 34th week of gestation delivery is the better choice of treatment for both mother and child.
We searched PubMed for articles with the keywords “preeclampsia AND animal models”, “treatment of preeclampsia”, “proteinuria in pregnancy”, “treatment of preeclampsia”, “markers for preeclampsia”, “podocyturia”, “physiological changes in pregnancy”, “plasmapheresis in pregnancy”. We also searched the bibliographies of the articles retrieved for further relevant references.
P- Reviewer: Khalil RA, Rasmussen S, Tsikouras P, Xiu QZ S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy: the need for a more pathophysiological approach. Obstet Gynecol. 2010;115:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Rasmussen PE, Nielsen FR. Hydronephrosis during pregnancy: A literature survey. Eur J Obstet Gynecol Reprod Biol. 1988;27:249-59. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 100] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Francis WJ. Disturbances of bladder function in relation to pregnancy. J Obstet Gynaecol Br Emp. 1960;67:353-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Smith MC, Moran P, Ward MK, Davison JM. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG. 2008;115:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Alper AB, Yi Y, Rahman M, Webber LS, Magee L, von Dadelszen P, Pridjian G, Aina-Mumuney A, Saade G, Morgan J. Performance of estimated glomerular filtration rate prediction equations in preeclamptic patients. Am J Perinatol. 2011;28:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Strevens H, Wide-Swensson D, Torffvit O, Grubb A. Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women. Indications of altered filtration process in pregnancy. Scand J Clin Lab Invest. 2002;62:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Kristensen K, Larsson I, Hansson SR. Increased cystatin C expression in the pre-eclamptic placenta. Mol Hum Reprod. 2007;13:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Saxena AR, Ananth Karumanchi S, Fan SL, Horowitz GL, Hollenberg NK, Graves SW, Seely EW. Correlation of cystatin-C with glomerular filtration rate by inulin clearance in pregnancy. Hypertens Pregnancy. 2012;31:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yamada T, Kojima T, Akaishi R, Ishikawa S, Takeda M, Kawaguchi S, Nishida R, Morikawa M, Yamada T, Minakami H. Problems in methods for the detection of significant proteinuria in pregnancy. J Obstet Gynaecol Res. 2014;40:161-166. [PubMed] |
| 10. | Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ. Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis. 2013;62:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Koopman MG, Krediet RT, Koomen GC, Strackee J, Arisz L. Circadian rhythm of proteinuria: consequences of the use of urinary protein: creatinine ratios. Nephrol Dial Transplant. 1989;4:9-14. [PubMed] |
| 12. | Côté AM, Brown MA, Lam E, von Dadelszen P, Firoz T, Liston RM, Magee LA. Diagnostic accuracy of urinary spot protein: creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ. 2008;336:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Aggarwal N, Suri V, Soni S, Chopra V, Kohli HS. A prospective comparison of random urine protein-creatinine ratio vs 24-hour urine protein in women with preeclampsia. Medscape J Med. 2008;10:98. [PubMed] |
| 14. | Eslamian L, Behnam F, Tehrani ZF, Jamal A, Marsoosi V. Random urine protein creatinine ratio as a preadmission test in hypertensive pregnancies with urinary protein creatinine ratio. Acta Med Iran. 2011;49:81-84. [PubMed] |
| 15. | Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008;112:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Durnwald C, Mucer B. A prospective comparison of total protein/creatinine ratio versus 24 hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Bianchi DW. Circulating fetal DNA: Its origin and diagnostic potential-a review. Placenta. 2004;25 Suppl A:S93-S101. |
| 18. | Piccoli GB, Attini R, Parisi S, Vigotti FN, Daidola G, Deagostini MC, Ferraresi M, De Pascale A, Porpiglia F, Veltri A. Excessive urinary tract dilatation and proteinuria in pregnancy: A common and overlooked association? BMC Nephrol. 2013;14:52. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Packham D, Fairley KF. Renal biopsy: indications and complications in pregnancy. Br J Obstet Gynaecol. 1987;94:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Day C, Hewins P, Hildebrand S, Sheikh L, Taylor G, Kilby M, Lipkin G. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant. 2008;23:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Piccoli GB, Daidola G, Attini R, Parisi S, Fassio F, Naretto C, Deagostini MC, Castelluccia N, Ferraresi M, Roccatello D. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG. 2013;120:412-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Gifford RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1757] [Cited by in RCA: 1842] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 23. | August P, Sibai BM. Preeclampsia: Clinical features and diagnosis [Internet]. Available from: http://www.uptodate.com/contents/preeclampsia-clinical-features-and-diagnosis. |
| 24. | Thornton CE, Makris A, Ogle RF, Tooher JM, Hennessy A. Role of proteinuria in defining pre-eclampsia: clinical outcomes for women and babies. Clin Exp Pharmacol Physiol. 2010;37:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Kihara M, Usui H, Tanaka H, Inoue H, Matsui H, Shozu M. Complicating preeclampsia as a predictor of poor survival of the fetus in complete hydatidiform mole coexistent with twin fetus. J Reprod Med. 2012;57:325-328. [PubMed] |
| 27. | Zhao M, Yin Y, Guo F, Wang J, Wang K, Chen Q. Placental expression of VEGF is increased in pregnancies with hydatidiform mole: possible association with developing very early onset preeclampsia. Early Hum Dev. 2013;89:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Arkwright PD, Rademacher TW, Dwek RA, Redman CW. Pre-eclampsia is associated with an increase in trophoblast glycogen content and glycogen synthase activity, similar to that found in hydatidiform moles. J Clin Invest. 1993;91:2744-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Nugent CE, Punch MR, Barr M, LeBlanc L, Johnson MP, Evans MI. Persistence of partial molar placenta and severe preeclampsia after selective termination in a twin pregnancy. Obstet Gynecol. 1996;87:829-831. [PubMed] |
| 30. | Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. High body mass index and hypercholesterolemia: Risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:543-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, Papiernik E. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J Reprod Immunol. 1993;24:1-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology. 1996;7:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Klebanoff MA, Levine RJ, Puri M, Moyer P. The puzzling association between smoking and hypertension during pregnancy. Am J Obstet Gynecol. 1999;181:1407-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Marcoux S, Brisson J, Fabia J. The effect of cigarette smoking on the risk of preeclampsia and gestational hypertension. Am J Epidemiol. 1989;130:950-957. [PubMed] |
| 36. | Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 320] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, CôtéAM , Douglas MJ, Gruslin A, Hutcheon JA, Joseph KS. Prediction of adverse maternal outcomes in pre-eclampsia: Development and validation of the fullPIERS model. Lancet. 2011;377:219-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 388] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 38. | Payne B, Magee LA, Côté AM, Hutcheon JA, Li J, Kyle PM, Menzies JM, Moore MP, Parker C, Pullar B. PIERS proteinuria: relationship with adverse maternal and perinatal outcome. J Obstet Gynaecol Can. 2011;33:588-597. [PubMed] |
| 39. | Chan P, Brown M, Simpson JM, Davis G. Proteinuria in pre-eclampsia: how much matters? BJOG. 2005;112:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Yancey LM, Withers E, Bakes K, Abbott J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 42. | Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramadan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynecol. 1994;83:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190:1464-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Epstein FH. Late vascular effects of toxemia of pregnancy. N Engl J Med. 1964;271:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383-392. [PubMed] |
| 46. | Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57:94-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169:1316-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 293] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Anton L, Brosnihan KB. Systemic and uteroplacental renin--angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis. 2008;2:349-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393-399. [PubMed] |
| 51. | Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 849] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 52. | Hanssens M, Keirse MJ, Spitz B, Van Assche FA. Measurement of individual plasma angiotensins in normal pregnancy and pregnancy-induced hypertension. J Clin Endocrinol Metab. 1991;73:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 610] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 54. | Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, Catrou PG, Bolin P, Parker K, Kendrick WT. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 55. | Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 56. | Di Iorio R, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349:328. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Hata T, Miyazaki K, Matsui K. Decreased circulating adrenomedullin in pre-eclampsia. Lancet. 1997;350:1600. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Simon M, Gröne HJ, Jöhren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240-F250. [PubMed] |
| 59. | Foster RR, Hole R, Anderson K, Satchell SC, Coward RJ, Mathieson PW, Gillatt DA, Saleem MA, Bates DO, Harper SJ. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol. 2003;284:F1263-F1273. [PubMed] |
| 60. | Müller-Deile J, Worthmann K, Saleem M, Tossidou I, Haller H, Schiffer M. The balance of autocrine VEGF-A and VEGF-C determines podocyte survival. Am J Physiol Renal Physiol. 2009;297:F1656-F1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196:320.e1-320.e7. |
| 62. | Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH. Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension. 2013;61:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Aita K, Etoh M, Hamada H, Yokoyama C, Takahashi A, Suzuki T, Hara M, Nagata M. Acute and transient podocyte loss and proteinuria in preeclampsia. Nephron Clin Pract. 2009;112:c65-c70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Skoberne A, Konieczny A, Schiffer M. Glomerular epithelial cells in the urine: what has to be done to make them worthwhile? Am J Physiol Renal Physiol. 2009;296:F230-F241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Kelder TP, Penning ME, Uh HW, Cohen D, Bloemenkamp KW, Bruijn JA, Scherjon SA, Baelde HJ. Quantitative polymerase chain reaction-based analysis of podocyturia is a feasible diagnostic tool in preeclampsia. Hypertension. 2012;60:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453:387-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199:122.e1-122.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Moslemi Zadeh N, Naghshvar F, Peyvandi S, Gheshlaghi P, Ehetshami S. PP13 and PAPP-A in the first and second trimesters: Predictive factors for preeclampsia? ISRN Obstet Gynecol. 2012;2012:263871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 70. | Muttukrishna S, North RA, Morris J, Schellenberg JC, Taylor RS, Asselin J, Ledger W, Groome N, Redman CW. Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum Reprod. 2000;15:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Bosio PM, Cannon S, McKenna PJ, O’Herlihy C, Conroy R, Brady H. Plasma P-selectin is elevated in the first trimester in women who subsequently develop pre-eclampsia. BJOG. 2001;108:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Wang Y, Adair CD, Coe L, Weeks JW, Lewis DF, Alexander JS. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J Soc Gynecol Investig. 1998;5:237-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Redman CW, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499-506. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1152] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 74. | Cozzi V, Garlanda C, Nebuloni M, Maina V, Martinelli A, Calabrese S, Cetin I. PTX3 as a potential endothelial dysfunction biomarker for severity of preeclampsia and IUGR. Placenta. 2012;33:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Cetin I, Cozzi V, Papageorghiou AT, Maina V, Montanelli A, Garlanda C, Thilaganathan B. First trimester PTX3 levels in women who subsequently develop preeclampsia and fetal growth restriction. Acta Obstet Gynecol Scand. 2009;88:846-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Akolekar R, Casagrandi D, Livanos P, Tetteh A, Nicolaides KH. Maternal plasma pentraxin 3 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Bodova KB, Biringer K, Dokus K, Ivankova J, Stasko J, Danko J. Fibronectin, plasminogen activator inhibitor type 1 (PAI-1) and uterine artery Doppler velocimetry as markers of preeclampsia. Dis Markers. 2011;30:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Johnson AD, Berberian PA, Bond MG. Effect of heat shock proteins on survival of isoated aortic cells from normal and atherosclerotic cynomolgus macaques. Atherosclerosis. 1990;84:111-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Fukushima A, Kawahara H, Isurugi C, Syoji T, Oyama R, Sugiyama T, Horiuchi S. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res. 2005;31:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Jirecek S, Hohlagschwandtner M, Tempfer C, Knöfler M, Husslein P, Zeisler H. Serum levels of heat shock protein 70 in patients with preeclampsia: a pilot-study. Wien Klin Wochenschr. 2002;114:730-732. [PubMed] |
| 81. | Molvarec A, Prohászka Z, Nagy B, Szalay J, Füst G, Karádi I, Rigó J. Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens. 2006;20:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Hirashima C, Ohkuchi A, Arai F, Takahashi K, Suzuki H, Watanabe T, Kario K, Matsubara S, Suzuki M. Establishing reference values for both total soluble Fms-like tyrosine kinase 1 and free placental growth factor in pregnant women. Hypertens Res. 2005;28:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2783] [Cited by in RCA: 2943] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 85. | Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2720] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 86. | Jenkins SM, Head BB, Hauth JC. Severe preeclampsia at & lt; 25 weeks of gestation: maternal and neonatal outcomes. Am J Obstet Gynecol. 2002;186:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1361] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 88. | Sifakis S, Zaravinos A, Maiz N, Spandidos DA, Nicolaides KH. First-trimester maternal plasma cell-free fetal DNA and preeclampsia. Am J Obstet Gynecol. 2009;201:472.e1-472.e7. |
| 89. | Carter J, Child A. Serum uric acid levels in normal pregnancy. Aust N Z J Obstet Gynaecol. 1989;29:313-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Powers RW, Bodnar LM, Ness RB, Cooper KM, Gallaher MJ, Frank MP, Daftary AR, Roberts JM. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194:160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Gallery ED, Hunyor SN, Györy AZ. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med. 1979;48:593-602. [PubMed] |
| 92. | Lam C, Lim KH, Kang DH, Karumanchi SA. Uric acid and preeclampsia. Semin Nephrol. 2005;25:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Pereira KN, Knoppka CK, da Silva JE. Association between uric acid and severity of pre-eclampsia. Clin Lab. 2014;60:309-314. [PubMed] |
| 94. | Schackis RC. Hyperuricaemia and preeclampsia: is there a pathogenic link? Med Hypotheses. 2004;63:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Gülmezoğlu AM, Hofmeyr GJ, Oosthuisen MM. Antioxidants in the treatment of severe pre-eclampsia: an explanatory randomised controlled trial. Br J Obstet Gynaecol. 1997;104:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 97. | Scantlebury DC, Schwartz GL, Acquah LA, White WM, Moser M, Garovic VD. The treatment of hypertension during pregnancy: When should blood pressure medications be started? Curr Cardiol Rep. 2013;15:412, 013-0412-0. |
| 98. | Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: A systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134.e1-134.20. [PubMed] |
| 99. | Wide-Swensson DH, Ingemarsson I, Lunell NO, Forman A, Skajaa K, Lindberg B. Calcium channel blockade (isradipine) in treatment of hypertension in pregnancy: A randomized placebo-controlled study. Am J Obstet Gynecol. 1995;173:872-878. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;CD000025. |
| 101. | The Magpie Trial Follow Up Study Management Group. The Magpie Trial follow up study: outcome after discharge from hospital for women and children recruited to a trial comparing magnesium sulphate with placebo for pre-eclampsia [ISRCTN86938761]. BMC Pregnancy Childbirth. 2004;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for women at 2 years. BJOG. 2007;114:300-309. [PubMed] |
| 103. | Wallenburg HC, Dekker GA, Makovitz JW, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986;1:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 360] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 104. | Roberge S, Villa P, Nicolaides K, Giguère Y, Vainio M, Bakthi A, Ebrashy A, Bujold E. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 105. | Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin -- a systematic review and meta-analysis of the main randomized controlled trials. Clinics (Sao Paulo). 2005;60:407-414. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Levine SE, Keesey JC. Successful plasmapheresis for fulminant myasthenia gravis during pregnancy. Arch Neurol. 1986;43:197-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Frampton G, Cameron JS, Thom M, Jones S, Raftery M. Successful removal of anti-phospholipid antibody during pregnancy using plasma exchange and low-dose prednisolone. Lancet. 1987;2:1023-1024. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Seyyed Majidi MR, Vafaeimanesh J. Plasmapheresis in acute fatty liver of pregnancy: An effective treatment. Case Rep Obstet Gynecol. 2013;2013:615975. |
| 110. | Jin F, Cao M, Bai Y, Zhang Y, Yang Y, Zhang B. Therapeutic effects of plasma exchange for the treatment of 39 patients with acute fatty liver of pregnancy. Discov Med. 2012;13:369-373. [PubMed] |
| 111. | Altun D, Eren G, Cukurova Z, Hergünsel O, Yasar L. An alternative treatment in hypertriglyceridemia-induced acute pancreatitis in pregnancy: Plasmapheresis. J Anaesthesiol Clin Pharmacol. 2012;28:252-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Shieh S, Fang YV, Becker JL, Holm A, Beutner EH, Helm TN. Pemphigus, pregnancy, and plasmapheresis. Cutis. 2004;73:327-329. [PubMed] |
| 113. | Katz VL, Watson WJ, Thorp JM, Hansen W, Bowes WA. Treatment of persistent postpartum HELLP syndrome with plasmapheresis. Am J Perinatol. 1992;9:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Schwartz ML. Possible role for exchange plasmapheresis with fresh frozen plasma for maternal indications in selected cases of preeclampsia and eclampsia. Obstet Gynecol. 1986;68:136-139. [PubMed] |
| 115. | Martin JN, Perry KG, Roberts WE, Norman PF, Files JC, Blake PG, Morrison JC, Wiser WL. Plasma exchange for preeclampsia: II. Unsuccessful antepartum utilization for severe preeclampsia with or without HELLP syndrome. J Clin Apher. 1994;9:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | d’Apice AJ, Reti LL, Pepperell RJ, Fairley KF, Kincaid-Smith P. Treatment of severe pre-eclampsia by plasma exchange. Aust N Z J Obstet Gynaecol. 1980;20:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Wang Y, Walli AK, Schulze A, Blessing F, Fraunberger P, Thaler C, Seidel D, Hasbargen U. Heparin-mediated extracorporeal low density lipoprotein precipitation as a possible therapeutic approach in preeclampsia. Transfus Apher Sci. 2006;35:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 119. | Jouppila P, Jouppila R, Koivula A. Albumin infusion does not alter the intervillous blood flow in severe pre-eclampsia. Acta Obstet Gynecol Scand. 1983;62:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 120. | Stratta P, Canavese C, Dogliani M, Gurioli L, Porcu MC, Todros T, Fianchino O, Benedetto C, Massobrio M, Balbi L. Repeated albumin infusions do not lower blood pressure in preeclampsia. Clin Nephrol. 1991;36:234-239. [PubMed] |
| 121. | Magann EF, Bass JD, Chauhan SP, Perry KG, Morrison JC, Martin JN. Accelerated recovery from severe preeclampsia: uterine curettage versus nifedipine. J Soc Gynecol Investig. 1994;1:210-214. [PubMed] |
| 122. | Harer WB, McIndoe DW. Postpartum eclampsia treated by curettage. Am J Obstet Gynecol. 1962;84:1349-1350. [PubMed] |
| 123. | Matsuo K, Kooshesh S, Dinc M, Sun CC, Kimura T, Baschat AA. Late postpartum eclampsia: report of two cases managed by uterine curettage and review of the literature. Am J Perinatol. 2007;24:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |