Published online Nov 12, 2016. doi: 10.5501/wjv.v5.i4.155
Peer-review started: April 23, 2016
First decision: June 6, 2016
Revised: July 25, 2016
Accepted: August 17, 2016
Article in press: August 18, 2016
Published online: November 12, 2016
Processing time: 200 Days and 5.6 Hours
To evaluate alterations of memory B cell subpopulations during a 48-wk period in human immunodeficiency virus type 1 (HIV-1) patients.
Forty-one antiretroviral naïve and 41 treated HIV-1 patients matched for age and duration of HIV infection were recruited. All clinical, epidemiological and laboratory data were recorded or measured. The different B cell subsets were characterized according to their surface markers: Total B cells (CD19+), memory B cells (CD19+CD27+, BMCs), resting BMCs (CD19+CD27+CD21high, RM), exhausted BMCs (CD19+CD21lowCD27-, EM), IgM memory B (CD19+CD27+IgMhigh), isotype-switched BMCs (CD19+CD27+IgM-, ITS) and activated BMCs (CD19+CD21low+CD27+, AM) at baseline on week 4 and week 48.
Mean counts of BMCs were higher in treated patients. There was a marginal upward trend of IgM memory B cell proportions which differed significantly in the treated group (overall trend, P = 0.004). ITS BMC increased over time significantly in all patients. Naive patients had of lower levels of EM B cells compared to treated, with a downward trend, irrespectively of highly active antiretroviral therapy (HAART) intake. Severe impairment of EM B cells was recorded to both treated (P = 0.024) and naive (P = 0.023) and patients. Higher proportions of RM cells were noted in HAART group, which differed significantly on week 4th (P = 0.017) and 48th (P = 0.03). Higher levels of AM were preserved in HAART naive group during the whole study period (week 4: P = 0.018 and 48: P = 0.035). HIV-RNA viremia strongly correlated with AM B cells (r = 0.54, P = 0.01) and moderately with RM cells (r = -0.45, P = 0.026) at baseline.
HIV disrupts memory B cell subpopulations leading to impaired immunologic memory over time. BMC, RM, EM and ITS BMC were higher in patients under HAART. Activated BMCs (AM) were higher in patients without HAART. Viremia correlated with AM and RM. Significant depletion was recorded in EM B cells irrespectively of HAART intake. Perturbations in BMC-populations are not fully restored by antiretrovirals.
Core tip: During the progress of human immunodeficiency virus (HIV) infection and viral replication functional irritations of memory B-cell (BMC) compartment occur. Depletion of BMCs is one hallmark of deregulation in HIV-1 infection. Diminished levels of IgM+ BMCs are also noted. Additionally, resting BMCs are severely impaired and defective B-cell subsets, like exhausted and activated BMCs circulate in peripheral blood. Significant fluctuations of these B cells’ frequencies are recorded over time and antiretroviral therapy may play a role on this observation. Assessing these populations could potentially lead to improvement in assessing vaccine responses and tracing vulnerable patients to certain infections.
- Citation: Tsachouridou O, Skoura L, Zebekakis P, Margariti A, Georgiou A, Bougiouklis D, Pilalas D, Galanos A, Daniilidis M, Metallidis S. Antiretroviral naive and treated patients: Discrepancies of B cell subsets during the natural course of human immunodeficiency virus type 1 infection. World J Virol 2016; 5(4): 155-160
- URL: https://www.wjgnet.com/2220-3249/full/v5/i4/155.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i4.155
During the chronic human immunodeficiency virus (HIV) infection and viral proliferation functional irritations of B-cells take place like impairment of isotype switching, polyclonal activation, divergences in the frequencies of circulating B cell-populations and diminished immune reactions to immunization[1-3].
The deprivation of memory B cells (CD19+CD21+CD27+, BMC) reflects some dysfunction in HIV patients[3,4]. Reduced IgM+ BMCs (CD19+CD27+IgMhigh) is also observed[2,3]. During the course of the infection, resting BMCs (CD19+CD21highCD27+, RM) are severely impaired[5]. Furthermore, dysfunctional B-cell subsets, accounting for activated BMCs (CD19+CD21lowCD27+, AM) and exhausted BMCs (CD19+CD21lowCD27-, EM), rise in these patients, while they appear at very low frequencies in healthy subjects[4].
The phenotype of B cells that serves the immune response to several antigens has been ambivalent[6-9]. The conflict primarily is targeted on the surface markers of the B cells that respond to the antigens[3,6,7,10-12]. It is currently challenged that IgM BMCs are merely in charge of antibody production; since other memory subsets such as isotype switched B cells (CD19+CD27+IgM-) also produce anti-polysaccharide antibodies in vitro[2].
Highly active antiretroviral therapy (HAART) reduces polyclonal B-cell activation but has only a constricted effect on the remediation of B-cells and remains to be elucidated which certain perturbations can be repaired[3,5]. The loss of memory is confirmed by in the reduction of antigen specific BMCs post vaccine administration, which is not reconstituted by HAART[13,14]. RM cells are preserved if HAART is initiated immediately in HIV confirmation of infection[14].
Aim of this study was to record and evaluate alterations of BMC subpopulations during a 48-wk period in HIV-1 patients. Moreover, we prospectively studied the impact of HAART on these cell populations seeking for significant changes.
This is a longitudinal study including 82 HIV patients matched for age and duration of HIV infection, 41 of whom were antiretroviral naive and 41 were under HAART, with successful viral suppression (HIV-1 viral load < 34 copies/mL). All rest data, including epidemiological (age, gender, HIV-1 transmission route, co morbidities) and laboratory results, HIV-1 viral load, current CD4 T-cell count, nadir CD4 cell count were recorded.
The Aristotle’s University Ethical Committee approved the protocol and a written informed consent was obtained from all participants. All study individuals were asked to give blood sample on day 0 and on week 4 and 48.
Mouse anti-human fluorochrome-conjugated monoclonal antibodies of Immunostep Company® were used: CD19-PerCP, CD27-PE, IgM-FITC, IgD-FITC and CD21-FITC to count total B cells and BMC subsets combined properly. One hundred microliter of blood samples after adding 10 μL of the above combined monoclonal antibodies were incubated in the dark for ten minutes. In turn, red blood cells were thawed upon ingestion of 2 mL of Lysis Buffer (BD Biosciences, San Jose, CA) and incubated for another twenty minutes in room temperature. B cells were assessed pre vaccination. The different B cell subsets were characterized as follows: Total B cells (CD19+), BMCs (CD19+CD27+), EM (CD19+CD21lowCD27-), IgM memory B (CD19+CD27+IgMhigh), RM (CD19+CD27+CD21high), IgM memory B (CD19+CD27+IgMhigh), AM (CD19+CD21low+CD27+) and ITS (CD19+CD27+IgM-) at baseline and on weeks four and forty eight. Results were expressed as B cell absolute counts or as a percentage of the total B cell population.
Sample processing and result extraction was performed in the XL Epics cytometer (Beckman Coulter™ Company, Florida, United States). The input capture, cell staining and flow cytometry were performed immediately in a blinded manner.
The comparison of variables at each time point was performed using the independent samples t test or the Mann-Whitney test in case of violation of normality. To indicate the trend in the one year period, the median percentage changes after 4 wk and 48 wk respectively were calculated. All tests are two-sided, a P-value of < 0.05 was used to denote statistical significance. All analyses were carried out using the statistical package SPSS vr 16.00 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, Ill., United States).
The demographics, clinical and rest data of eighty two HIV individuals are illustrated in Table 1.
| HAART naïve patients (n = 41) | Treated patients (n = 41) | P value | |
| Age (yr) | 31.76 ± 7.16 | 34.15 ± 6.17 | 0.168 |
| Gender (male/female), n (%) | 41 (100.0)/0 (0.0) | 36 (87.8)/ 4 (12.2) | 0.065 |
| Years on HIV infection | 3.27 ± 2.78 | 3.6 = 98 ± 4.41 | 0.415 |
| Nadir CD4 cell count | 573.6 ± 223.4 | 326 ± 187.3 | 0.0005 |
| ART duration in months | NA | 34.4 ± 14.18 | NA |
| HCV infection, n (%) | 2 (4.8) | 3 (7.3) | 0.345 |
| HBV infection, n (%) | 6 (14.6) | 4 (9.7) | 0.167 |
In order to confirm whether the percentages of B-cell subsets were altered among treated and antiretroviral naive HIV-1 individuals, the percentages of memory B, activated memory, resting memory, exhausted memory as well as isotype-switched and total B-cells were assessed. Significant differences were observed between the groups in relation to B cell subsets.
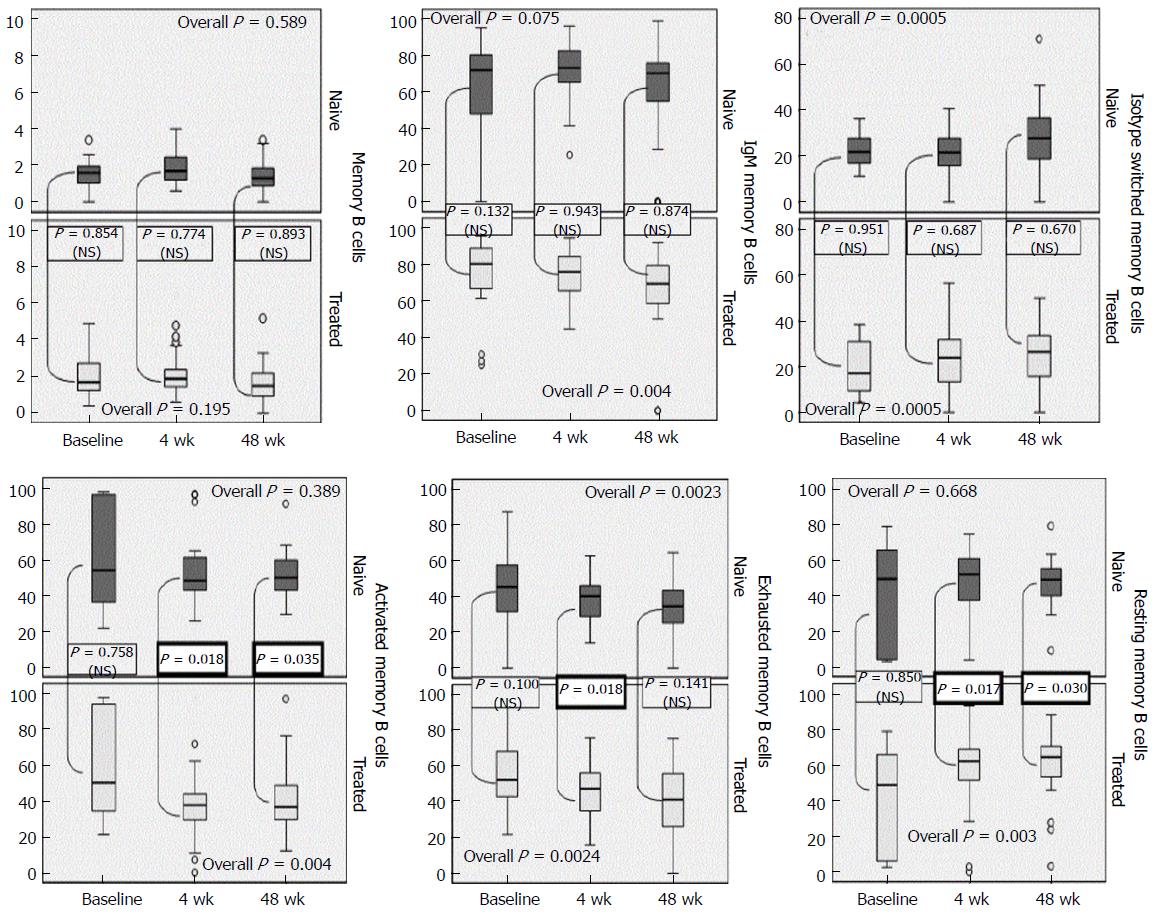
Mean counts of BMCs (CD19+CD27+) were higher in treated patients throughout the 48 wk (P = 0.987, NS), with a gradual declining trend by the end of the 48th week. Mean fraction of IgM memory B (CD19+CD27+IgMhigh) cell-population found higher in the treated group at baseline. There was a marginal upward trend of the proportions which differed significantly in the treated group (overall trend, P = 0.004) (Figure 1). Isotype-switched BMC (CD19+CD27+IgM-) were slightly elevated in patients without HAART compared to the other group. The time trend variation was equivalent in both groups, irrelevantly of HAART intake (P = 0.808). ITS B cell compartment raised significantly in all patients, concerning baseline levels (overall significance, P = 0.0005) (Figure 1).
HAART patients preserved higher proportions of EM B cells (CD19+CD21lowCD27-) compared those without treatment, with a downward trend along with the progression of the disease, irrespectively of HAART intake. These changes were not significant among groups (overall significance, P = 0.876). Significant depletion of EM B cells was recorded to both ART-naive (P = 0.023) and rest individuals (P = 0.024) (Figure 1). The fraction of RM cells (CD19+CD21high+CD27+) in patients under HAART were higher and significantly different on week 4th (P = 0.017) and 48th (P = 0.03). The fluctuation over time of RM was nearly the same though, in both groups (P = 0.201) with treated patients having a significant overall increase (P = 0.003). Patients HAART-naive maintained higher levels of AM (CD19+CD21low+CD27+) during the whole study period, with the downward trend being significant in the treated group (P = 0.004) (Figure 1).
HIV-RNA viremia strongly correlated with AM B cells (r = 0.54, P = 0.01) and moderately with RM B cells (r = -0.45, P = 0.026) at baseline, supporting the impact of viral replication on these subsets (data not shown).
HIV infection impels to a broad amplitude of B cell defects, like cell switching, depleted numbers of B cells, production of uncommon B cell populations and disfunctional immune responses even in patients under HAART[3,6,7]. Furthermore, it is generally accepted to augments the risk of several infections. Very scarce and conflicting data are currently available illustrating the significance of B cell subsets that mediate satisfactory and protective immune responses[6,7].
Our study promotes the scouting and assessment of specific BMC subpopulations interfered in humoral responses, confirming few other authors claiming that, not solely ITS and IgM BMC, but also AM and RM, might contribute to impaired protection against certain bacteria[7-12,14]. Recent studies focus on B cell memory and immunological response post immunizations, though most constitute solely cross-sectional studies[8-12,14].
BMCs were increased in patients on HAART compared to naive on an annual basis, as confirmed in other studies as well[2,15]. We showed rise of BMCs in both groups, which depleted throughout time in a similar pattern, that is in line with previous studies[7].
Interestingly, slight rise in IgM BMC was confirmed in all patients. Patients on treatment preserved high frequencies of them, confirming authors suggesting that HAART preserves the levels of this specific subset[15]. Both treated and naive patients maintained their IgM BMC over time, which is controversial in literature[15].
EM B cells are believed to be increased in naive patients[2,6]. Although in our study we did not confirmed the former observation, gradual decrease has been recorded irrespectively of HAART. Patients under HAART had decreased AM cells compared to those without treatment, explaining the effect of HAART which restricts their expansion during the progress of HIV infection[2]. We additionally confirmed that AM B cells are preserved in continuous viral replication[13]. Even though effective HAART is regarded to have no impact on RM, in our study maintenance of high levels especially in the treated group, implies that some restoration may be feasible upon HAART initiation, regardless being not during primary infection[5,14].
Studies have shown that isotype switched B cells are not affected in healthy individuals[7], but these are dramatically impaired in HIV infection irrespectively of HAART[15]. Despite few studies that confirmed high frequencies of ITS B cells in patients under HAART[2,15], our study confirmed more recent authors[15] showing no effect of HAART, which underlies the need for further investigation on this specific memory subset.
HAART introduction lead to further investigation on these populations, concluding that most divergences are reversible, implying that viremia has a causal relationship. Viremia has been associated with the elevated frequency of AM cells[16]. However, the impact of HIV viremia has not been fully explained, apart from in limited studies[14]. Our study in lineage with other authors has shown that viremia was linked to certain B cell populations[14].
In conclusion, the data of our study points out that significant divergences occur in specific BMC populations in HIV patients. Natural course of HIV infection has an immunological impact on distinct B cells, sparking modifications on their absolute numbers and functions in the peripheral blood of HIV adults. Furthermore, HAART administration affects subsets like RM and AM which are significant in secondary immune responses, while has controversial implication in other BMC-compartments. We propose that evaluation of BMC might implicate in immunization and have clinical utility in forecasting all susceptible HIV adults to bacterial and other viral infections.
The significance of the paper lies to the fact that HAART prompt initiation may alter few of the disturbances that HIV infection itself promotes. Similar findings for the significance of immediate initiation of antivirals have been published recently, which insist that HAART is necessary to be started once the diagnosis of HIV infections has been confirmed[17].
Additionally, concerning the HIV vaccine development design, new scientific trends lean towards the role of B cells in HIV pathogenesis and their possible use to design and develop a proper vaccine for preventing HIV infections. Multiple studies try to assess and isolate the responsible B cell subsets that interfere to the pathogenesis of HIV infection and will lead to the vaccine development[18,19].
The authors would like acknowledge all the laboratory staff members of the National AIDS Reference Centre for their valuable co-operation throughout the study procedure.
Human immunodeficiency virus (HIV) causes several phenotypic and functional perturbations on B lymphocytes, like hyperactivation leading to hypergammaglobulinemia. Simultaneously, B cells also display hyporesponsiveness to vaccines. Memory B cell (BMC) react and secrete antibodies specific to the antigen faced, with improved pertinence when meeting the same antigen twice, offering protection against several infections. Highly active antiretroviral therapy (HAART) partially restores B cell perturbation, especially when initiation is prompt. Moreover, antivirals are cannot retrieve humoral response in HIV adults, and re-immunization might be mandatory. Optimizing vaccine strategy may improve BMC responses to immunizations. The role of some recently described BMC subsets is not fully understood and remains to be elucidated. Health care providers should consider prompt HAART initiation in order to restrict HIV-associated BMC impairment.
Previous studies have assessed the role of early antiretroviral therapy (ART) administration showing that some populations may be benefited and protected from functional and phenotypic perturbations, with conflicting results.
This study aimed to assess over time the fluctuation of significant BMC subsets for humoral responses in HIV patients and not in single time slot. The study revealed certain time-trend differences among antiretroviral naive and treated patients.
Literature is still ambiguous concerning the precise role of certain BMC populations and the significance of HAART in restoring or preventing some disturbances on them. ART intake affects subsets like resting BMC (RM) and activated BMC (AM) which are significant in secondary immune responses, while has controversial implication in other BMC. Evaluation of BMCs might intervene in immunizations and have clinical utility in pointing out the susceptible HIV adults to bacterial and other infections.
CD27+ BMC comprise of CD21+ cells (RM) and CD21- cells (AM). RM are depleted while AM rise during the natural course of HIV infection. The classical CD27+ BMC are classified as ITS and un-switched subpopulations, while the isotype-switched BMC subset represent BMC that have switched their immunoglobulin from IgM and IgD to other classes. TLM (CD19+CD10- CD27-CD21low), rise in HIV infected patients.
The article is well prepared and makes a pleasant and useful reading for those in the field.
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Maggi F, Pandey KK S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Shen X, Tomaras GD. Alterations of the B-cell response by HIV-1 replication. Curr HIV/AIDS Rep. 2011;8:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O’Shea MA, Kottilil S. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116:5571-5579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Hu Z, Luo Z, Wan Z, Wu H, Li W, Zhang T, Jiang W. HIV-associated memory B cell perturbations. Vaccine. 2015;33:2524-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12-19; quiz 20-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O’Shea MA, Roby G, Mican JM, Kottilil S, Chun TW. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 726] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 7. | Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212-8220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Lee KY, Tsai MS, Kuo KC, Tsai JC, Sun HY, Cheng AC, Chang SY, Lee CH, Hung CC. Pneumococcal vaccination among HIV-infected adult patients in the era of combination antiretroviral therapy. Hum Vaccin Immunother. 2014;10:3700-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Leggat DJ, Iyer AS, Ohtola JA, Kommoori S, Duggan JM, Georgescu CA, Khuder SA, Khaskhely NM, Westerink MJ. Response to Pneumococcal Polysaccharide Vaccination in Newly Diagnosed HIV-Positive Individuals. J AIDS Clin Res. 2015;6:pii: 419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhang L, Li Z, Wan Z, Kilby A, Kilby JM, Jiang W. Humoral immune responses to Streptococcus pneumoniae in the setting of HIV-1 infection. Vaccine. 2015;33:4430-4436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Tsachouridou O, Skoura L, Zebekakis P, Margariti A, Metallidis S. Memory B Cell Divergences upon Immunization Against Streptococcus pneumoniae in HIV-1-Infected Adults. AIDS Res Hum Retroviruses. 2015;31:1053-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Tsachouridou O, Skoura L, Zebekakis P, Margariti A, Georgiou A, Daniilidis M, Malisiovas N, Metallidis S. The controversial impact of B cells subsets on immune response to pneumococcal vaccine in HIV-1 patients. Int J Infect Dis. 2015;38:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev. 2013;254:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Pensieroso S, Galli L, Nozza S, Ruffin N, Castagna A, Tambussi G, Hejdeman B, Misciagna D, Riva A, Malnati M. B-cell subset alterations and correlated factors in HIV-1 infection. AIDS. 2013;27:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | D’Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Amu S, Ruffin N, Rethi B, Chiodi F. Impairment of B-cell functions during HIV-1 infection. AIDS. 2013;27:2323-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1887] [Cited by in RCA: 2119] [Article Influence: 211.9] [Reference Citation Analysis (0)] |
| 18. | Haynes BF, Moody MA, Liao HX, Verkoczy L, Tomaras GD. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends Mol Med. 2011;17:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Zhang R, Verkoczy L, Wiehe K, Munir Alam S, Nicely NI, Santra S, Bradley T, Pemble CW, Zhang J, Gao F. Initiation of immune tolerance-controlled HIV gp41 neutralizing B cell lineages. Sci Transl Med. 2016;8:336ra62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |