Published online Sep 25, 2024. doi: 10.5527/wjn.v13.i3.98300
Revised: July 26, 2024
Accepted: August 5, 2024
Published online: September 25, 2024
Processing time: 87 Days and 3.2 Hours
Acute pyelonephritis (APN) is a bacterial infection resulting in kidney inflammation, typically arising as a complication of an ascending urinary tract infection that ascends from the bladder to the kidneys. Clinical diagnosis is generally based on clinical and laboratory findings. Recent guidelines recommend not performing diagnostic imaging unless a complicated APN is suspected or the infection affects high-risk patients such as the elderly, immunocompromised individuals, or diabetics. Contrast-enhanced ultrasound (CEUS) is a valuable tool in both the diagnosis and follow-up of APN. It aids in distinguishing small simple nephritic involvement from abscess complications and monitoring their evolution over time during antibiotic therapy. Given its lack of ionizing radiation and nephrotoxicity, CEUS is a valid diagnostic modality for approaching and monitoring pyelo
Core Tip: Acute pyelonephritis (APN) is a kidney inflammation typically due to an ascending urinary tract bacterial infection. The diagnosis is suggested by clinical and laboratory findings. Diagnostic imaging should be performed in case of a complicated APN. Contrast-enhanced ultrasound (CEUS) is a valid diagnostic tool for evaluating APN and performing a follow-up. This review aims to summarize the main evidence on the use of ultrasound and CEUS in the diagnosis of APN and its follow-up.
- Citation: Boccatonda A, Stupia R, Serra C. Ultrasound, contrast-enhanced ultrasound and pyelonephritis: A narrative review. World J Nephrol 2024; 13(3): 98300
- URL: https://www.wjgnet.com/2220-6124/full/v13/i3/98300.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i3.98300
Acute pyelonephritis (APN) is a bacterial infection resulting in kidney inflammation, typically arising as a complication of an ascending urinary tract infection (UTI) that ascends from the bladder to kidneys[1]. In the United States, the estimated annual incidence of APN ranges from 459000 cases to 1138000 cases, with 10.5 million to 25.9 million cases occurring globally each year[2]. The most commonly affected group is young, sexually active women, with almost all cases related to Escherichia coli infection[3]. Conversely, pyelonephritis in men > 18 years, elderly women, subjects affected by urological issues, and hospitalized individuals often results from less virulent E. coli strains, other Gram-negative bacilli, Gram-positive bacilli, and Candida[3]. The most common symptoms encompass fever, pain on the flank, nausea and vomiting, dysuria, and pollakiuria[3]. If there is no clinical improvement after 72 hours of intravenous antibiotic therapy or if a urinary tract obstruction is present complications should be considered. Clinical diagnosis is generally based on clinical and laboratory findings; a urinalysis and culture should always be performed in patients suspected of APN before administering antibiotics. Nevertheless, urine culture may be negative in up to 30% of pyelonephritis cases, possibly due to the administration of outpatient antibiotics[4]. Blood exams such as a complete blood cell count are sent to look for leukocytosis and laboratory signs of sepsis. Creatinine and blood urea nitrogen should be required to assess kidney function. There are no serum biomarkers available specific for pyelonephritis, although urinary neutrophil gelatinase-associated lipocalin may be a useful and sensitive indicator of APN in children and possibly in adults[5,6]. If a complicated APN is suspected or if the patient falls into high-risk categories, such as immunocompromised individuals, elderly or diabetics, diagnostic imaging should be performed[7].
The European Association of Urology guidelines on urological infections published in 2024 indicate that ultrasound should be performed to exclude obstruction of the urinary tract or the presence of renal stones[8]; additional investigations such as contrast-enhanced computed tomography (CT) or urography should be considered in patients who remain febrile and/or do not improve after 72 hours of therapy or in those with worsening clinical status[8]. Moreover, ultrasound or resonance imaging is suggested for the diagnosis of complicating factors during pyelonephritis in pregnant women[8]. As reported by the American College of Radiology, CT abdomen and pelvis without and with contrast is usually appropriate for the initial imaging of complicated patients with suspected APN[9]. The use of routine United States for APN is questioned as reported in a recent systematic review and meta-analysis by Yu et al[10]. Therefore, there are few discordant data, and there is no mention of ultrasound as the first evaluation/screening method for suspected pyelonephritis, much less for contrast-enhanced ultrasound (CEUS) in the guidelines.
In our previous experience, CEUS was used as the first and only method of evaluating suspected pyelonephritis in 35.7% of cases[11]. In our opinion, these data are even more relevant in clinical reality, as in our work, many examinations were performed as controls on patients who came from other hospitals where it was not possible to perform a CEUS. In some cases, the minority, both CEUS and contrast-enhanced CT were requested due to clinical-radiological inconsistencies (14.2%)[11].
Diagnostic imaging can evaluate the location and extent of lesions and indicate possible underlying causes[7]. Despite the B-mode ultrasound can detect alterations in pyelonephritis with complications, it is less accurate than CT, which is still the preferred diagnostic tool[12]. Due to concerns about radiation exposure and potential nephrotoxicity, CEUS has emerged as an alternative imaging modality for accurately detecting renal abnormalities in APN[12]. A 2007 study demonstrated that CEUS has a diagnostic performance similar to that of CT, particularly for focal involvement[13]. The 2017 update from the European Federation of Societies for Ultrasound in Medicine and Biology recommends CEUS for assessing renal abscesses in complicated APN[14]. However, renal abscesses represent only one possible inflammatory alteration in the renal parenchyma due to APN.
On B-mode ultrasound, the kidneys are examined using axial and longitudinal scans[15]. B-mode exploration helps to exclude hydronephrosis and to evaluate echo-structural pathological changes, such as perirenal fluid collections[15]. This assessment is complemented by a color-Doppler evaluation[15].
Abnormalities detected by color and power Doppler sonography in a cohort of infants with APN poorly correlated with Tc-99 m dimercaptosuccinic acid (DMSA) renal scintigraphy findings[16]. However, the sensitivity of color and power Doppler sonography is highly age-related and can be a non-invasive helpful tool for early diagnosis of APN in infants older than 6 months[16].
Moreover, microvascular Doppler ultrasonography showed improved detectability of hypoperfused areas in pediatric APN, providing higher diagnostic confidence[17].
Kidneys affected by APN showed a mean resistive index (RI) of 0.744 ± 0.06 in infants, 0.745 ± 0.03 in preschool children, and 0.733 ± 0.09 in school-age patients with upper UTI[18]. The mean RI values were significantly higher in patients with upper UTI (P < 0.001)[18]. There was a highly significant correlation between RI values and the severity of the renal lesion as ranked by DMSA scintigraphy (P < 0.001)[18]. When the cut-off RI value was 0.715, there was an 80% sensitivity and an 89% specificity for diagnosing upper UTI. Refluxing kidneys and scarred kidneys also had higher RI values[18].
In another study, kidneys with permanent damage had a mean RI of 0.71 ± 0.06, while the RI value for non-scarred kidneys was 0.66 ± 0.06 (P = 0.02)[19]. The best cutoff point of the RI value was 0.715, with a sensitivity of 70%, a specificity of 87.7%, and positive and negative predictive values of 32% and 97%, respectively[19].
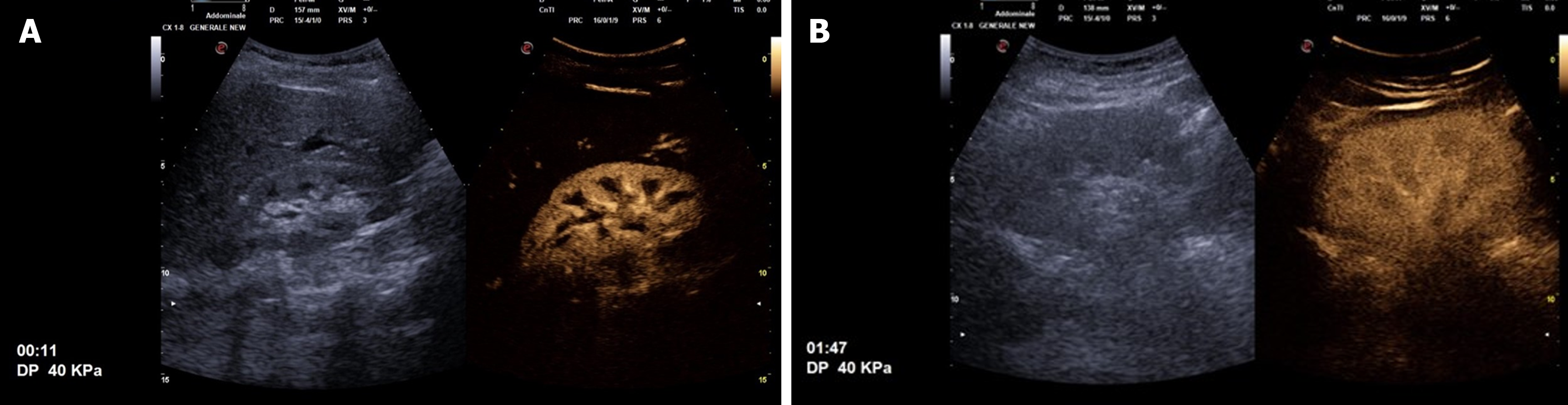
After completing the B-mode exam, the CEUS study can be conducted. A sulfur hexafluoride-based contrast medium, SonoVueâ (Bracco, Italy), is rapidly injected intravenously at a volume of either 2.4 mL or 4.8 mL, followed by a 10 mL saline flush. Following the ultrasound contrast agent bolus injection, the kidneys enhance quickly and intensely. The contrast enhancement is visible in the arterial vessels 10-15 seconds following injection, and then by the renal cortex a few seconds later, while the pyramids remain not perfused. The kidney pyramids gradually present a wash-in, thus displaying the same perfusion with the cortex within 30-40 seconds following SonoVueâ infusion. Initially, the kidneys appear hyperechoic compared to the liver or spleen. Subsequently, the kidneys display lower perfusion than the near parenchyma, particularly the spleen (Figure 1)[20]. Since SonoVueâ is not metabolized and excreted in the urinary system, the kidney collecting system never presents contrast enhancement.
Cortical round hyperechoic lesion is the most frequent ultrasound finding in complicated APNs, typically accompanied by cortical bulging. This feature is characteristic of APNs, and it can also be detected in APNs with small-size abscesses. Larger abscesses are characterized by inhomogeneous or hypoechoic areas and cortical bulging. Color-power-Doppler evaluation reveals these pathological areas as low-perfused lesions[21]. B-mode ultrasound is less sensitive and specific compared to other diagnostic imaging modalities, failing to detect APN lesions in up to 50% of patients[22,23]. The application of power-Doppler ultrasound displays a sensitivity of 89% and specificity of 53% in identifying kidney inflammatory changes[24]. Additionally, ultrasound with tissue harmonic imaging is characterized by a sensitivity of 97% and specificity of 80% in the evaluation of APNs[25].
Depending on the kidney involvement, APNs may be categorized as focal, multifocal, or diffuse. In the diffuse form of APN, CEUS diagnostic accuracy appears to be lower if compared to CT because it does not allow simultaneous com
| Ref. | Year | Country | PMID | Study design | Aim of the study | Sample size | Mean age in years | Sensitivity | Specificity |
| Kim et al[25] | 2001 | Korea | 11149528 | Prospective study | CEUS in APN compared with CT | 30 | NA | NA | |
| Stunell et al[7] | 2006 | Ireland | 16937102 | Comparative study | CEUS in APN compared with CT | ||||
| Mitterberger et al[13] | 2007 | Austria | 17941932 | Prospective study | CEUS in APN compared with CT | 100 | 30.2 | 98 | 100 |
| Granata et al[23] | 2011 | Italy | 20659906 | Prospective study | CEUS in APN compared with MRI | 56 | 50.1 | 95 | 100 |
| Fontanilla et al[21] | 2012 | Spain | 21792579 | Observational study | CEUS in evaluation and follow-up of APN | 48 | NA | NA | |
| Hosokawa et al[28] | 2020 | Japan | 32162084 | Observational study | US compared with CT in AFNB or APN | 11 | |||
| Boccatonda et al[11] | 2023 | Italy | 37958043 | Retrospective study | CEUS as follow-up of APN | 28 | 49.2 | NA | NA |
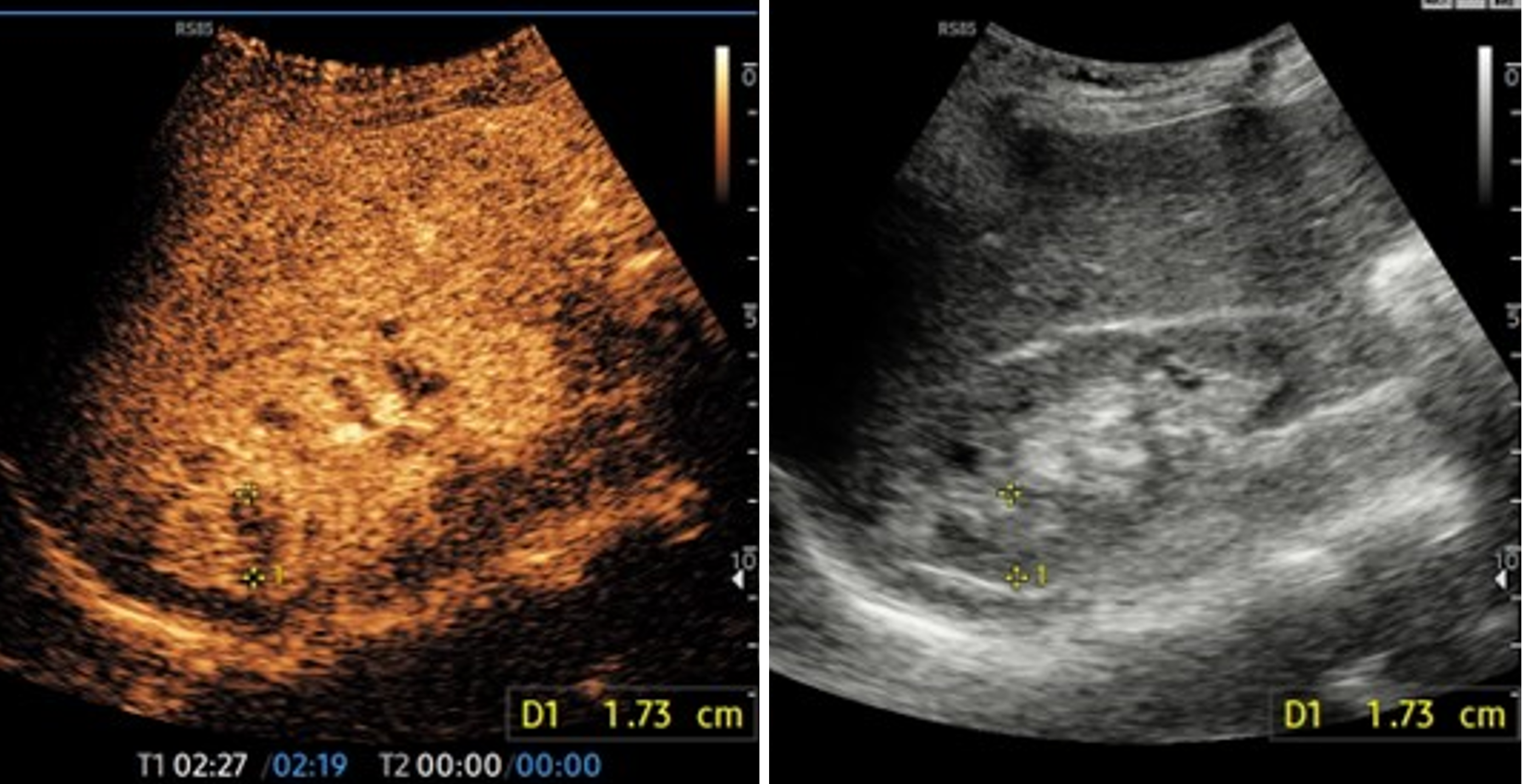
Focal pyelonephritis presents as wedge-shaped and/or round hypoechoic cortical lesions or affecting both the cortex and medulla (Figure 2). In terms of enhancement patterns, these lesions are most clearly seen during the late parenchymal phase, with variable findings during other phases.
Typically, the lesions are low perfused in the early phase, then become isoechoic to kidney parenchyma, and finally return to being hypoechoic in the late phase. Moreover, the abscess appears as a non-perfused area in all phases with a round shape; a peripheral rim enhancement may be observed in the early phase.
In the context of transplanted kidneys, CEUS has shown significant value for the early diagnosis of APN. It helps to limit the utilization of iodinated or paramagnetic contrast media, thereby preventing the risk of kidney damage and toxicity, particularly in subjects with chronic kidney disease[26,27]. In a work by Granata et al[23], 56 subjects with suspected APN in transplanted kidneys were evaluated by CEUS in comparison with magnetic resonance imaging (MRI) with gadolinium. The study demonstrated good diagnostic accuracy for CEUS, with a sensitivity of 95% and specificity of 100%[23]. CEUS can identify early complicated lesions such as acute focal bacterial nephritis (AFBN), enabling the selection of an appropriate therapeutic regimen. In a study by Hosokawa et al[28], involving a small cohort of pediatric patients with UTIs, ultrasound proved feasible in differentiating AFBN from APN, using contrast-enhanced CT as the reference standard. Notably, the study found a significant difference in the presence of ultrasound-detected focal loss of corticomedullary differentiation among subjects with AFBN and those with APN (P = 0.01)[28].
In the emergency setting, the early detection of those findings by CEUS can help to reduce exposure to radiation and iodinated contrast medium related to CT, which is particularly beneficial for pediatric patients.
Furthermore, CEUS can be used to evaluate the right placement of a nephrostomy tube by administering the contrast medium into the drainage catheter. This technique allows for the verification of unobstructed drainage, ensuring the proper function of the nephrostomy tube[29].
Ultrasound is typically the initial diagnostic imaging used in the assessment of patients suspected of having upper UTIs. However, the signs of renal infection are often not assessable on B-mode ultrasound, so diagnosis primarily relies on clinical and laboratory data along with indirect ultrasound findings[1]. In cases of complicated APN, ultrasound imaging is employed to ascertain the involvement of the inflammatory process over the kidney or to detect ureteral obstruction[12].
Historically, CT is considered the gold-standard imaging tool for diagnosing APN complications[12]. Otherwise, CT displays notable drawbacks, including exposure to radiation, especially concerning young patients, as well as the potential nephrotoxicity of contrast media.
While CT remains valuable in diagnosing complicated APN, the drawbacks associated with radiation exposure and contrast media toxicity have led to a growing interest in alternative imaging modalities. CEUS has emerged as a promising tool, offering excellent diagnostic accuracy comparable to CT but without the associated radiation exposure or risk of contrast-induced nephrotoxicity. CEUS provides detailed visualization of renal parenchymal perfusion and can effectively identify early complications of APN, aiding in timely and accurate diagnosis without the drawbacks of CT.
In this scenario, CEUS assumes a pivotal role due to its avoidance of ionizing radiation and non-nephrotoxic contrast agents. Recent works showed a comparable diagnostic accuracy of CEUS to CT in evaluating kidney parenchymal changes related to pyelonephritis[13,30]. CEUS can depict cortical areas with low perfusion related to infection, with studies indicating high accuracy and a 100% PPV for APN diagnosis, with CT scan as the reference standard[7].
Fontanilla et al[21] provided detailed descriptions of CEUS findings in APN, offering insights into typical enhancement features of various parenchymal lesions. This enables differentiation between abscesses and focal pyelonephritis and facilitates the detection of even small abscesses within pyelonephritic areas[21]. This highlights the valuable diagnostic capabilities of CEUS in the assessment of APN, allowing for precise characterization of lesions without the drawbacks associated with CT imaging.
In the emergency setting, CEUS exhibits high sensitivity and specificity in diagnosing renal infarction[31]. CEUS is increasingly utilized for distinguishing kidney infarction and necrotic areas, particularly when vascular disease is suspected to induce acute kidney failure[31]. Additionally, time-intensity curve software allows quantification of renal perfusion, offering a reliable tool for post-therapeutic monitoring to assess residual inflammatory infiltrate[32].
For patients with contraindications to contrast media administration or transplant recipients, diffusion-weighted (DW) MRI emerges as an optimal diagnostic tool for identifying APNs. Research by Faletti et al[33] investigated the role of DW MRI in managing APN in transplanted kidneys. Their study revealed significant differences in apparent diffusion coefficient parameters between normal cortical areas and APNs, and to differentiate APN from abscesses[33]. DW MRI thus proves to be a valuable tool in the diagnosis and management of APN, particularly in populations with specific contraindications or considerations regarding contrast media usage[33].
Focal pyelonephritis typically presents as a hyperechoic focal lesion with cortical bulging on grey-scale ultrasound, related to the high number of inflammatory cells in the interstitium and tubules[30]. Moreover, relevant capillary damage in association with leukocyte infiltration and fibrin plugs has been observed[12]. Those tissue changes are responsible for the hypoechoic feature and reduced enhancement of APN areas on CEUS, compared to healthy kidney. Focal APNs are better detectable in the late phase of CEUS, as they appear more hypoechoic during this phase.
Abscesses manifest as inhomogeneous and/or hypoechoic areas with cortical bulging. Ultrasound and CEUS enable the assessment of measure, structure and extent of abscesses. Since an abscess is a necrotic cavity containing pus and debris, no enhancement is observed inside throughout all phases. However, enhancement of septa may be visible if the abscess is partially liquefied. Peripheral rim enhancement in the cortical phase may also be observed.
The morphological features observed on CEUS in complicated APN are highly specific, leading to few potential differential diagnoses. When distinguishing between focal APN, abscesses, and infarcts, it's crucial to consider the enhan
In cases of peri- and para-renal involvement, patients typically undergo CT or MRI. MRI is suggested for young subjects with peri-renal disease extension. It's important to note that SonoVueTM is not related to kidney damage, and it can be administered to subjects with reduced renal function and in renal transplant recipients safely. Caution should be exercised in pregnant and lactating patients, as there is currently no available clinical data about the use of CEUS in those populations.
CEUS plays a crucial role in the follow-up of patients with complicated APN. Utilizing CEUS for follow-up examinations reduces the patient's exposure to a significant dose of radiation[11,34].
The European Association of Urology guidelines on urological infections published in 2024 do not give any recom
In our previous work, all patients who underwent a CEUS follow-up for APN experienced resolution of the lesion[11]. With the limitations of the retrospective nature of the study, the times of follow-up and therefore ultrasound demon
Furthermore, it was interesting to note that the ultrasound findings did not prompt any therapeutic modifications or lead to the prescription of additional laboratory tests in any instance[11].
This certainly raises doubts about the real clinical usefulness of CEUS control, if we exclude the desire to have imaging confirmation of the complete healing of the affected portion of the kidney.
Despite its advantages, CEUS has limitations, primarily related to patient factors such as body habitus and operator experience. Issues like obesity and bowel gas can affect the image quality of parenchymal perfusion, and those factors are crucial in determining the best diagnostic strategy for those subjects (CEUS vs CT or MRI).
In conclusion, CEUS proves to be a valuable technique in the diagnosis and follow-up of APNs. It aids in distinguishing focal pyelonephritic lesions from abscesses and monitoring their changes after antibiotic therapy. However, it is essential to acknowledge that CT and MRI continue to play a crucial role in evaluating peri-renal complications. The major limitation of the CEUS method compared to CT is certainly the poor panoramic view and the difficulty in evaluating both kidneys, especially if the symptoms are not localized, with a single bolus of contrast. Future studies will have to evaluate in which categories of patients reporting symptoms of UTI it is appropriate to perform an ultrasound and a CEUS. Furthermore, it is essential to understand the timing of the first imaging examination in relation to the onset of symptoms and subsequently set the correct follow-up time.
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17072] [Article Influence: 1896.9] [Reference Citation Analysis (2)] |
| 2. | Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis. 2007;45:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Johnson JR, Russo TA. Acute Pyelonephritis in Adults. N Engl J Med. 2018;378:1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Khoo KSM, Lim ZY, Chai CY, Mahadevan M, Kuan WS. Management of acute pyelonephritis in the emergency department observation unit. Singapore Med J. 2021;62:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Arambašić J, Mandić S, Debeljak Ž, Mandić D, Horvat V, Šerić V. Differentiation of acute pyelonephritis from other febrile states in children using urinary neutrophil gelatinase-associated lipocalin (uNGAL). Clin Chem Lab Med. 2016;54:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Moon JH, Yoo KH, Yim HE. Urinary neutrophil gelatinase-associated lipocalin: a marker of urinary tract infection among febrile children. Clin Exp Pediatr. 2021;64:347-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol. 2007;17:1820-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Kranz J, Bartoletti R, Bruyère F, Cai T, Geerlings S, Köves B, Schubert S, Pilatz A, Veeratterapillay R, Wagenlehner FME, Bausch K, Devlies W, Horváth J, Leitner L, Mantica G, Mezei T, Smith EJ, Bonkat G. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur Urol. 2024;86:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 9. | Expert Panel on Urological Imaging, Smith AD, Nikolaidis P, Khatri G, Chong ST, De Leon AD, Ganeshan D, Gore JL, Gupta RT, Kwun R, Lyshchik A, Nicola R, Purysko AS, Savage SJ, Taffel MT, Yoo DC, Delaney EW, Lockhart ME. ACR Appropriateness Criteria® Acute Pyelonephritis: 2022 Update. J Am Coll Radiol. 2022;19:S224-S239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 10. | Yu J, Sri-Ganeshan M, Smit V, Mitra B. Ultrasound for acute pyelonephritis: a systematic review and meta-analysis. Intern Med J. 2024;54:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 11. | Boccatonda A, Venerato S, D'Ardes D, Cocco G, Schiavone C, Vicari S. Contrast-Enhanced Ultrasound Follow-Up for Acute Pyelonephritis Patients. Healthcare (Basel). 2023;11:2899. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Craig WD, Wagner BJ, Travis MD. Pyelonephritis: radiologic-pathologic review. Radiographics. 2008;28:255-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Mitterberger M, Pinggera GM, Colleselli D, Bartsch G, Strasser H, Steppan I, Pallwein L, Friedrich A, Gradl J, Frauscher F. Acute pyelonephritis: comparison of diagnosis with computed tomography and contrast-enhanced ultrasonography. BJU Int. 2008;101:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D'Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, Seidel G, Sidhu PS, ter Haar G, Timmerman D, Weskott HP. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 678] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 15. | Ferro F, Vezzali N, Comploj E, Pedron E, Di Serafino M, Esposito F, Pelliccia P, Rossi E, Zeccolini M, Vallone G. Pediatric cystic diseases of the kidney. J Ultrasound. 2019;22:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Chen MG, Yang Y, Yang Q, Zhuang JQ, Ye XH, Zheng WJ. New strategy of color and power doppler sonography combined with DMSA in the assessment of acute pyelonephritis in infants. BMC Nephrol. 2021;22:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Choi G, Je BK, Hong D, Cha J. Microvascular Doppler ultrasound in children with acute pyelonephritis. Med Ultrason. 2021;23:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ozçelik G, Polat TB, Aktaş S, Cetinkaya F. Resistive index in febrile urinary tract infections: predictive value of renal outcome. Pediatr Nephrol. 2004;19:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Mohammadjafari H, Aalaee A, Salehifar E, Shiri A, Khademloo M, Shahmohammadi S. Doppler ultrasonography as a predictive tool for permanent kidney damage following acute pyelonephritis: comparison with dimercaptosuccinic acid scintigraphy. Iran J Kidney Dis. 2011;5:386-391. [PubMed] |
| 20. | Nilsson A. Contrast-enhanced ultrasound of the kidneys. Eur Radiol. 2004;14 Suppl 8:P104-P109. [PubMed] |
| 21. | Fontanilla T, Minaya J, Cortés C, Hernando CG, Arangüena RP, Arriaga J, Carmona MS, Alcolado A. Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging. 2012;37:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Majd M, Nussbaum Blask AR, Markle BM, Shalaby-Rana E, Pohl HG, Park JS, Chandra R, Rais-Bahrami K, Pandya N, Patel KM, Rushton HG. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology. 2001;218:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Granata A, Andrulli S, Fiorini F, Basile A, Logias F, Figuera M, Sicurezza E, Gallieni M, Fiore CE. Diagnosis of acute pyelonephritis by contrast-enhanced ultrasonography in kidney transplant patients. Nephrol Dial Transplant. 2011;26:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Basiratnia M, Noohi AH, Lotfi M, Alavi MS. Power Doppler sonographic evaluation of acute childhood pyelonephritis. Pediatr Nephrol. 2006;21:1854-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kim B, Lim HK, Choi MH, Woo JY, Ryu J, Kim S, Peck KR. Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med. 2001;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Como G, Da Re J, Adani GL, Zuiani C, Girometti R. Role for contrast-enhanced ultrasound in assessing complications after kidney transplant. World J Radiol. 2020;12:156-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, Drudi FM, Malpassini F, Isidori A, Meloni FM, Calliada F, D'Ambrosio F. Growing indications for CEUS: The kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Hosokawa T, Tanami Y, Sato Y, Oguma E. Comparison of imaging findings between acute focal bacterial nephritis (acute lobar nephronia) and acute pyelonephritis: a preliminary evaluation of the sufficiency of ultrasound for the diagnosis of acute focal bacterial nephritis. Emerg Radiol. 2020;27:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Di Serafino M, Iacobellis F, Schillirò ML, Ronza R, Verde F, Grimaldi D, Dell'Aversano Orabona G, Caruso M, Sabatino V, Rinaldo C, Romano L. The Technique and Advantages of Contrast-Enhanced Ultrasound in the Diagnosis and Follow-Up of Traumatic Abdomen Solid Organ Injuries. Diagnostics (Basel). 2022;12:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Demertzis J, Menias CO. State of the art: imaging of renal infections. Emerg Radiol. 2007;14:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Girometti R, Stocca T, Serena E, Granata A, Bertolotto M. Impact of contrast-enhanced ultrasound in patients with renal function impairment. World J Radiol. 2017;9:10-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 32. | McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012;67:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Faletti R, Cassinis MC, Gatti M, Giglio J, Guarnaccia C, Messina M, Bergamasco L, Fonio P. Acute pyelonephritis in transplanted kidneys: can diffusion-weighted magnetic resonance imaging be useful for diagnosis and follow-up? Abdom Radiol (NY). 2016;41:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Rinaldo C, Grimaldi D, Di Serafino M, Iacobellis F, Verde F, Caruso M, Sabatino V, Orabona GD, Schillirò ML, Vallone G, Cantisani V, Romano L. An update on pyelonephritis: role of contrast enhancement ultrasound (CEUS). J Ultrasound. 2023;26:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |