Revised: January 7, 2013
Accepted: January 11, 2013
Published online: May 12, 2013
Processing time: 155 Days and 3.3 Hours
Anaemia and thrombocytopenia are haematological disorders that can be detected in many human immunodeficiency virus (HIV)-positive patients during the development of HIV infection. The progressive decline of erythrocytes and platelets plays an important role both in HIV disease progression and in the clinical and therapeutic management of HIV-positive patients. HIV-dependent impairment of the megakaryocyte and erythrocyte lineages is multifactorial and particularly affects survival, proliferation and differentiation of bone marrow (BM) CD34+ haematopoietic progenitor cells, the activity of BM stromal cells and the regulation of cytokine networks. In this review, we analyse the major HIV-related mechanisms that are involved in the genesis and development of the anaemia and thrombocytopenia observed in HIV positive patients.
- Citation: Gibellini D, Clò A, Morini S, Miserocchi A, Ponti C, Re MC. Effects of human immunodeficiency virus on the erythrocyte and megakaryocyte lineages. World J Virol 2013; 2(2): 91-101
- URL: https://www.wjgnet.com/2220-3249/full/v2/i2/91.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i2.91
Human immunodeficiency virus (HIV) is the causative agent of acquired immunodeficiency syndrome (AIDS), which is characterised by the progressive and fatal impairment of immune system function and the occurrence of opportunistic infections and tumours[1]. Although the dysfunction of the immune system and the decline in the number and activity of CD4+ T cells represent the hallmark of HIV infection, it is noteworthy that HIV can also interfere with other cell lineages and tissues[2-5]. In addition to progressive depletion of CD4+ T lymphocytes, peripheral blood cytopenias, such as anaemia, neutropenia and thrombocytopenia, occur in most patients with AIDS[6,7] and in some HIV-positive naive individuals during the early phases of disease progression, especially when high plasma levels of HIV RNA are detectable. Interestingly, isolated thrombocytopenia can represent the first clinical manifestation in otherwise asymptomatic HIV positive patients[8] whereas anaemia and neutropenia are more common in the late stages of HIV disease[9]. These peripheral blood cytopenias have been observed even in the absence of tumours, chemotherapeutic treatment or opportunistic infections suggesting that HIV infection may be directly associated with the induction of these haematological abnormalities[10]. The progressive depletion of these cell lineages in the blood has been related to several HIV-driven mechanisms: (1) the impairment of survival and proliferation of haematopoietic progenitor cells (HPCs); (2) the inhibition of the differentiation of HPCs into certain cell lineages or direct action on mature cells; (3) the impairment of stromal cells; and (4) the dysregulation of cytokine production and the appearance of autoimmune responses. In this report, we analyse several aspects of these major HIV-related mechanisms that are involved in the impairment of the erythrocyte and megakaryocyte (MK) lineages.
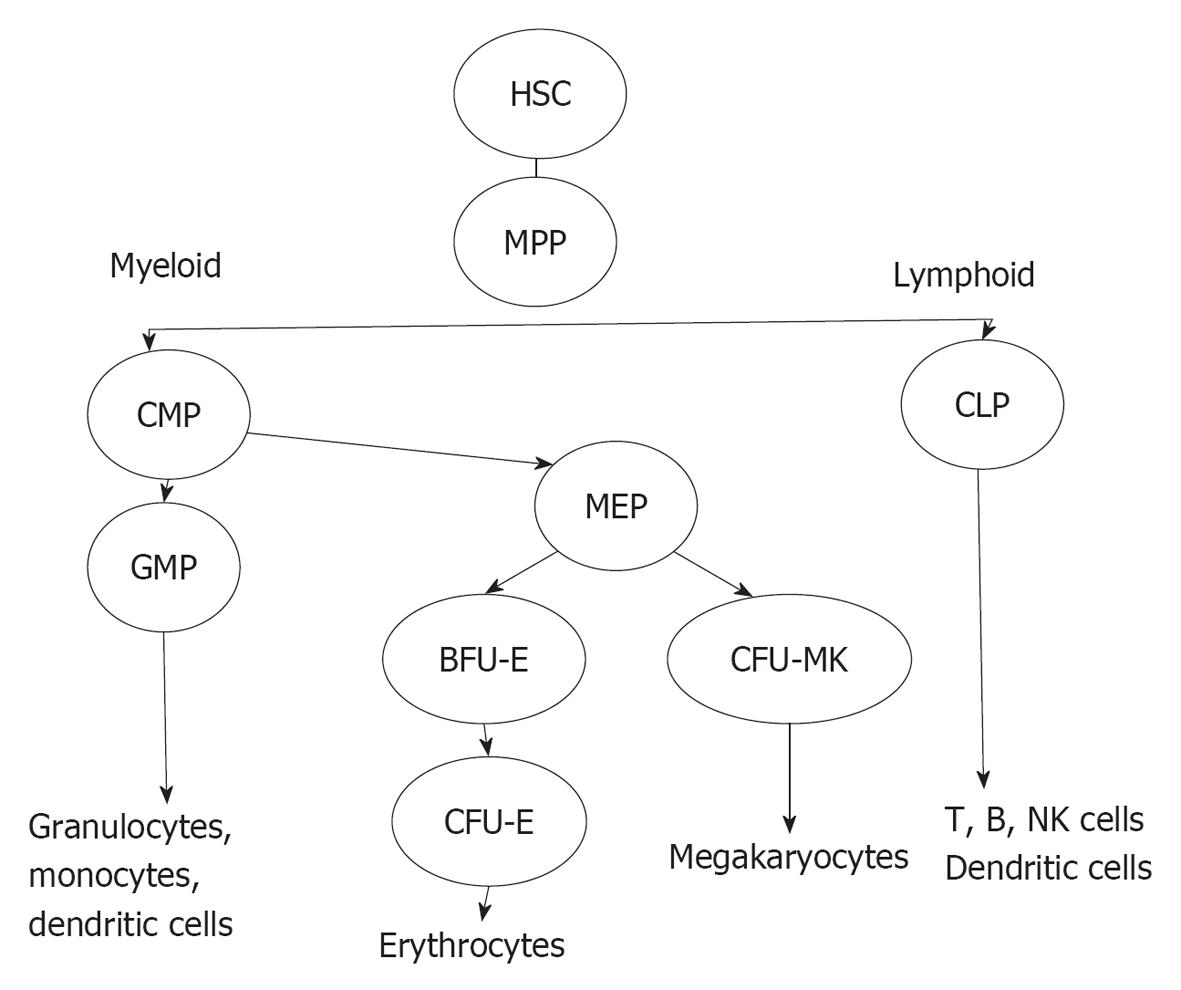
The bone marrow (BM) forms a suitable environment for stem cell survival, growth and differentiation. The cellular components of BM include HPCs, HPC-derived cell lineages and stromal cells. HPCs represent a heterogeneous CD34+ cell population in the BM that includes the most primitive CD34+ haematopoietic stem cells (HSCs), which are characterised by pluripotency and a high capacity for self-renewal, and the CD34+ multi-potent progenitors (MPPs), which originate from HSCs and are multipotent but have a more limited capacity for self-renewal (Figure 1). MPPs can differentiate into common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). CLPs can differentiate into B and T cells, natural killer cells and plasmacytoid dendritic progenitor cells. T cell differentiation occurs in the thymus whereas CMPs differentiate in the BM, through specific differentiation stages, into several cell lineages including granulocytes, erythrocytes, MKs and monocytes[11]. CMP-derived cell lineages migrate into the blood with the exception of MKs, which are maintained in the BM. The differentiation of HSCs is regulated by specific haematopoietic growth factors that induce the survival, proliferation and maturation of specific cell lineages. These factors share several common properties and act hierarchically at different stages of differentiation, and they often show synergistic or additive interactions with other growth factors. Stromal cells are the major source of these factors with the exceptions of erythropoietin (EPO) and thrombopoietin (TPO), which are largely produced in the kidneys and the liver, respectively.
The incidence of peripheral blood cytopenias in HIV positive individuals has led to hypothesis that HIV can impair BM homeostasis and affect the biology and activity of HPCs. Early studies have observed that HIV infection is correlated with the depletion of HPCs and a significant reduction in the in vitro growth of HPCs that have been purified from HIV-infected patients[12-17], suggesting that the multiple peripheral cytopenias may be related, at least in part, to a productive HIV infection of BM HPCs. HIV infection may determine a progressive HPC depletion due to cell lysis, which in turn leads to the derangement of the differentiation towards various cellular lineages. This hypothesis of a potential HIV infection of HPCs may further imply an important feature in the dynamics of HIV disease: long-lived HPCs may harbour proviral HIV DNA genomes in their own genomes and act as an additional reservoir of HIV. Interestingly, cellular HIV receptors and co-receptors can be detected on HPC cell membrane. Flow cytometry analyses showed that 25%-65% of CD34+ HPCs that had been purified from the BM of healthy donors, expressed detectable levels of CD4 protein on their cell membranes[18,19]. Moreover, the CD4 protein was functionally active, and it effectively bound the HIV-1 gp120 anti-receptor[19]. The major co-receptors CXCR4 and CCR5 were also expressed on HPC cell membranes[20-22], and CXCR4 and CCR5 proteins were expressed in 53% and 35% of isolated CD34+ HPCs, respectively[23]. However, the analysis of CXCR4 and CCR5 expression was dependent on the differentiation stage. When the expression levels of CXCR4 and CCR5 were determined in CD34+/CD38- and CD34+/CD38+ HPC subsets, the CXCR4 protein expression level was relatively constant in both subsets whereas CCR5 was detected in 2% of more primitive CD34+/CD38- cells and in 35% of more mature CD34+/CD38+ subset, which indicated that CCR5 but not CXCR4 is up-regulated during differentiation from HSC into MPP[23]. The expression of HIV receptors and co-receptors on the cell membranes of CD34+ HPCs suggested that these cells could be considered a possible target of HIV infection.
To explore this hypothesis, two major experimental approaches were undertaken by several groups: (1) the challenge of BM or cord blood CD34+ HPCs, isolated from uninfected donors, with HIV strains; and (2) the detection of HIV nucleic acids and/or viral proteins in BM CD34+ HPCs isolated from HIV-positive patients. These studies were based on the isolation and purification of CD34+ HPCs that represent a heterogeneous cell population[24,25] because the CD34+ marker could be detected not only on HSCs and MPPs but also on more committed myeloid progenitors such as CFU-GEMM, CFU-GM, BFU-E and CFU-MK progenitors.
Several reports showed that CD34+ BM HPCs, purified from uninfected donors, were resistant to HIV infection. Polymerase chain reaction (PCR) or reverse transcriptase-PCR analysis of proviral HIV DNA or HIV RNA in HPCs that had been challenged with different HIV-1 strains did not reveal significant evidence of HIV infection[9,12,26-29]. In partial contrast to these data, Chelucci and coworkers[30] have purified CD34+ HPCs from the peripheral blood of healthy donors, cultured them with EPO + granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3) and SCF and then challenged with different HIV-1 strains. The analysis of p24 protein showed that 12% of CFU-GM and less than 1% of BFU-E colonies were positive whereas the CFU-GEMM progeny were negative.
Interestingly, early stem cells in the CD34+ HPCs, which are arrested in the G0 phase of the cell cycle, were not permissive for HIV infection[23], and other reports showed that the more primitive CD34+/CD38- HPC subset was not susceptible for HIV-1 or HIV-2 infection[31,32]. However, a limited infection was revealed in the first weeks of long-term culture in CD34+/CD38+ HPCs, which suggested that HIV infects at low extent only the more committed HPC subset but not the more primitive HPCs[31].
The analysis of HIV infection in BM HPCs, purified from HIV-positive patients, was carried out to determine whether these patients could harbour proviral HIV DNA in HPCs. Two studies[33,34], based on PCR assays to detect proviral HIV DNA in BM HPCs, reported that 1 out of 14 patients and 1 out of 11 patients, respectively, were HIV DNA positive. Similar percentages of HIV proviral DNA positive samples were detected in subsequent reports[12,13,35]. In contrast with these results, a higher percentage of HIV-1 infection of CD34+ HPCs was observed in some groups of HIV-1 positive individuals especially in patients with the more advanced stages of the disease[36,37]. This discrepancy could be related to the use of different PCR assays with different sensitivities, and the possible presence of contaminating HIV-infected BM stromal cells.
Notwithstanding these controversial results, the consensus on HPC susceptibility to HIV infection, was that in vitro infection of HPCs occurred, under some experimental conditions, in a low fraction of HPCs, and these HPCs were the more committed HPCs, whereas the more primitive HPCs were not considered a significant HIV target. Moreover, in vivo infection of HPCs was infrequent suggesting a negligible role of HIV-infection of HPCs in BM derangement and the induction of cytopenias[7].
Several mechanisms have been proposed to explain HPC resistance to HIV infection. HPCs secrete the CCR5 ligands macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and regulated on activation normal T cell expressed and secreted (RANTES)[37,38] and the CXCR4 ligand stromal-derived factor 1 (SDF-1)[22], which may compete with R5- or X4-tropic HIV-1 strain infection by interfering with gp120/co-receptor-binding. In addition, an analysis of the interference between gp120 and mAb directed against CXCR4 in HPCs, suggested the lack of a real CD4/CXCR4 complex on HPC membranes, which excluded the formation of the trimeric complex with gp120, essential for HIV binding and infection[22]. Zhang and coworkers have also showed that the cellular cyclin-dependent kinase inhibitor p21 protein restricts HPC infection and interferes with the integration of the proviral HIV-1 genome[39].
However, recent studies have challenged the consensus about HPC resistance to HIV infection[40-44]. A report has described the HIV-1 subtype C infection in CD34+ HPCs, and the analysis of proviral HIV DNA in peripheral blood CD34+ cells showed that 12 out of 19 patients were positive. Interestingly, HIV-1 subtype B strains were not able to infect HPCs, suggesting that only specific HIV subtypes could be associated with direct infection of HPCs[40]. Carter et al[41] challenged purified HPCs with a molecular HIV clone p89.6 derived from the dual tropic HIV strain 89.6. A small percentage (1%-6%) of HPCs exhibited HIV-1 gag protein expression 72 h post infection. A similar infection rate was found, in contrast with previous studies, even in the more primitive CD133+CD34+CD38- HPC subset. However, the methodological approach of this study was subsequently criticised for the choice of the sole criterion of gag analysis, the infection protocol and the pseudo-viruses that were used[45]. These results were substantially confirmed by the same group in a subsequent study[42] that showed HIV infection in approximately 2% of primitive CD133+CD34+high HPC subset cells. HIV infection was detectable when X4-tropic HIV subtype B strains were used, whereas R5-tropic HIV strains were ineffective on CD133+CD34+CD38- HPC subset cells, suggesting that the infection of HPCs might be detectable when X4-tropic HIV strains appear during the progression of HIV infection. These X4-tropic HIV strains are generally observed in the late stages of HIV infection and are related to more rapid disease progression and a poorer prognosis.
Carter et al[41] have also studied BM HPCs, isolated from six HIV-positive patients with high HIV RNA load. HIV-1 gag protein was detected in three of the six samples. When these cells were cultured with GM-CSF and tumour necrosis factor-α (TNF-α) to induce myeloid differentiation, all six of the samples were positive for the gag protein. In the same report, fresh BM HPCs, isolated from nine combination antiretroviral therapy (cART)-treated HIV positive individuals with undetectable viral loads for longer than 6 mo, were analysed using a quantitative real time PCR assay for integrated proviral HIV DNA. Four of the nine samples were positive with the number of proviral HIV genomes ranging between 2.5-40 copies/10000 CD34+ HPCs. These data suggested a new interpretation of the interaction between HIV and HPCs, in which a low number of HSCs and HPCs are susceptible to HIV infection and may represent an HIV reservoir. The rate of infection could be under-recorded because the data from Carter et al[41] indicate that HIV challenge is cytotoxic for HPCs. Moreover, their studies showed that even HIV-1 subtype B strains could infect these HPC subsets, which is in contrast to the previous study indicated above[40], suggesting that the HIV-1 subtype B cytotoxicity could explain this phenomenon. The infection of HPCs indicated that these cells could be a reservoir of HIV. Unfortunately, the hypothesis of HPCs as a viral reservoir was not confirmed by two subsequent analyses on proviral HIV-1 DNA in HPCs[46,47]. In these studies, CD34+ HPCs were purified from 11 and 8 HIV-positive patients treated with long-term suppressive cART. High sensitivity PCR assays demonstrated no HIV-1 proviral DNA in these cells[46,47].
Altogether, these recent studies have reconsidered the relationship between HIV infection and HPCs, but the data still remain controversial and further studies are needed to evaluate whether HIV infection of HPCs may be associated with the onset of blood cytopenias or may represent an additional HIV infection reservoir.
In addition to analyses of the direct infection of HPCs by HIV, several studies have been performed on granulocyte-macrophage CFUs (CFU-GMs), mixed lineage CFUs (CFU-GEMMs) or erythroid burst-forming units (BFU-Es). In this context, HPCs from HIV-1 infected patients, showed impaired in vitro BFU-E, CFU-GM and BFU-MK growth[12,25,27,34,36,48,49]. These results were confirmed even in HPCs purified from HIV-negative individuals and challenged with HIV even though other studies did not observe growth inhibition[26,30,50,51] probably due to the different HIV strains and cell culture conditions that were used. The significant reduction of CFU-GEMM, CFU-GM, BFU-E, and BFU-MK growth suggested an alteration of HPC proliferation with the possible involvement of apoptosis in the induction of cytopenias. Apoptosis plays an important role in the depletion of CD4+ T lymphocytes even through the interaction of HIV gp120 and CD4. HIV gp120 is also able to induce the activation of apoptosis in endothelial cells, osteoblasts, and neurons[52-54], and several studies have been performed on the effects of heat-inactivated HIV and certain viral proteins such as gp120 and Tat on the myelosuppression observed in HIV-positive patients. HIV-1 gp120 and heat-inactivated HIV-1[28,31,55,56] impaired the in vitro clonogenic capacity and induced apoptosis. This negative regulation of proliferation and survival was associated with transforming growth factor β1 (TGFβ1) increased production by HPCs and the occurrence of a Fas-dependent mechanism[57,58]. This reduction in survival and proliferation due to apoptosis could at least partially explain the decrease of HPCs and circulating precursors that has been noted in HIV-positive patients[13,34,36,56-62].
The cellular components of the BM include HPCs at all stages of differentiation and stromal cells. BM stromal cells are a mixed population composed of mesenchymal stem cells (MSCs), endothelial cells, macrophages, fibroblasts, adipocytes, osteoblasts and osteoclasts, as well as dendritic cells and B and T lymphocytes that migrate from the blood to the BM. Stromal cells are essential for proper homeostasis and the regulation of BM haematopoiesis through a complex cellular cross-talk that is modulated by cytokines. In vitro experiments using long-term BM cultures showed that HIV-infected BM stroma was unable to support uninfected CD34+ HPC growth and differentiation compared to uninfected cultures[49,63,64]. In addition, the stromal structure of the BM in HIV patients shows morphological variations including an increased number of macrophages and a decreased number of fibroblasts[10,65]. This impairment of stromal activity and structure affects HPC differentiation and growth and it is due to the complex interaction between HIV and the different BM stromal cells that lead to a derangement of cytokine regulation. In particular, certain cell types, such as T cells, MSCs, macrophages and endothelial cells, are targeted, directly and indirectly, by HIV and its proteins including Tat and gp120[66-69]. BM MSCs can differentiate towards several cell lineages such as osteoblasts, adipocytes, fibroblasts, etc. In vitro experiments have demonstrated that HIV, gp120 and Tat can elicit a derangement of the clonogenic capacity of BM MSCs. In particular, the osteoblast differentiation is inhibited whereas adipocyte differentiation is increased. The alteration of the clonogenic activity may also explain the decreased number of fibroblasts that are detectable in the BM of HIV patients[10,65]. T cells, macrophages, endothelial cells and MKs are productively infected by HIV to different degrees in the BM. Endothelial cells are permissive for HIV infection and BM endothelial cells are infected in HIV patients at every stage of HIV disease. Endothelial cell infection was related to BM impairment in HIV-positive subjects because they exhibited a reduced ability to respond to BM micro-environmental regulatory signals that positively up-regulated the number of blood cells[69].
CD4+ T cells and macrophages are the major targets of HIV replication, and the release of specific cytokines and haematopoietic factors is affected by HIV infection. TNFα, TGFβ1, interferon-γ (IFN-γ), IL-1, IL-6, IL-10, IL-18, TNF-related apoptosis-inducing ligand and monocyte colony-stimulating factor are dysregulated by HIV in T cells and monocyte models[70-75]. Similarly, viral proteins such as Tat and/or gp120 increase the expression of IL-6, TNFα and IL-1[76-81]. The impairment of several cytokines during HIV infection was confirmed by clinical studies in which higher levels of IL-1, IL-18, TNFα and IL-6 in the plasma of HIV-positive patients were detected compared to uninfected individuals[82-85]. It is noteworthy that the pro-inflammatory cytokines TNFα, IL-1, and IL-6 and the chemokines MIP-1α, MIP-1β and RANTES were up-regulated in the BM of HIV-positive patients[65,86]. TNFα involvement in the HIV-1-induced suppression of haematopoiesis, was also suggested in neutralisation studies[87]. Tat is able to elicit a significant activation of the TGFβ1 expression in macrophages that have been isolated from BM. BM macrophage culture supernatants were added to BM HPC cultures thus inducing an inhibition of HPC growth in the liquid cultures[88]. This chronic derangement of cytokine modulation can elicit several negative effects on HPCs and their differentiation into various cell lineages, cooperating in the pathogenesis of anaemia and thrombocytopenia in HIV-infected patients.
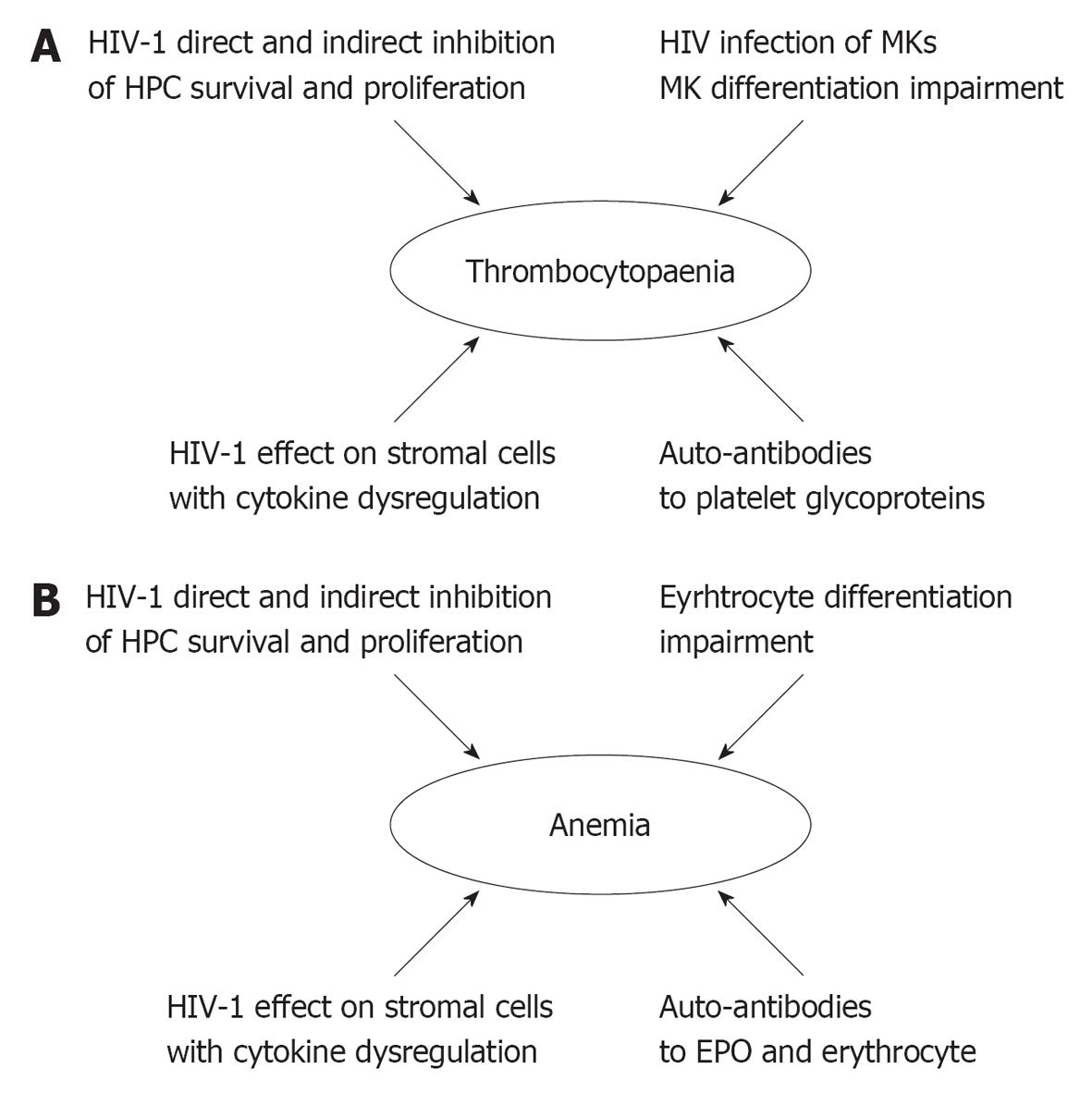
Chronic thrombocytopenia is detectable during HIV disease in approximately 10% of HIV positive patients and 15%-60% of patients with AIDS[89-92]. This haematological disorder may represent the first manifestation of HIV infection and it may progress over time and lead to severe bleeding[91]. HIV-associated thrombocytopenia is related to reduced platelet survival, ineffective platelet production and the impairment of the survival of BM MKs and their precursors. HIV targets the MK cell lineage by interfering throughout the differentiation of mature MKs (Figure 2A). As described above, HIV decreases the number and activity of HPCs and induces a growth deficit in CFU-MKs in HIV patients. An analysis of the impact of HIV-1 and gp120 during TPO-induced cord blood-derived HPC differentiation into MKs has demonstrated that gp120 treatment led to the induction of apoptosis in the CD41+ and CD61+ subsets due to TGFβ1 increase and APRIL down-regulation[55]. These data confirmed the induction of apoptosis through the gp120 engagement of CD4, observed in BM GPIIb/IIa+ (CD41+) megakaryocytic cells and in megakaryocytic cell line models[93-95]. Moreover, a reduction of c-mpl expression in the MK lineage due to V3 loop region of gp120 was observed in MK lineage thus indicating a further mechanism involved in the impairment of megakaryocytopoiesis[96].
HIV and gp120 altered the maturation of MKs, and decreased the number of MKs with higher ploidy[55]. Electron microscopy analysis of MKs from HIV-infected individuals with thrombocytopenia clearly demonstrated ultrastructural abnormalities, such as blebbing of the surface membrane and vacuolisation of the peripheral cytoplasm[97]. Mature MKs can be infected by HIV through binding the CD4 receptor[97-102], and HIV genomes have been detected in MKs purified from BM of HIV-positive patients[103]. The infection of MKs is not strain-restricted because both R5- and X4-tropic HIV-1 strains are able to infect MKs thus indicating that the infection may occur early in the development of HIV infection[99]. In addition to these direct effects of HIV on the MK cell lineage, HIV also supports chronic thrombocytopenia through autoimmune mechanisms[89-92], particularly evident in early stages of the disease[104,105]. Autoimmune mechanisms are related to anti-HIV antibodies cross-reacting with platelet-membrane glycoproteins, supporting the basic role of molecular mimicry in the induction of these antibodies[106-110]. In particular, an autoantibody directed against integrin GPIIIa49-66 induced a platelet lysis[110] and cross-reacted with some peptides derived from Nef and gp120[111]. The anti-GPIIIa49-66 antibody isolated from HIV-1 patients down-regulated MK proliferation in in vitro culture of human cord blood CD34+ cells driven by TPO[112].
Platelets can bind HIV-1 gp120 through its CXCR4 and fibronectin surface receptors, and platelet-bound HIV may infect permissive cells suggesting a possible role for platelets as carriers in the spread of HIV infection[113]. The interaction between platelets and HIV leads to the activation of platelets and an altered platelet morphology, which is likely due to CXCR4 binding because this protein is the receptor of SDF-1, a factor involved in enhancing platelet activation by agonists[114]. Platelet activation was detected in HIV patients and the degree of activation in circulating platelets was higher in AIDS patients than patients in earlier stages of HIV infection[115]. Activated platelets also represent a source of some pro-inflammatory cytokines. Their activation led to a strong induction of IL-1β and IL-18 secretion eliciting a further cytokine regulation derangement[114,116]. These alterations of platelet activity were also related to the impairment of coagulation homeostasis, thus increasing the complexity of the HIV/MK/platelet/coagulation interactions. These studies demonstrated that the MK lineage is a direct and indirect target of HIV and its proteins throughout their entire differentiation and development. This targeting affects platelet maturation and activity, explaining why thrombocytopenia is a major cytopenia in HIV-positive patients.
Anaemia is a clinical complication detectable in many HIV patients[117]. The overall incidence of anaemia in HIV-positive individuals is 10% in asymptomatic patients and up to 92% in patients with AIDS[6,117]. cART treatment has reduced but not solved the problem of anaemia in HIV patients. In a cohort of 1624 patients in the EuroSIDA study, the prevalence of anaemia during HAART decreased from 65% in naive patients to 53% after 6 mo of therapy and 45% after 1 year of therapy[118]. Although anaemia does not generally cause death in HIV patients, it is well known that anaemia can increase morbidity in these subjects. HIV patients with anaemia have a higher risk of reduced survival compared to non-anaemic in HIV positive patients[117,119]. The symptoms of anaemia during HIV infection are not different from the symptoms that are observed in HIV negative patients, and the diagnosis of anaemia is often a laboratory diagnosis based on a reduction of the haemoglobin (Hb) value and erythrocyte count. The anaemia is generally mild with Hb concentrations between 8-14 g/dL for men and 8-12 g/dL for women, although the degree of anaemia is dependent on the immunosuppressive context and disease stage[120]. The erythrocyte morphology does not exhibit consistent variations in the peripheral blood[121]. Microcytosis is rarely observed, whereas macrocytosis is found in HIV-positive patients treated with zidovudine (AZT). AZT treatment was related to BM suppression, and the HER and WIHS studies observed a significant increase in anaemia in AZT-treated patients[122,123]. Some reports indicated the presence of poikilocytosis, anisocytosis and ruleaux formation, but, in general, HIV-associated anaemia is characterised by normocytosis, low reticulocyte counts and an ineffective erythropoiesis with an hyporegenerative BM[6,124]. The pathogenesis of anaemia in HIV-positive patients is multifactorial (Figure 2B): the different mechanisms that are involved in the anaemia induction are characterised by the impairment of erythrocyte production and increased erythrocyte destruction[120]. HIV is directly involved in the induction of anaemia even though neoplastic diseases, vitamin deficiencies, iron metabolism impairment, pharmacological treatments and opportunistic infections are implicated in anaemia onset during HIV infection. The involvement of HPCs in the cytopenias has been illustrated above, however, it is noteworthy that Cleveland and coworkers observed the expression of CD4 on the cell membranes of erythroid differentiating cells. The co-expression of CD4 and glycophorin A indicates that some erythroid-committed cells could represent a target for HIV infection[125]. In addition, the expression of functional CXCR4[37] was detected in CD34+ BFU-Es even though its expression level decreased during erythroid differentiation. Interestingly, Tat treatment of cord blood-isolated HPCs up-regulated CXCR4 protein expression indicating a complex effect of HIV activity on erythrocyte lineage survival and differentiation[126]. Moreover, the dysfunction of erythroid differentiation could be related to BM microenvironment damage and stromal cell impairment[7,71]. IL-1β, IFN-γ, TGFβ1 and TNFα suppress the growth of progenitor cells in vitro and may play an important role in the induction of HIV-associated anaemia[116,126,127]. Some papers have suggested that HIV could impair the EPO-related feedback mechanisms that regulate the red blood cell homeostasis. Decreasing the Hb concentration induces EPO production, whereas in many HIV patients the presence of anaemia is coupled with a decrease in the serum EPO concentration that is independent of kidney damage[121,128,129]. Moreover, in vitro experiments demonstrated that HIV-1 reduced EPO synthesis[130]. Different mechanisms have been considered to explain this EPO reduction. HIV-related up-regulation of pro-inflammatory cytokines IL-1β and TNF-α directly down-regulates EPO expression in vitro[131] through the cytokine-mediated formation of reactive oxygen species, which in turn impair the binding affinities of EPO-inducing transcription factors. In addition, circulating antibodies to EPO are detectable in approximatively 23% of HIV-infected patients, and a prospective study on 113 patients showed that anti-EPO antibodies could be considered an independent risk factor for anaemia[132,133]. The presence of these auto-antibodies, directed against several targets, was associated with molecular mimicry and the dysregulation of the immune system. Recent reports demonstrated that the anti-EPO antibodies recognised three major EPO molecule epitopes that span three regions including the amino acids domains 1-20 (EP1), 54-72 (EP5) and 147-166 (EP12) of which EP1 and EP12 are the domains that are involved in the EPO-EPOR interaction[134]. The region corresponding to EP1 shows a 63% sequence homology with the 34-52 amino acid sequence of HIV gag p17, and a cross-reaction between anti-EP-1 auto-antibodies and the gag fragment was detected suggesting a role for mimicry by this protein in the occurrence of anaemia[134]. HIV-associated anaemia could also be induced by haemolysis. In HIV patients, cases of haemolysis have been observed that are linked to CID, glucose-6-dehydrogenase deficiency, auto-antibodies against red blood cells, thrombotic thrombocytopenia purpura and pharmacological treatment. Furthermore, some HIV positive patients exhibited the presence of a broad panel of specific and non-specific anti-erythrocyte antibodies, and, in some cases, erythrocyte lysis was mediated by complement activation. Although consistent haemolysis is rare in HIV patients, the damage and lysis of red blood cells by auto-antibodies can be considered an additional mechanism of HIV-associated anaemia[135-137].
In conclusion, the occurrence of thrombocytopenia and anaemia represent major pathological manifestations in HIV patients. The pathogenesis of these cytopenias is multifactorial, and several targets such as HPCs, cell lineage differentiation, cytokine dysregulation and stromal cell impairment cooperate in the occurrence of these haematopoietic defects. The investigation of the different mechanisms that are involved in the genesis of these cytopenias has provided important findings on HIV pathogenesis even though some pivotal items such as the susceptibility of HPCs to HIV infection and their role as HIV infection reservoirs are still under debate and deserve additional experimental analysis. Further studies will be essential to better characterise these mechanisms and to identify useful targets for supportive therapy and management of HIV-positive patients.
P- Reviewer Giannecchini S S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Levy JA. HIV pathogenesis: 25 years of progress and persistent challenges. AIDS. 2009;23:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Borderi M, Gibellini D, Vescini F, De Crignis E, Cimatti L, Biagetti C, Tampellini L, Re MC. Metabolic bone disease in HIV infection. AIDS. 2009;23:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Gandhi RT, Sax PE, Grinspoon SK. Metabolic and cardiovascular complications in HIV-infected patients: new challenges for a new age. J Infect Dis. 2012;205 Suppl 3:S353-S354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Maggi P, Bartolozzi D, Bonfanti P, Calza L, Cherubini C, Di Biagio A, Marcotullio S, Montella F, Montinaro V, Mussini C. Renal complications in HIV disease: between present and future. AIDS Rev. 2012;14:37-53. [PubMed] |
| 5. | Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Zon LI, Arkin C, Groopman JE. Haematologic manifestations of the human immune deficiency virus (HIV). Br J Haematol. 1987;66:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 192] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Moses A, Nelson J, Bagby GC. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91:1479-1495. [PubMed] |
| 8. | Ratner L. Human immunodeficiency virus-associated autoimmune thrombocytopenic purpura: a review. Am J Med. 1989;86:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Davis BR, Zauli G. Effect of human immunodeficiency virus infection on haematopoiesis. Baillieres Clin Haematol. 1995;8:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4:e1000215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Zauli G, Re MC, Davis B, Sen L, Visani G, Gugliotta L, Furlini G, La Placa M. Impaired in vitro growth of purified (CD34+) hematopoietic progenitors in human immunodeficiency virus-1 seropositive thrombocytopenic individuals. Blood. 1992;79:2680-2687. [PubMed] |
| 13. | Louache F, Henri A, Bettaieb A, Oksenhendler E, Raguin G, Tulliez M, Vainchenker W. Role of human immunodeficiency virus replication in defective in vitro growth of hematopoietic progenitors. Blood. 1992;80:2991-2999. [PubMed] |
| 14. | Banda NK, Simon GR, Sipple JD, Terrell KL, Archer P, Shpall EJ, Akkina RK, Myers AM, Harrison GS. Depletion of CD34+ CD4+ cells in bone marrow from HIV-1-infected individuals. Biol Blood Marrow Transplant. 1999;5:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zauli G, Vitale M, Re MC, Furlini G, Zamai L, Falcieri E, Gibellini D, Visani G, Davis BR, Capitani S. In vitro exposure to human immunodeficiency virus type 1 induces apoptotic cell death of the factor-dependent TF-1 hematopoietic cell line. Blood. 1994;83:167-175. [PubMed] |
| 16. | Thiebot H, Louache F, Vaslin B, de Revel T, Neildez O, Larghero J, Vainchenker W, Dormont D, Le Grand R. Early and persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J Virol. 2001;75:11594-11602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Costantini A, Giuliodoro S, Mancini S, Butini L, Regnery CM, Silvestri G, Greco F, Leoni P, Montroni M. Impaired in-vitro growth of megakaryocytic colonies derived from CD34 cells of HIV-1-infected patients with active viral replication. AIDS. 2006;20:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Louache F, Debili N, Marandin A, Coulombel L, Vainchenker W. Expression of CD4 by human hematopoietic progenitors. Blood. 1994;84:3344-3355. [PubMed] |
| 19. | Zauli G, Furlini G, Vitale M, Re MC, Gibellini D, Zamai L, Visani G, Borgatti P, Capitani S, La Placa M. A subset of human CD34+ hematopoietic progenitors express low levels of CD4, the high-affinity receptor for human immunodeficiency virus-type 1. Blood. 1994;84:1896-1905. [PubMed] |
| 20. | Deichmann M, Kronenwett R, Haas R. Expression of the human immunodeficiency virus type-1 coreceptors CXCR-4 (fusin, LESTR) and CKR-5 in CD34+ hematopoietic progenitor cells. Blood. 1997;89:3522-3528. [PubMed] |
| 21. | Carr JM, Ramshaw HS, Li P, Burrell CJ. CD34+ cells and their derivatives contain mRNA for CD4 and human immunodeficiency virus (HIV) co-receptors and are susceptible to infection with M- and T-tropic HIV. J Gen Virol. 1998;79:71-75. [PubMed] |
| 22. | Aiuti A, Turchetto L, Cota M, Cipponi A, Brambilla A, Arcelloni C, Paroni R, Vicenzi E, Bordignon C, Poli G. Human CD34(+) cells express CXCR4 and its ligand stromal cell-derived factor-1. Implications for infection by T-cell tropic human immunodeficiency virus. Blood. 1999;94:62-73. [PubMed] |
| 23. | Shen H, Cheng T, Preffer FI, Dombkowski D, Tomasson MH, Golan DE, Yang O, Hofmann W, Sodroski JG, Luster AD. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73:728-737. [PubMed] |
| 24. | Kitano K, Abboud CN, Ryan DH, Quan SG, Baldwin GC, Golde DW. Macrophage-active colony-stimulating factors enhance human immunodeficiency virus type 1 infection in bone marrow stem cells. Blood. 1991;77:1699-1705. [PubMed] |
| 25. | Steinberg HN, Crumpacker CS, Chatis PA. In vitro suppression of normal human bone marrow progenitor cells by human immunodeficiency virus. J Virol. 1991;65:1765-1769. [PubMed] |
| 26. | Molina JM, Scadden DT, Sakaguchi M, Fuller B, Woon A, Groopman JE. Lack of evidence for infection of or effect on growth of hematopoietic progenitor cells after in vivo or in vitro exposure to human immunodeficiency virus. Blood. 1990;76:2476-2482. [PubMed] |
| 27. | Cen D, Zauli G, Szarnicki R, Davis BR. Effect of different human immunodeficiency virus type-1 (HIV-1) isolates on long-term bone marrow haemopoiesis. Br J Haematol. 1993;85:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Zauli G, Vitale M, Gibellini D, Capitani S. Inhibition of purified CD34+ hematopoietic progenitor cells by human immunodeficiency virus 1 or gp120 mediated by endogenous transforming growth factor beta 1. J Exp Med. 1996;183:99-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kaushal S, La Russa VF, Gartner S, Kessler S, Perfetto S, Yu Z, Ritchey DW, Xu J, Perera P, Kim J. Exposure of human CD34+ cells to human immunodeficiency virus type 1 does not influence their expansion and proliferation of hematopoietic progenitors in vitro. Blood. 1996;88:130-137. [PubMed] |
| 30. | Chelucci C, Hassan HJ, Locardi C, Bulgarini D, Pelosi E, Mariani G, Testa U, Federico M, Valtieri M, Peschle C. In vitro human immunodeficiency virus-1 infection of purified hematopoietic progenitors in single-cell culture. Blood. 1995;85:1181-1187. [PubMed] |
| 31. | Weichold FF, Zella D, Barabitskaja O, Maciejewski JP, Dunn DE, Sloand EM, Young NS. Neither human immunodeficiency virus-1 (HIV-1) nor HIV-2 infects most-primitive human hematopoietic stem cells as assessed in long-term bone marrow cultures. Blood. 1998;91:907-915. [PubMed] |
| 32. | Hariharan D, Li Y, Campbell DE, Douglas SD, Starr SE, Ho W. Human immunodeficiency virus infection of human placental cord blood CD34+AC133+ stem cells and their progeny. AIDS Res Hum Retroviruses. 1999;15:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | von Laer D, Hufert FT, Fenner TE, Schwander S, Dietrich M, Schmitz H, Kern P. CD34+ hematopoietic progenitor cells are not a major reservoir of the human immunodeficiency virus. Blood. 1990;76:1281-1286. [PubMed] |
| 34. | Davis BR, Schwartz DH, Marx JC, Johnson CE, Berry JM, Lyding J, Merigan TC, Zander A. Absent or rare human immunodeficiency virus infection of bone marrow stem/progenitor cells in vivo. J Virol. 1991;65:1985-1990. [PubMed] |
| 35. | Neal TF, Holland HK, Baum CM, Villinger F, Ansari AA, Saral R, Wingard JR, Fleming WH. CD34+ progenitor cells from asymptomatic patients are not a major reservoir for human immunodeficiency virus-1. Blood. 1995;86:1749-1756. [PubMed] |
| 36. | Stanley SK, Kessler SW, Justement JS, Schnittman SM, Greenhouse JJ, Brown CC, Musongela L, Musey K, Kapita B, Fauci AS. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992;149:689-697. [PubMed] |
| 37. | Majka M, Rozmyslowicz T, Lee B, Murphy SL, Pietrzkowski Z, Gaulton GN, Silberstein L, Ratajczak MZ. Bone marrow CD34(+) cells and megakaryoblasts secrete beta-chemokines that block infection of hematopoietic cells by M-tropic R5 HIV. J Clin Invest. 1999;104:1739-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Majka M, Rozmyslowicz T, Ratajczak J, Dobrowsky A, Pietrzkowski Z, Gaulton GN, Janowska-Wieczorek A, Ratajczak MZ. The limited infectability by R5 HIV of CD34(+) cells from thymus, cord, and peripheral blood and bone marrow is explained by their ability to produce beta-chemokines. Exp Hematol. 2000;28:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007;117:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Redd AD, Avalos A, Essex M. Infection of hematopoietic progenitor cells by HIV-1 subtype C, and its association with anemia in southern Africa. Blood. 2007;110:3143-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Carter CC, McNamara LA, Onafuwa-Nuga A, Shackleton M, Riddell J, Bixby D, Savona MR, Morrison SJ, Collins KL. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe. 2011;9:223-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | McNamara LA, Ganesh JA, Collins KL. Latent HIV-1 infection occurs in multiple subsets of hematopoietic progenitor cells and is reversed by NF-κB activation. J Virol. 2012;86:9337-9350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Li L, Qiu C, Li L, Liu A, Zhou M, Han Z, Qiu C, Zhang X, Xu J, Zhu H. In vitro infection of human umbilical cord blood CD34+ hematopoietic progenitor cells by HIV-1 CRF07_BC enveloped pseudovirus. Curr HIV Res. 2012;10:572-577. [PubMed] |
| 45. | Zhang J, Crumpacker CS. Hematopoietic stem and progenitor cells in HIV/AIDS and immune reconstitution. Cell Res. 2010;20:745-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Durand CM, Ghiaur G, Siliciano JD, Rabi SA, Eisele EE, Salgado M, Shan L, Lai JF, Zhang H, Margolick J. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis. 2012;205:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Josefsson L, Eriksson S, Sinclair E, Ho T, Killian M, Epling L, Shao W, Lewis B, Bacchetti P, Loeb L. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. J Infect Dis. 2012;206:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Maciejewski JP, Weichold FF, Young NS. HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha. J Immunol. 1994;153:4303-4310. [PubMed] |
| 49. | Schwartz GN, Kessler SW, Szabo JM, Burrell LM, Francis ML. Negative regulators may mediate some of the inhibitory effects of HIV-1 infected stromal cell layers on erythropoiesis and myelopoiesis in human bone marrow long term cultures. J Leukoc Biol. 1995;57:948-955. [PubMed] |
| 50. | Marandin A, Canque B, Coulombel L, Gluckman JC, Vainchenker W, Louache F. In vitro infection of bone marrow-adherent cells by human immunodeficiency virus type 1 (HIV-1) does not alter their ability to support hematopoiesis. Virology. 1995;213:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Kojouharoff G, Ottmann OG, von Briesen H, Geissler G, Rübsamen-Waigmann H, Hoelzer D, Ganser A. Infection of granulocyte/monocyte progenitor cells with HIV1. Res Virol. 1991;142:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Gibellini D, De Crignis E, Ponti C, Cimatti L, Borderi M, Tschon M, Giardino R, Re MC. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Chen L, Liu J, Xu C, Keblesh J, Zang W, Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents. PLoS One. 2011;6:e25994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | MacEneaney OJ, Connick E, DeSouza CA. Effects of HIV-1 gp120 and protease inhibitors on apoptotic susceptibility of CD34+ hematopoietic progenitor cells. J Acquir Immune Defic Syndr. 2011;56:e49-e50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Banda NK, Tomczak JA, Shpall EJ, Sipple J, Akkina RK, Steimer KS, Hami L, Curiel TJ, Singer Harrison G. HIV-gp120 induced cell death in hematopoietic progenitor CD34+ cells. Apoptosis. 1997;2:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Gibellini D, Vitone F, Buzzi M, Schiavone P, De Crignis E, Cicola R, Conte R, Ponti C, Re MC. HIV-1 negatively affects the survival/maturation of cord blood CD34(+) hematopoietic progenitor cells differentiated towards megakaryocytic lineage by HIV-1 gp120/CD4 membrane interaction. J Cell Physiol. 2007;210:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Zauli G, Re MC, Visani G, Furlini G, La Placa M. Inhibitory effect of HIV-1 envelope glycoproteins gp120 and gp160 on the in vitro growth of enriched (CD34+) hematopoietic progenitor cells. Arch Virol. 1992;122:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Zauli G, Re MC, Visani G, Furlini G, Mazza P, Vignoli M, La Placa M. Evidence for a human immunodeficiency virus type 1-mediated suppression of uninfected hematopoietic (CD34+) cells in AIDS patients. J Infect Dis. 1992;166:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Stella CC, Ganser A, Hoelzer D. Defective in vitro growth of the hemopoietic progenitor cells in the acquired immunodeficiency syndrome. J Clin Invest. 1987;80:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Lunardi-Iskandar Y, Georgoulias V, Bertoli AM, Augery-Bourget Y, Ammar A, Vittecoq D, Rosenbaum W, Meyer P, Jasmin C. Impaired in-vitro proliferation of hemopoietic precursors in HIV-1-infected subjects. Leuk Res. 1989;13:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Donahue RE, Johnson MM, Zon LI, Clark SC, Groopman JE. Suppression of in vitro haematopoiesis following human immunodeficiency virus infection. Nature. 1987;326:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 156] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Bahner I, Kearns K, Coutinho S, Leonard EH, Kohn DB. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90:1787-1798. [PubMed] |
| 64. | Gill V, Shattock RJ, Scopes J, Hayes P, Freedman AR, Griffin GE, Gordon-Smith EC, Gibson FM. Human immunodeficiency virus infection impairs hemopoiesis in long-term bone marrow cultures: nonreversal by nucleoside analogues. J Infect Dis. 1997;176:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Isgrò A, Aiuti A, Mezzaroma I, Addesso M, Riva E, Giovannetti A, Mazzetta F, Alario C, Mazzone A, Ruco L. Improvement of interleukin 2 production, clonogenic capability and restoration of stromal cell function in human immunodeficiency virus-type-1 patients after highly active antiretroviral therapy. Br J Haematol. 2002;118:864-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Cotter EJ, Chew N, Powderly WG, Doran PP. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Gibellini D, Alviano F, Miserocchi A, Tazzari PL, Ricci F, Clò A, Morini S, Borderi M, Viale P, Pasquinelli G. HIV-1 and recombinant gp120 affect the survival and differentiation of human vessel wall-derived mesenchymal stem cells. Retrovirology. 2011;8:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Gibellini D, Miserocchi A, Tazzari PL, Ricci F, Clò A, Morini S, Ponti C, Pasquinelli G, Bon I, Pagliaro P. Analysis of the effects of HIV-1 Tat on the survival and differentiation of vessel wall-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Moses AV, Williams S, Heneveld ML, Strussenberg J, Rarick M, Loveless M, Bagby G, Nelson JA. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87:919-925. [PubMed] |
| 70. | Birx DL, Redfield RR, Tencer K, Fowler A, Burke DS, Tosato G. Induction of interleukin-6 during human immunodeficiency virus infection. Blood. 1990;76:2303-2310. [PubMed] |
| 71. | Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159-7167. [PubMed] |
| 72. | Gibellini D, Zauli G, Re MC, Milani D, Furlini G, Caramelli E, Capitani S, La Placa M. Recombinant human immunodeficiency virus type-1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF-beta 1 mRNA expression and protein synthesis in peripheral blood monocytes. Br J Haematol. 1994;88:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-gamma and tumor necrosis factor-alpha secretion. Blood. 1994;84:2622-2631. [PubMed] |
| 74. | Scala G, Ruocco MR, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, Dragonetti E, Quinto I, Venuta S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994;179:961-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 199] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Barqasho B, Nowak P, Tjernlund A, Kinloch S, Goh LE, Lampe F, Fisher M, Andersson J, Sönnerborg A. Kinetics of plasma cytokines and chemokines during primary HIV-1 infection and after analytical treatment interruption. HIV Med. 2009;10:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Weiss L, Haeffner-Cavaillon N, Laude M, Gilquin J, Kazatchkine MD. HIV infection is associated with the spontaneous production of interleukin-1 (IL-1) in vivo and with an abnormal release of IL-1 alpha in vitro. AIDS. 1989;3:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Lafeuillade A, Poizot-Martin I, Quilichini R, Gastaut JA, Kaplanski S, Farnarier C, Mege JL, Bongrand P. Increased interleukin-6 production is associated with disease progression in HIV infection. AIDS. 1991;5:1139-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133-150. [PubMed] |
| 79. | Ahmad R, Sindhu ST, Toma E, Morisset R, Ahmad A. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J Virol. 2002;76:12448-12456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Torre D, Pugliese A. Interleukin 18 and cardiovascular disease in HIV-1 infection: a partner in crime. AIDS Rev. 2010;12:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol. 2008;180:6675-6684. [PubMed] |
| 82. | Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load & lt; or=20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. 2008;68:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Tebas P, Henry WK, Matining R, Weng-Cherng D, Schmitz J, Valdez H, Jahed N, Myers L, Powderly WG, Katzenstein D. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk. PLoS One. 2008;3:e2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Bastard JP, Soulié C, Fellahi S, Haïm-Boukobza S, Simon A, Katlama C, Calvez V, Marcelin AG, Capeau J. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther. 2012;17:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 86. | Wang H, English NJ, Reid CD, Merson JE, Knight SC. Role of beta-chemokines in HIV-1 infection of dendritic cells maturing from CD34+ stem cells. J Acquir Immune Defic Syndr. 1999;21:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Yurasov SV, Pettoello-Mantovani M, Raker CA, Goldstein H. HIV type 1 infection of human fetal bone marrow cells induces apoptotic changes in hematopoietic precursor cells and suppresses their in vitro differentiation and capacity to engraft SCID mice. AIDS Res Hum Retroviruses. 1999;15:1639-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Zauli G, Davis BR, Re MC, Visani G, Furlini G, La Placa M. tat protein stimulates production of transforming growth factor-beta 1 by marrow macrophages: a potential mechanism for human immunodeficiency virus-1-induced hematopoietic suppression. Blood. 1992;80:3036-3043. [PubMed] |
| 89. | Bierling P, Bettaieb A, Oksenhendler E. Human immunodeficiency virus-related immune thrombocytopenia. Semin Thromb Hemost. 1995;21:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Najean Y, Rain JD. The mechanism of thrombocytopenia in patients with HIV infection. J Lab Clin Med. 1994;123:415-420. [PubMed] |
| 91. | Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Vannappagari V, Nkhoma ET, Atashili J, Laurent SS, Zhao H. Prevalence, severity, and duration of thrombocytopenia among HIV patients in the era of highly active antiretroviral therapy. Platelets. 2011;22:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Zauli G, Catani L, Gibellini D, Re MC, Milani D, Borgatti P, Bassini A, La Placa M, Capitani S. The CD4 receptor plays essential but distinct roles in HIV-1 infection and induction of apoptosis in primary bone marrow GPIIb/IIIa+ megakaryocytes and the HEL cell line. Br J Haematol. 1995;91:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Zauli G, Catani L, Gibellini D, Re MC, Vianelli N, Colangeli V, Celeghini C, Capitani S, La Placa M. Impaired survival of bone marrow GPIIb/IIa+ megakaryocytic cells as an additional pathogenetic mechanism of HIV-1-related thrombocytopenia. Br J Haematol. 1996;92:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Gibellini D, Re MC, Bassini A, Guidotti L, Catani L, La Placa M, Zauli G. HIV-1 gp120 induces the activation of both c-fos and c-jun immediate-early genes in HEL megakaryocytic cells. Br J Haematol. 1999;104:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Zhang M, Evans S, Yuan J, Ratner L, Koka PS. HIV-1 determinants of thrombocytopenia at the stage of CD34+ progenitor cell differentiation in vivo lie in the viral envelope gp120 V3 loop region. Virology. 2010;401:131-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Zucker-Franklin D, Cao YZ. Megakaryocytes of human immunodeficiency virus-infected individuals express viral RNA. Proc Natl Acad Sci USA. 1989;86:5595-5599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Chelucci C, Federico M, Guerriero R, Mattia G, Casella I, Pelosi E, Testa U, Mariani G, Hassan HJ, Peschle C. Productive human immunodeficiency virus-1 infection of purified megakaryocytic progenitors/precursors and maturing megakaryocytes. Blood. 1998;91:1225-1234. [PubMed] |
| 99. | Voulgaropoulou F, Pontow SE, Ratner L. Productive infection of CD34+-cell-derived megakaryocytes by X4 and R5 HIV-1 isolates. Virology. 2000;269:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Sakaguchi M, Sato T, Groopman JE. Human immunodeficiency virus infection of megakaryocytic cells. Blood. 1991;77:481-485. [PubMed] |
| 101. | Basch RS, Kouri YH, Karpatkin S. Expression of CD4 by human megakaryocytes. Proc Natl Acad Sci USA. 1990;87:8085-8089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Kouri YH, Borkowsky W, Nardi M, Karpatkin S, Basch RS. Human megakaryocytes have a CD4 molecule capable of binding human immunodeficiency virus-1. Blood. 1993;81:2664-2670. [PubMed] |
| 103. | Zucker-Franklin D, Termin CS, Cooper MC. Structural changes in the megakaryocytes of patients infected with the human immune deficiency virus (HIV-1). Am J Pathol. 1989;134:1295-1303. [PubMed] |
| 104. | Murphy MF, Metcalfe P, Waters AH, Carne CA, Weller IV, Linch DC, Smith A. Incidence and mechanism of neutropenia and thrombocytopenia in patients with human immunodeficiency virus infection. Br J Haematol. 1987;66:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 105. | Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 106. | Karpatkin S, Nardi M, Lennette ET, Byrne B, Poiesz B. Anti-human immunodeficiency virus type 1 antibody complexes on platelets of seropositive thrombocytopenic homosexuals and narcotic addicts. Proc Natl Acad Sci USA. 1988;85:9763-9767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 107. | Bettaieb A, Fromont P, Louache F, Oksenhendler E, Vainchenker W, Duédari N, Bierling P. Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune thrombocytopenic purpura. Blood. 1992;80:162-169. [PubMed] |
| 108. | Hohmann AW, Booth K, Peters V, Gordon DL, Comacchio RM. Common epitope on HIV p24 and human platelets. Lancet. 1993;342:1274-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Karpatkin S, Nardi MA, Hymes KB. Sequestration of anti-platelet GPIIIa antibody in rheumatoid factor immune complexes of human immunodeficiency virus 1 thrombocytopenic patients. Proc Natl Acad Sci USA. 1995;92:2263-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia. Blood. 2005;106:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Pan R, Wang J, Nardi MA, Li Z. The inhibition effect of anti-GPIIIa49-66 antibody on megakaryocyte differentiation. Thromb Haemost. 2011;106:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Pugliese A, Savarino A, Cantamessa C, Torre D. Influence of fibronectin on HIV-1 infection and capability of binding to platelets. Cell Biochem Funct. 1996;14:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Gresele P, Falcinelli E, Momi S. Potentiation and priming of platelet activation: a potential target for antiplatelet therapy. Trends Pharmacol Sci. 2008;29:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Holme PA, Müller F, Solum NO, Brosstad F, Frøland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79-89. [PubMed] |
| 116. | Ahmad R, Iannello A, Samarani S, Morisset R, Toma E, Grosley M, Ahmad A. Contribution of platelet activation to plasma IL-18 concentrations in HIV-infected AIDS patients. AIDS. 2006;20:1907-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Kreuzer KA, Rockstroh JK. Pathogenesis and pathophysiology of anemia in HIV infection. Ann Hematol. 1997;75:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio Monforte A, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 119. | Forsyth BW, Andiman WA, O’Connor T. Development of a prognosis-based clinical staging system for infants infected with human immunodeficiency virus. J Pediatr. 1996;129:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 121. | Spivak JL, Bender BS, Quinn TC. Hematologic abnormalities in the acquired immune deficiency syndrome. Am J Med. 1984;77:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 122. | Levine AM, Berhane K, Masri-Lavine L, Sanchez M, Young M, Augenbraun M, Cohen M, Anastos K, Newman M, Gange SJ. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26:28-35. [PubMed] |
| 123. | Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis. 2002;34:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 124. | Castella A, Croxson TS, Mildvan D, Witt DH, Zalusky R. The bone marrow in AIDS. A histologic, hematologic, and microbiologic study. Am J Clin Pathol. 1985;84:425-432. [PubMed] |
| 125. | Cleveland RP, Liu YC. CD4 Expression by erythroid precursor cells in human bone marrow. Blood. 1996;87:2275-2282. [PubMed] |
| 126. | Gibellini D, Re MC, Vitone F, Rizzo N, Maldini C, La Placa M, Zauli G. Selective up-regulation of functional CXCR4 expression in erythroid cells by HIV-1 Tat protein. Clin Exp Immunol. 2003;131:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 127. | Watanabe D, Uehira T, Yonemoto H, Bando H, Ogawa Y, Yajima K, Taniguchi T, Kasai D, Nishida Y, Shirasaka T. Sustained high levels of serum interferon-γ during HIV-1 infection: a specific trend different from other cytokines. Viral Immunol. 2010;23:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | Camacho J, Poveda F, Zamorano AF, Valencia ME, Vázquez JJ, Arnalich F. Serum erythropoietin levels in anaemic patients with advanced human immunodeficiency virus infection. Br J Haematol. 1992;82:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Kreuzer KA, Rockstroh JK, Jelkmann W, Theisen A, Spengler U, Sauerbruch T. Inadequate erythropoietin response to anaemia in HIV patients: relationship to serum levels of tumour necrosis factor-alpha, interleukin-6 and their soluble receptors. Br J Haematol. 1997;96:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Wang Z, Goldberg MA, Scadden DT. HIV-1 suppresses erythropoietin production in vitro. Exp Hematol. 1993;21:683-688. [PubMed] |
| 131. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2165] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 132. | Sipsas NV, Kokori SI, Ioannidis JP, Kyriaki D, Tzioufas AG, Kordossis T. Circulating autoantibodies to erythropoietin are associated with human immunodeficiency virus type 1-related anemia. J Infect Dis. 1999;180:2044-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 133. | Tsiakalos A, Kordossis T, Ziakas PD, Kontos AN, Kyriaki D, Sipsas NV. Circulating antibodies to endogenous erythropoietin and risk for HIV-1-related anemia. J Infect. 2010;60:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Tsiakalos A, Routsias JG, Kordossis T, Moutsopoulos HM, Tzioufas AG, Sipsas NV. Fine epitope specificity of anti-erythropoietin antibodies reveals molecular mimicry with HIV-1 p17 protein: a pathogenetic mechanism for HIV-1-related anemia. J Infect Dis. 2011;204:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 135. | McGinniss MH, Macher AM, Rook AH, Alter HJ. Red cell autoantibodies in patients with acquired immune deficiency syndrome. Transfusion. 1986;26:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Rarick MU, Espina B, Mocharnuk R, Trilling Y, Levine AM. Thrombotic thrombocytopenic purpura in patients with human immunodeficiency virus infection: a report of three cases and review of the literature. Am J Hematol. 1992;40:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Sasadeusz J, Buchanan M, Speed B. Reactive haemophagocytic syndrome in human immunodeficiency virus infection. J Infect. 1990;20:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |