Published online Jun 28, 2019. doi: 10.5500/wjt.v9.i2.35
Peer-review started: February 12, 2019
First decision: March 15, 2019
Revised: March 26, 2019
Accepted: May 14, 2019
Article in press: May 14, 2019
Published online: June 28, 2019
Processing time: 142 Days and 11.9 Hours
The adverse renal effects of proton pump inhibitors (PPIs) are increasingly recognized in both the general population and patients with chronic kidney disease. Several pharmacokinetic studies have also raised concerns regarding the interaction between PPIs and immunosuppressive drugs in transplant patients. Whether the adverse effects of PPIs have a clinical significance in kidney transplant recipients remains unclear. We performed this meta-analysis to assess the risk of adverse effects in kidney transplant recipients on PPI compared with those without PPI exposure.
To investigate the risk of acute rejection, graft loss, hypomagnesemia, renal dysfunction, and overall mortality in kidney transplant recipients on PPI compared with those without PPI exposure.
A systematic review was conducted in MEDLINE, EMBASE, and Cochrane databases from inception through October 2018 to identify studies that evaluated the adverse effects of PPIs in kidney transplant recipients, including biopsy-proven acute rejection, graft loss, hypomagnesemia, renal function, and overall mortality. Effect estimates from the individual studies were extracted and combined using random-effect, generic inverse variance method of DerSimonian and Laird. The protocol for this meta-analysis is registered with PROSPERO, No. CRD42018115676.
Fourteen observational studies with 6786 kidney transplant recipients were enrolled. No significant association was found between PPI exposure and the risk of biopsy-proven acute rejection at ≥ 1 year [pooled odds ratio (OR), 1.25; 95% confidence interval (CI), 0.82-1.91, I2 = 55%], graft loss at 1 year (pooled OR = 1.30, 95%CI: 0.75-2.24, I2 = 0%) or 1-year mortality (pooled OR = 1.53, 95%CI: 0.90-2.58, I2 = 34%). However, PPI exposure was significantly associated with hypomagnesemia (pooled OR = 1.56, 95%CI: 1.19-2.05, I2 = 27%). Funnel plots and Egger regression asymmetry test were performed and showed no publication bias.
PPI use was not associated with significant risks of higher acute rejection, graft loss, or 1-year mortality. However, the risk of hypomagnesemia was significantly increased with PPI use. Thus, future studies are needed to assess the impact of PPIs on long-term outcomes.
Core tip: Several pharmacokinetic studies have raised concerns regarding the interaction between proton pump inhibitors (PPIs) and immunosuppressive drugs in transplant patients. Whether the adverse effects of PPIs have a clinical significance in kidney transplant recipients remains unclear. We performed this meta-analysis to assess the risk of adverse effects in kidney transplant recipients on PPI compared with those without PPI exposure. We demonstrate that PPI use is not associated with significant risks of higher acute rejection, graft loss, or 1-year mortality. However, PPI use is associated with 1.56-fold increased risk of hypomagnesemia. Thus, future studies are needed to assess the impact of PPIs on long-term outcomes.
- Citation: Boonpheng B, Thongprayoon C, Bathini T, Sharma K, Mao MA, Cheungpasitporn W. Proton pump inhibitors and adverse effects in kidney transplant recipients: A meta-analysis. World J Transplant 2019; 9(2): 35-47
- URL: https://www.wjgnet.com/2220-3230/full/v9/i2/35.htm
- DOI: https://dx.doi.org/10.5500/wjt.v9.i2.35
Proton pump inhibitors (PPIs) are commonly prescribed after transplantation for prophylaxis against peptic ulcer disease and for treatment of gastro-esophageal reflux disease or dyspepsia. Prolonged exposure to this class of medication has been shown to be associated with kidney dysfunction[1,2], as well as other non-renal adverse outcomes, including hypomagnesemia[3], fracture[4], or dementia[5] in the general population. The risk of kidney dysfunction associated with PPIs is particularly concerning to kidney transplant recipients who are already at risk for acute kidney injury.
Mycophenolate mofetil (MMF) is an antimetabolite that is commonly used as part of the maintenance immunosuppression in kidney transplant recipients[6]. MMF is a prodrug that is hepatically metabolized to the active compound mycophenolic acid (MPA) after oral administration. MPA exerts its immunosuppressive effects by reversibly inhibiting the de novo synthesis of purine nucleotides, leading to reduced proliferation of B- and T-cell lymphocytes, induction of activated T lymphocyte apoptosis, and downregulation of adhesion molecule expression, resulting in lower leukocyte trafficking and recruitment[7]. Because gastrointestinal discomfort is a common side effect of MMF, PPIs are commonly prescribed to alleviate the sym-ptoms. However, pharmacokinetic studies[8-12] have shown that PPIs reduce the absorption of MMF and lower the exposure to MPA presumably by its potent inhibition of gastric acidification compared with another class of acid suppressant, the H2-receptor antagonists[13,14]. Randomized controlled trials[15,16] and observational studies[17-19] have also shown that reduced exposure to MPA is associated with higher risk of acute rejection and overall worse allograft outcome in kidney transplant recipients. However, the clinical significance of this drug interaction in kidney transplant recipients is unknown. Several studies[20,21] have shown a possible increased risk of acute rejection with PPI exposure whereas others have not[22-24].
Some studies[25,26] have shown that concurrent PPI can increase tacrolimus drug concentration, leading to higher risk of toxicity through cytochrome or p-glycoprotein inhibition in patients with certain Cytochrome P450 2C19 (CYP2C19) and/or CYP3A5 genotypes. However, this is not expected to increase the risk of rejection, but calcineurin inhibitor toxicity may lead to renal dysfunction. Other commonly used immunosuppressive drugs are not known to have significant interaction with PPIs.
PPI may also interfere with magnesium absorption in the gastrointestinal tract, causing hypomagnesemia[3]. The mechanism of renal dysfunction related to PPIs is not clear although acute interstitial nephritis (AIN) associated with PPIs has been purposed[1,2].
Therefore, we conducted this systematic review and meta-analysis to investigate the adverse outcomes in kidney transplant recipients on PPI compared with those without PPI exposure. The outcomes of interest include biopsy-proven acute rejection, graft loss, kidney dysfunction, hypomagnesemia, and overall mortality.
The protocol for this meta-analysis is registered with PROSPERO, No. CRD420-18115676. PRISMA statement guidelines were followed for conducting and reporting meta-analysis data[27]. A systematic review was conducted in MEDLINE, EMBASE, and Cochrane databases from inception to October 2018 to identify studies that evaluated adverse effects of PPIs in kidney transplant recipients by using the search terms “kidney transplant” and “proton pump inhibitor,” as described in the online supplementary data without any language restriction. References of selected articles were also manually searched for additional studies.
Studies were eligible for this meta-analysis if the following inclusion criteria were met: (1) Randomized controlled trial, cohort (either prospective or retrospective), case–control study or cross-sectional study published as an original study to evaluate the outcomes of kidney transplantation in patients on PPIs; (2) Odds ratios (ORs), relative risk (RR), hazard ratio (HR), and standardized incidence ratio (SIR) with 95% confidence intervals (CIs) or sufficient raw data to calculate these ratios were provided; and (3) Subjects not on PPIs were used as comparators in cohort and cross-sectional studies.
Study eligibility was independently evaluated by the investigators (BB and CT). Any disagreement was resolved by mutual consensus. The quality of each study was appraised using the Newcastle–Ottawa quality scale[28]. This scale assesses each study in three domains, including the: (1) Representativeness of the subjects; (2) Com-parability between the study groups; and (3) Ascertainment of the exposure of interest for the case–control study and the outcome of interest for the cohort study. The modified version of the Newcastle–Ottawa scale as described by Herzog et al[29] was used for cross-sectional studies.
The two study investigators independently reviewed the titles and abstracts of all retrieved articles. Articles that apparently did not fulfill the inclusion criteria were excluded. Only potentially relevant articles underwent full-text review to determine eligibility. A standardized data collection form was used to extract the following information from the included studies: First author’s name, year of publication, year of study, country where the study was conducted, study design, source of population, number of subjects, baseline characteristics of the subjects, and effect estimates. This data extraction process was performed by both investigators to ensure accuracy.
All statistical analyses were performed using Comprehensive Meta-analysis version 3 software (Eaglewood, NJ, United States). The pooled RRs of acute rejection, graft loss, hypomagnesemia, and overall mortality in kidney transplant recipients on PPIs compared with subjects not on PPIs were calculated using the generic inverse method of DerSimonian and Laird[30]. The random-effects model was used, given the high likelihood of between-study variance due to the difference in underlying population and methodology. Cochran's Q-test, which was supplemented by I2 statistics, was used to evaluate statistical heterogeneity. I2 statistics quantify the proportion of the total variation across studies, that is, due to true heterogeneity rather than chance. An I2 value of 0% to 25% represents insignificant heterogeneity, > 25% to ≤ 50% represents low heterogeneity, > 50% to ≤ 75% represents moderate heterogeneity, and > 75% represents high heterogeneity[31].
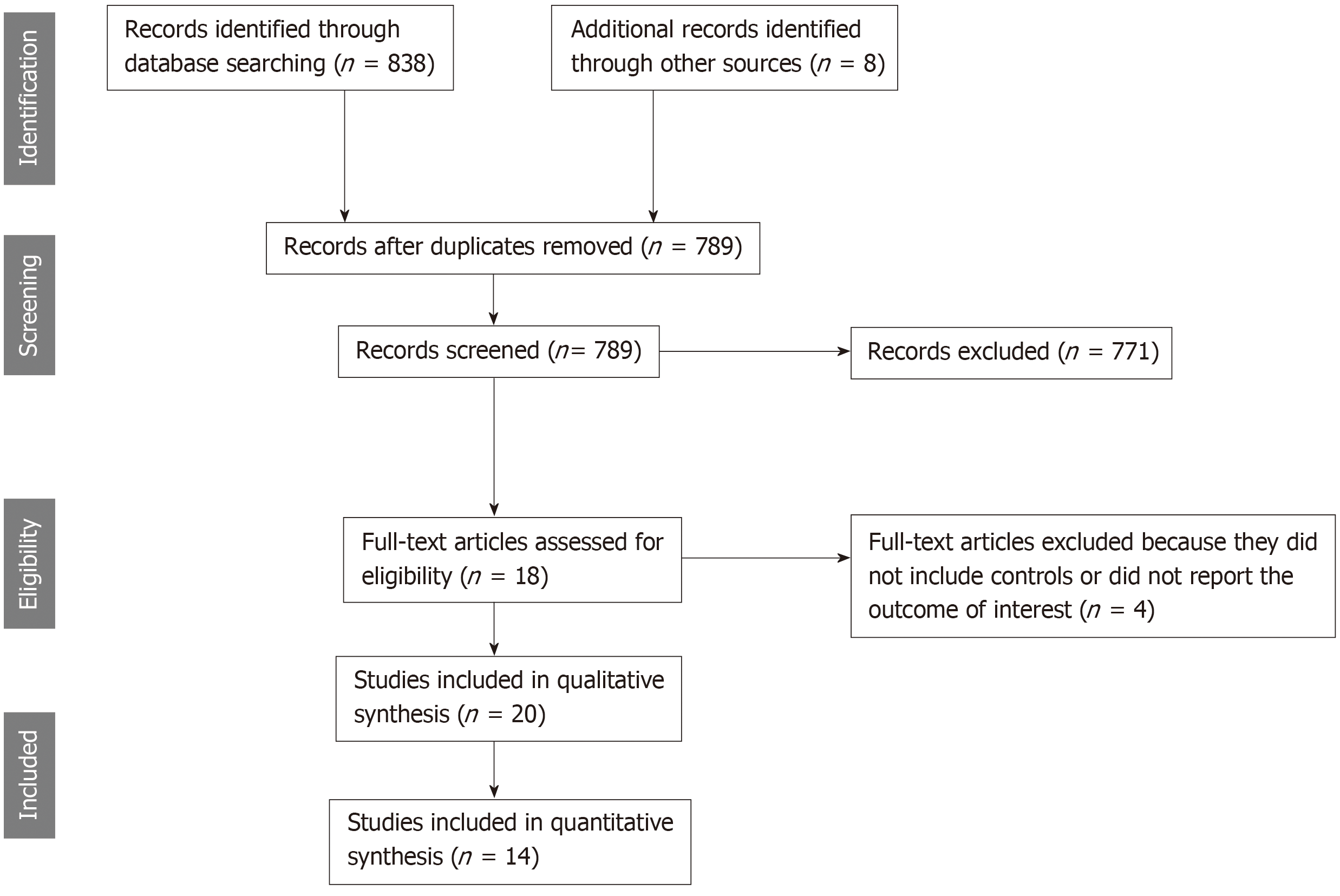
The initial search yielded 838 articles, all of which underwent title and abstract review (Figure 1). Most of the articles were excluded at this step because they were case reports, letters to the editor, review articles, or interventional studies, which clearly did not fulfill our inclusion criteria. Eighteen studies underwent full-length article review, and four were excluded because they did not include controls or did not report the outcome of interest. Therefore, 14 studies met our inclusion criteria[20-24,32-40] and were included in the meta-analysis. The baseline characteristics of the included studies are summarized in Table 1. These 14 observational studies consisted of 6786 kidney transplant recipients (> 1907 with PPI exposure and 2528 without PPI exposure).
| Ref. | Country | Type | Total N | Race | Immuno-suppre-ssive regimen | CNI use (% Cyclos-porine) | PPI | No PPI | Quality Scale3 | ||||
| N | Age | M/F | N | Age | M/F | ||||||||
| Patel et al[32] 2012 | United States | Retrospec-tive | 561 | NR | Tacroli-mus, MMF, Predni-sone | 0% | 155 | 52±131 | NR | 406 | 48±14 | NR | 3-2-2 |
| Knorr et al[20] 2014 | United States | Retrospec-tive | 597 | 52% Black | rATG, MMF, Tacroli-mus, Predni-sone | <3% | 213 | 55±12 | 122/91 | 384 | 55±13 | 210/174 | 4-2-3 |
| van Boekel et al[22] 2014 | The Netherlands | Retrospec-tive | 202 | 98.5% Caucasi-an | Tacroli-mus, MMF. Predni-sone | 0% | 125 | 47.7±12.8 | 61.6%/38.4% | 77 | 46.7±13.3 | 66.2%/43.8% | 4-2-3 |
| Van Ende et al[33] 2014 | Belgium | Cross-sectional | 512 | 98% Caucasi-an | Varies | 47% (tacroli-mus 35 %) | 101 | 53 ± 13 | 59%/41% | 411 | 53 ± 13 | 59%/41% | 4-2-3 |
| Alhosaini et al[34] 2015 | United States | Retrospec-tive | 83 | 59% Caucasi-an, 19% Black | CNI (Tacroli-mus, Cyclospo-rine), MPA, Predni-sone | 5/83 (6%) | 43 | 54 ± 15.1 | 25/18 | 40 | 49.7 ± 16.4 | 24/16 | 4-2-3 |
| Sezer et al[35] 2015 | Turkey | Retrospec-tive | 354 | NR | NR | NR | 164 | 38.6 ±1 0.7 | NR | 96 | NR | 38.6 ±1 0.7 | 3-2-2 |
| Courson et al[21] 2016 | United States | Retrospec-tive | 286 | 51% Caucasi-an, 17% Black, 10% Asian | Tacroli-mus, MMF or MPS, early steroid withdra-wal | 0% | 171 | 56±13 | 118/53 | 115 | 54±13 | 88/27 | 4-2-3 |
| Patel et al[23] 2017 | United States | Retrospec-tive | 522 | 24% Black | Tacroli-mus, reduced-dose MMF, predni-sone | 11/522 (2%) convert-ed to cyclospo-rine | 183 | 54 (44-63)2 | 102/81 | 339 | 53 (43-60) | 219/120 | 4-2-3 |
| Shabaka et al[36] 2017 | Spain | Cross-sectional | 938 | NR | CNI-based regimen | NR | NR | NR | NR | NR | NR | NR | 3-2-2 |
| Rouse et al[24] 2017 | United States | Retrospec-tive | 211 | 55% Caucasi-an, 30% Black | Tacroli-mus, MMF or MPS, Predni-sone | 0% | 35 | 55±10.7 | 25/10 | 176 | 63±14 | 124/52 | 4-2-3 |
| Uludag et al[37] 2017 | Turkey | Retrospec-tive | 292 | NR | NR | NR | 223 | 36±10 | 129/104 | 69 | 33±11 | 42/27 | 3-2-2 |
| Kipp et al[39] 2018 | United States | Retrospec-tive | 819 | NR | NR | NR | 404 | NR | NR | 415 | NR | NR | 3-1-2 |
| Douwes et al[40] 2018 | The Nether-lands | Cross-sectional | 706 | NR | NR | NR | NR | 53 ± 13 | 57%/43% | NR | 53 ± 13 | 57%/43% | 3-1-2 |
| Gomes-Neto et al[38] 2018 | The Nether-lands | Cross-sectional | 703 | NR | NR | NR | NR | 53 ± 13 | 57%/43% | NR | 53 ± 13 | 57%/43% | 3-1-2 |
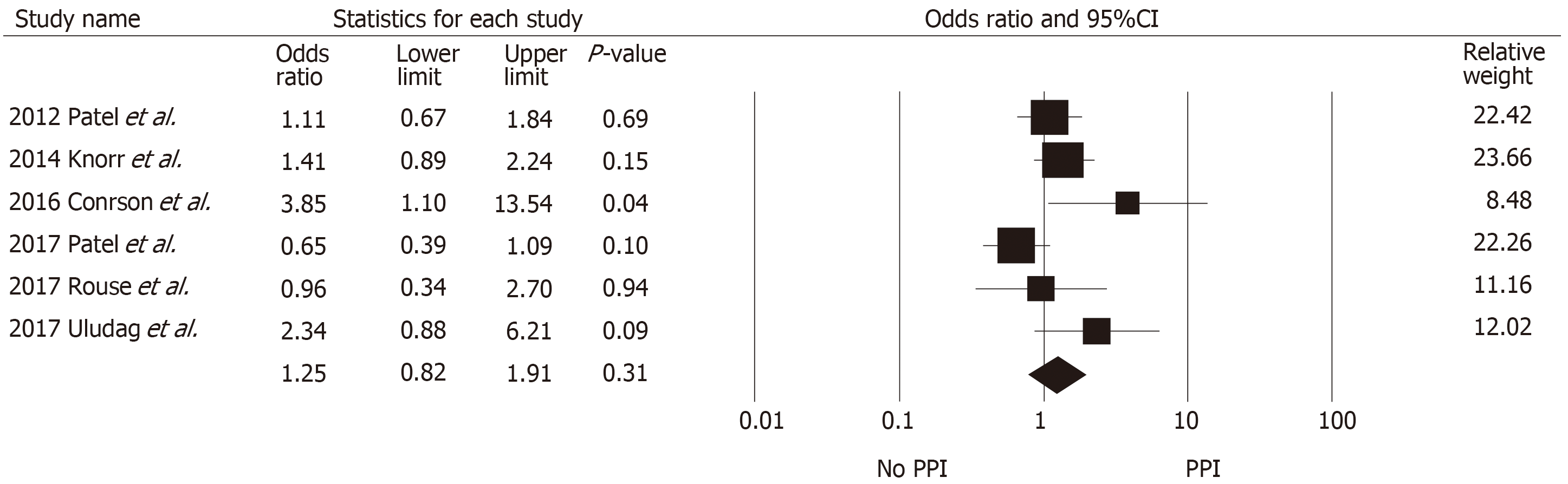
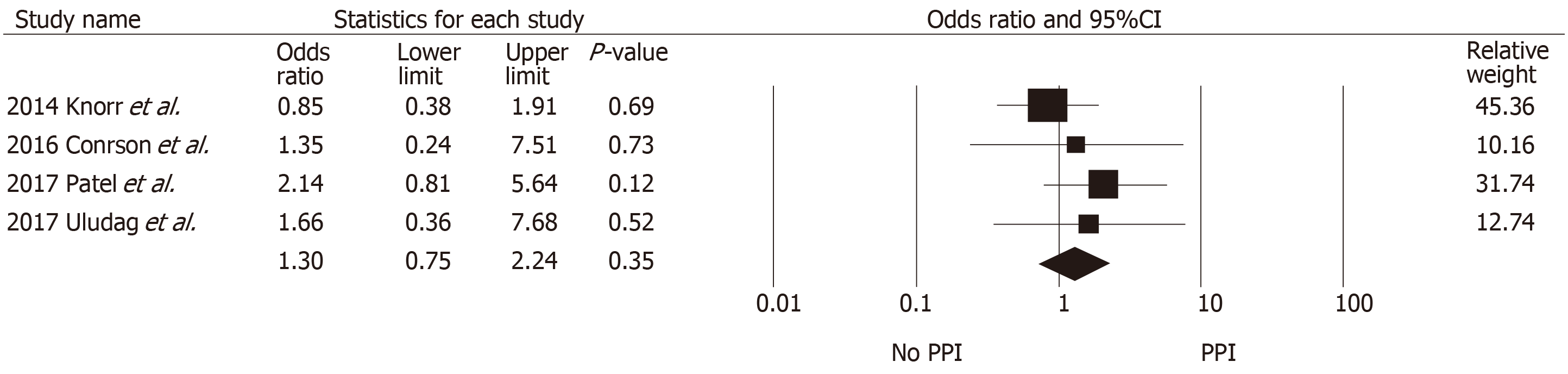
Table 2 summarizes the findings across the studies that reported allograft outcomes. Definitions of biopsy-proven acute rejection and presumed rejection across included studies are also shown in Supplementary Table S1. Pooled data for acute rejection at ≥ 1 year were available from six studies with 2427 kidney transplant recipients (980 with PPI exposure and 1447 without PPI exposure). No significant association was found between PPI exposure and the risk of biopsy-proven acute rejection at ≥ 1 year (pooled OR = 1.25, 95%CI: 0.82-1.91, I2 = 55%, Figure 2). At 3 mo, acute rejection risk was also not significantly different between the two groups (pooled OR = 1.54, 95%CI: 0.64-3.82). Acute cellular rejection was more common than antibody-mediated rejection (AMR) and the rejection rates were similar between the two groups, except in studies by Courson et al[21] and Rouse et al[24] which demonstrated higher rates of AMR among the PPI group. The median time to rejection was reported to be similar between the two groups across four studies (approximately 3-4 mo post-transplant). Graft loss at 1 year was also not different between those with and without PPI ex-posure (pooled OR = 1.30, 95%CI: 0.75-2.24, I2 = 0%, Figure 3).
| Ref. | Biopsy-proven acute rejection at ⩾ 1 yr (%) | Biopsy-proven or presumed rejection at 3 mo (%) | Median time to rejection | Antibody mediated rejection (%) | Graft loss (%) |
| Patel et al[32] 2012 | |||||
| PPI | 25 (16%) | NR | 4.1 mo | 3.3% | NR |
| No PPI | 60 (15%) | NR | 3.3 mo | 3.1% | NR |
| P | 0.69 | - | NS | NS | - |
| Knorr et al[20] 2014 | |||||
| PPI | 32/213 (15%) | NR | 110 ± 91 d | 1/32 (3.1%) | 9/213 (4.2%) |
| H2A | 46/384 (12%) | NR | 110 ± 112 d | 2/46 (4.3%) | 19/384 (4.9%) |
| P | 0.15 | - | 1.0 | NR | 0.84 |
| van Boekel et al[22] 2014 | |||||
| PPI | NR | 25/125 (20%) BPAR: 13/125 (10.4%) | NR | NR | NR |
| H2RA | NR | 15/77 (19.5%) BPAR: 7/77 (9.1%) | NR | NR | NR |
| P | - | NS | - | - | - |
| Courson et al[21] 2014 | |||||
| PPI | 16/171 (9.4%) | NR | 116±92 d1 | 5/16 (31%) | 4/171 (2.3%) |
| H2RA | 3/115 (2.6%) | NR | both | 0 | 2/115 (1.7%) |
| P | 0.029 | - | NS | 0.53 | 1 |
| Patel et al[23] 2017 | |||||
| PPI | 11/183 (19%) | 12/183 (4.9%) | 106 (57-286) days2 | 1/11 (9.1%) | 9/183 (4.9%) |
| H2RA | 28/339 (14%) | 9/339 (3.5%) | 139 (96-339) days | 2/28 (7.1%) | 8/339 (2.4%) |
| P | 0.35 | 0.44 | 0.28 | NR | 0.12 |
| Rouse et al[24] 2017 | |||||
| PPI | 5/35 NR | NR | 2/5 (40%) | NR | |
| H2RA | 26/176 | NR | NR | 3/26 (12%) | NR |
| P | 1.0 | - | - | 0.03 | - |
| Uludag et al[37] 2017 | |||||
| PPI | 36/233 (15.5%) | NR | NR | NR | 11/233 (4.7%) |
| No PPI | 5/69 (7.2%) | NR | NR | NR | 2/69 (2.9%) |
| P | 0.08 | - | - | - | 0.51 |
All but one study reported no significant short term (3 mo to 1 year) difference in renal function, as summarized in Table 3. Uludag et al[37], which had the most extended follow-up period of all included studies (median, 109 mo; interquartile range, 82-156 mo), however demonstrated that the serum creatinine level in the PPI group was higher than that in the non-PPI group (1.44 ± 0.99 vs 1.24 ± 0.46 mg/dL).
| Ref. | eGFR | Cr | ||||
| PPI | No PPI | P | PPI | No PPI | P | |
| Knorr et al[20] | 53.1 ± 20.21 | 55.1 ± 20.6 | 0.29 | NR | NR | - |
| van Boekel et al[22] | 49.5 ± 12.3 | 50.7 ± 12.5 | NS | 1.5 ± 0.4 at 3 mo | 1.5 ± 0.4 | NS |
| Patel et al[23] | 49.0 (39.4–63.2)2 | 49.9 (39.3–60.8) | 0.78 | NR | NR | - |
| Uludag et al[37] | - | - | - | 1.49 ± 0.99 mg/dL | 1.24 ± 0.46 mg/dL | 0.017 |
| Alhosaini et al[34] | 49.4 ± 14.9 | 52.8 ± 14.3 | 0.29 | - | - | - |
| Kipp et al[39] | NR | NR | - | 1.896 ± 1.53 | 1.812 ± 1.25 | P = 0.4098 |
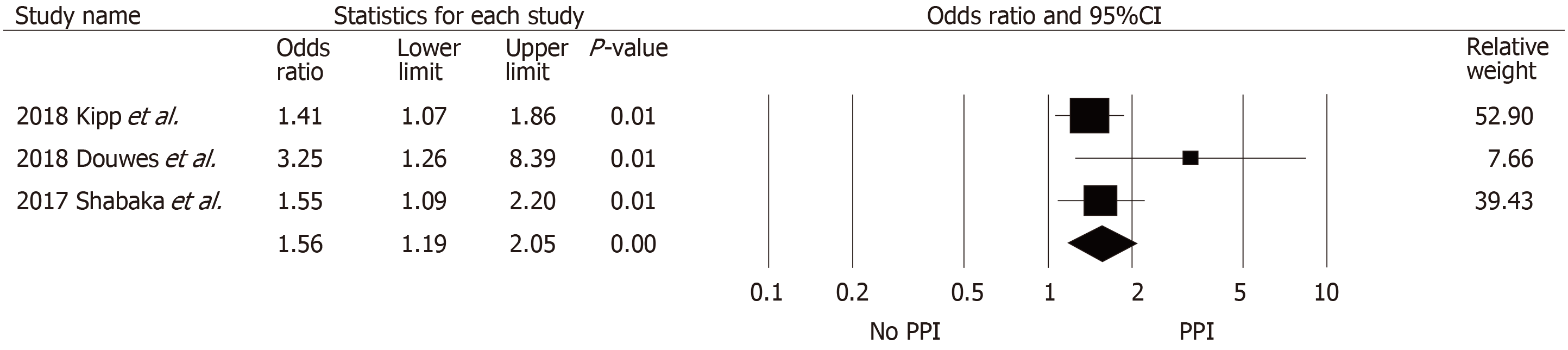
Table 4 summarizes data across eight studies. The risk of hypomagnesemia in the PPI group was significantly higher than in the non-PPI group (pooled OR = 1.56, 95%CI: 1.19-2.05, I2 = 27%, Figure 4) based on three studies. Sezer et al[35], Van Ende et al[33], and Uludag et al[37] did not report a significant difference in the magnesium level between those with and without PPI exposure, whereas Alhosaini et al[34] reported a significant difference between the two groups (magnesium: 1.70 ± 0.12 vs 1.79 ± 0.17 for those with PPI and without PPI exposure; P = 0.006). Gomes-Neto et al[38] and Douwes et al[40] (who analyzed data from an overlapping set of patients) reported a significant inverse correlation between PPI use and plasma magnesium level. The proportion of hy-pomagnesemia also did not differ between the two groups, but a study by Shabaka et al[36] noted that those with PPI exposure seemed to develop significantly more severe hypomagnesemia (defined as magnesium level < 1.3 mg/dL) compared with those without PPI exposure (21% vs 5%).
| Ref. | Serum / Plasma magnesium level | Hypomagnesemia | Magnesium supplemen-tation | ||||||
| PPI | No PPI | P | Definition of hypomagne-semia | PPI | No PPI | P | Correlation between PPI and hypomagne-semia | ||
| Sezer et al[35] | 1.5 ± 0.04 mg/dl | 1.7 ± 0.02 mg/dl | P < 0.05 | NR | NR | NR | NR | NR | |
| Shabaka et al[36] | NR | NR | NR | OR 1.55, (95%CI 1.09-2.20) | 1 | NR | NR | ||
| Kipp et al[39] | NR | NR | NR | 215 (53.1%) | 185 (44.6%) | P < 0.013 | NR | NR | |
| Alhosaini et al[34] | 1.70 ± 0.12 | 1.79 ± 0.17 | 0.006 | Serum Mg < 1.8 mg/dL | 33/43 | 24/40 | P > 0.05 | NR | Use of Mg supplement: PPI 47% vs Non-PPI 21% (P = 0.02) |
| Serum Mg < 1.3 mg/dL | 9/43 (21%) | 2/40 (5%) | P = 0.03 | ||||||
| Uludag et al[37] | 0.728 mmol/L | vs 0.755 mmol/L, | P = 0.061 | NR | NR | NR | NR | NR | |
| Van Ende et al[33] | NR | NR | Serum Mg < 1.7 mg/dL | β: −0.84 (0.26; 2.71), P = 0.78 | β: −0.84 (0.26; 2.71), P = 0.78 | NR | |||
| Douwes et al[40] | NR | NR | Serum Mg < 1.8 mg/dL (0.75 mmol/L) | HR 3.25 (1.26-8.39) | 1 | β: -0.08, P = 0.046 | Mean Mg intake: 330 ± 85 mg/d, (P = 0.204) | ||
| Gomes-Neto et al[38] | NR | NR | NR | β: -0.05, P = 0.04 | NR | β: -0.05, P = 0.04 | NR | ||
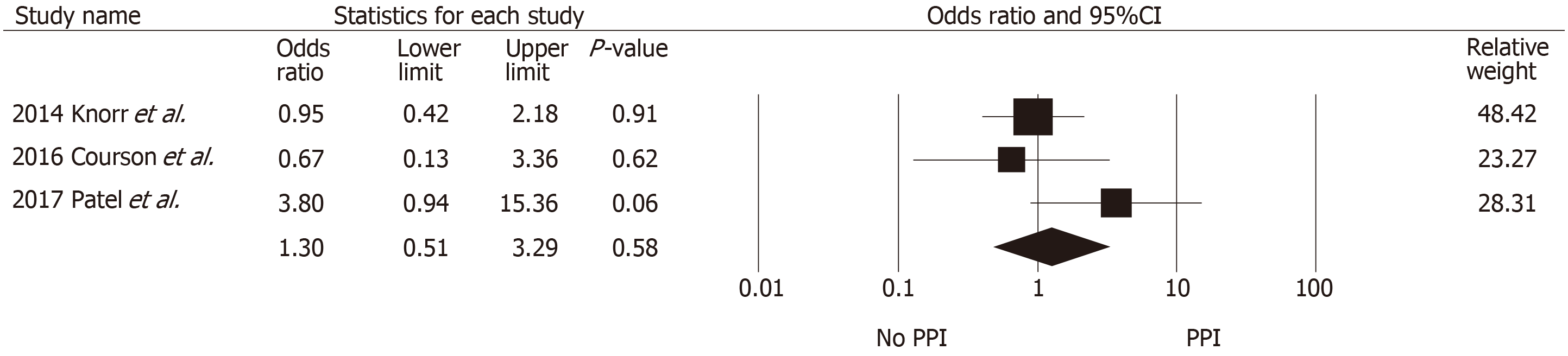
All-cause mortality data were available from five studies (Table 5), with three studies reporting 1-year survival and two reporting longer-term all-cause mortality. One-year mortality did not significantly differ between PPI and non-PPI use (pooled OR = 1.30, 95%CI: 0.51-3.29, I2 = 41.4%; Figure 5). The two studies that reported long-term mortality outcomes (Douwes et al[40] and Gomes-Neto et al[38]) seemed to analyze data from a highly overlapping set of patients (n = 706 vs 703); hence, pooled HR was not calculated. With a median follow-up duration of 5.4 years (range, 4.8-6.1 years) in both studies, the adjusted HRs for all-cause mortality was significantly associated with PPI use (HR = 1.94, 95%CI: 1.32-2.88, and HR = 2.01, 95%CI: 1.43-2.83, re-spectively).
The funnel plots (Supplementary Figure S1 to Figure S4) and Egger’s regression asymmetry test were performed and showed no significant publication bias (P > 0.05 for all outcomes).
Sensitivity analysis was performed by excluding one study at a time to investigate the effect of each study on the pooled OR for each outcome assessed. The pooled effect estimate from this sensitivity analysis remained essentially unchanged.
This meta-analysis showed no significant association between exposure to PPIs and higher risk of acute biopsy-proven rejection, graft loss, or overall mortality, but a significantly higher risk of hypomagnesemia among those with PPI exposure was noted. No short-term difference in renal function was found between the two groups.
Despite several pharmacokinetic studies that have clearly showed significantly reduced MPA exposure following concomitant administration of PPIs and MMF in both healthy volunteers[12,41] and in immediate post-transplant kidney transplant recipients[10,11], there was no significant association between PPI use and increased risk of acute rejection in our study, suggesting that the effect may not be large enough to be clinically significant. Because none of the included studies reported MPA drug level or direct gastric pH measurement, it is difficult to ascertain whether a significant interaction between PPIs and MMF exists in the real-world setting. Three studies (van Boekel et al[22], Courson et al[21], and Patel et al[23]) reported the total cumulative MMF exposure or mean daily dose between the two groups. In all three studies, despite the PPI group receiving a slightly lower cumulative MMF dose compared to the non-PPI group (non-significant in the study by van Boekel et al[22] and Patel et al[23]; significant in the study by Courson et al[21]), no significant difference in acute rejection was found. Interestingly, in black patients, PPI was found to be significantly associated with a higher risk of acute rejection in one study[20].
Another potential reason for the lack of a significant association between PPI use and acute biopsy-proven rejection is that the majority of the kidney transplant recipients enrolled in the included studies were on tacrolimus, with none or only a small percentage of recipients on cyclosporine. The use of tacrolimus as the cal-cineurin inhibitor instead of cyclosporine may help lower the risk of reduced MPA exposure with PPI use. Cyclosporine, unlike tacrolimus, can reduce the enterohepatic recirculation of MPA in the gastrointestinal tract[42,43], thus further lowering total MPA exposure. The enteric-coated mycophenolate sodium does not appear to have a significant interaction with PPI[8,41,44], unlike MMF.
We did not demonstrate a significant difference in renal function as measured by estimated glomerular filtration rate or serum creatinine between the PPI and the non-PPI group in the short term (3 mo to 1 year). Extrapolating from observational studies in the general population, this is not unexpected as the risk of kidney dysfunction seems to be associated with more prolonged PPI use and may have a long latent period[1,45]. Uludag et al[37] also confirmed this observation by noting a significantly higher serum creatinine level in PPI users compared with non-users at a longer median follow-up of 109 mo.
The risk of hypomagnesemia in the PPI group was significantly higher than that in the non-PPI group in our study. This is consistent with studies in the general population that report hypomagnesemia with prolonged PPI use[3]. The exact mechanism of PPI-induced hypomagnesemia is unknown. Urinary magnesium excretion has been shown to be low in patients with hypomagnesemia related to PPI use[46], suggesting that reduced absorption from the gastrointestinal tract is the main cause. It is hypothesized that the TRMP6 (transient receptor potential melastatin) pathway in gut epithelial cells, which mediates magnesium absorption, is inhibited by the high pH milieu caused by PPI use[47]. This inhibition is more pronounced in certain individuals with additional polymorphisms of the related cellular pathway proteins or other risk factors, which explains why the incidence and degree of hypo-magnesemia vary among PPI users[47]. Some studies have also reported that high-dose oral magnesium supplementation can correct hypomagnesemia associated with PPI[48], suggesting that the paracellular passive absorption in the bowel remains intact.
In kidney transplant recipients, hypomagnesemia has been shown to be associated with various adverse consequences[49]. Low magnesium level has been associated with accelerated decline of allograft function and a higher rate of graft loss in patients with cyclosporine-induced nephropathy[50], consistent with animal studies showing a higher degree of renal tissue fibrosis associated with low magnesium[51] that appears to be partially correctable with magnesium supplementation[51,52]. Hypomagnesemia may also lead to a higher incidence of new-onset diabetes after transplant[53], which is a separate risk factor for allograft loss and overall mortality.
Our study did not show a significant difference in the 1-year overall mortality, as expected, because the risks of acute rejection, graft loss, and kidney dysfunction did not significantly differ between the PPI and non-PPI groups. Only hypomagnesemia was found to be significantly associated with PPI use; hence, this may not be clinically significant to drive a mortality difference at least in the short term. However, Douwes et al[40] and Gomes-Neto et al[38] reported a significant association between PPI use and long-term all-cause mortality despite adjustment for confounders. Furthermore, both studies also showed a significant interaction between PPI use and hypomagnesemia. As noted previously, Uludag et al[37] has also reported significantly worse kidney function in the PPI group with longer follow-up (median, 109 mo). Hypomagnesemia or renal dysfunction may be a late manifestation associated with prolonged exposure to PPIs, which may eventually be clinically significant enough to cause higher mortality. Further studies are needed to clarify this question.
Although we believe the literature review process was rigorous and the included studies were of high quality, this meta-analysis has some limitations. Therefore, the interpretation of the results needs to be performed with caution. First, this meta-analysis is based solely on observational studies. Although this is appropriate for our clinical question, it may be inherently subject to selection bias and unadjusted confounders. Second, certain important baseline characteristics could not be obtained or compared across all studies. Of interest to transplant recipients, comparison of different immunosuppressive regimens, drug level, dosage, and adherence to both immunosuppressive drugs or acid suppressive therapy between the two groups was not possible in most included studies due to either their observational or retrospective design. Third, the definitions of various outcomes of interest varied across studies, such as the cut-off value for hypomagnesemia, definition of severe rejection, or the use of different criteria for the classification of AMR and cell-mediated rejection. Finally, most of the included studies only reported follow-up data for a relatively short-term period (approximately 1 year). Therefore, we cannot rule out the possibility that prolonged exposure of PPIs (longer than a year) may lead to adverse outcomes. Further study is needed to address whether long-term PPI exposure in kidney transplant recipients is associated with worse outcomes.
In conclusion, PPI use was not associated with significant risks of higher acute rejection, graft loss, or 1-year mortality. However, the risk of hypomagnesemia was significantly increased with PPI use.
Adverse renal effects of PPIs are increasingly recognized in clinical practice. Pharmacokinetic studies have also raised concerns regarding the interaction between PPIs and immuno-suppressive drugs in transplant patients. Whether the adverse effects of PPIs have a clinical significance in kidney transplant recipients remains unclear.
Proton pump inhibitors are commonly used after transplantation for prophylaxis against peptic ulcer disease and for treatment of gastro-esophageal reflux disease or dyspepsia. Prolonged exposure to this class of medication has been shown to be associated with kidney dysfunction, as well as other non-renal adverse outcomes, including hypomagnesemia, fracture, or dementia in the general population. The clinical significance of this drug interaction in kidney transplant recipients is unknown. Several studies have shown a possible increased risk of acute rejection with PPI exposure whereas others have not.
We performed this systematic review and meta-analysis to investigate the adverse outcomes in kidney transplant recipients on PPI compared with those without PPI exposure.
A systematic review was conducted in MEDLINE, EMBASE, and Cochrane databases from inception to October 2018 to identify studies that evaluated adverse effects of PPIs in kidney transplant recipients. The outcomes of interest include biopsy-proven acute rejection, graft loss, kidney dysfunction, hypomagnesemia, and overall mortality. The protocol for this meta-analysis is registered with PROSPERO, No. CRD42018115676.
The authors found no significant association between exposure to PPIs and higher risk of acute biopsy-proven rejection, graft loss, or overall mortality, but a significantly 1.56-fold higher risk of hypomagnesemia among those with PPI exposure was noted. No short-term difference in renal function was found between the two groups.
PPI use was not associated with significant risks of higher acute rejection, graft loss, or 1-year mortality. However, the risk of hypomagnesemia was significantly increased with PPI use. In the long-term, PPI use may also be associated with kidney dysfunction and increased overall mortality.
This study demonstrated significant hypomagnesemia in kidney transplant recipients who received PPIs. Since hypomagnesemia is associated with new onset diabetes new-onset diabetes after transplantation, future large-scale clinical studies are needed to assess the impact of PPIs on long-term outcomes.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hibberd AD, Boteon YL S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 499] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 2. | Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, Chongruksut W, Thavorn K. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 3. | Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, Edmonds PJ, Ungprasert P, O'Corragain OA, Korpaisarn S, Erickson SB. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Hussain S, Siddiqui AN, Habib A, Hussain MS, Najmi AK. Proton pump inhibitors' use and risk of hip fracture: a systematic review and meta-analysis. Rheumatol Int. 2018;38:1999-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Proton pump inhibitors and risk of dementia. Ann Transl Med. 2016;4:240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Kasiske BL, Israni AK. Kidney. Am J Transplant. 2016;16 Suppl 2:11-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80:S181-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Gabardi S, Olyaei A. Evaluation of potential interactions between mycophenolic acid derivatives and proton pump inhibitors. Ann Pharmacother. 2012;46:1054-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Schaier M, Scholl C, Scharpf D, Hug F, Bönisch-Schmidt S, Dikow R, Schmitt WH, Schwenger V, Zeier M, Sommerer C. Proton pump inhibitors interfere with the immunosuppressive potency of mycophenolate mofetil. Rheumatology (Oxford). 2010;49:2061-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kiberd BA, Wrobel M, Dandavino R, Keown P, Gourishankar S. The role of proton pump inhibitors on early mycophenolic acid exposure in kidney transplantation: evidence from the CLEAR study. Ther Drug Monit. 2011;33:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Suzuki T, Habuchi T. Influence of lansoprazole and rabeprazole on mycophenolic acid pharmacokinetics one year after renal transplantation. Ther Drug Monit. 2008;30:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kees MG, Steinke T, Moritz S, Rupprecht K, Paulus EM, Kees F, Bucher M, Faerber L. Omeprazole impairs the absorption of mycophenolate mofetil but not of enteric-coated mycophenolate sodium in healthy volunteers. J Clin Pharmacol. 2012;52:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Miner PB, Allgood LD, Grender JM. Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) o.m. famotidine 10 mg (Pepcid AC) b.d. and famotidine 20 mg b.d. over 14 days of treatment. Aliment Pharmacol Ther. 2007;25:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | McRorie JW, Kirby JA, Miner PB. Histamine2-receptor antagonists: Rapid development of tachyphylaxis with repeat dosing. World J Gastrointest Pharmacol Ther. 2014;5:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hené RJ, Verpooten GA, Navarro MT, Hale MD, Nicholls AJ. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 419] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, Lohmus A, Sommerer C, Hartmann A, Le Meur Y, Oellerich M, Holt DW, Tönshoff B, Keown P, Campbell S, Mamelok RD. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation. 2008;86:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, Etienne I, Westeel PF, Hurault de Ligny B, Rostaing L, Thervet E, Szelag JC, Rérolle JP, Rousseau A, Touchard G, Marquet P. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Kiberd BA, Lawen J, Fraser AD, Keough-Ryan T, Belitsky P. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant. 2004;4:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | van Gelder T, Tedesco Silva H, de Fijter JW, Budde K, Kuypers D, Arns W, Soulillou JP, Kanellis J, Zelvys A, Ekberg H, Holzer H, Rostaing L, Mamelok RD. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation. 2010;89:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Knorr JP, Sjeime M, Braitman LE, Jawa P, Zaki R, Ortiz J. Concomitant proton pump inhibitors with mycophenolate mofetil and the risk of rejection in kidney transplant recipients. Transplantation. 2014;97:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Courson AY, Lee JR, Aull MJ, Lee JH, Kapur S, McDermott JK. Routine prophylaxis with proton pump inhibitors and post-transplant complications in kidney transplant recipients undergoing early corticosteroid withdrawal. Clin Transplant. 2016;30:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | van Boekel GA, Kerkhofs CH, van de Logt F, Hilbrands LB. Proton pump inhibitors do not increase the risk of acute rejection. Neth J Med. 2014;72:86-90. [PubMed] |
| 23. | Patel KS, Stephany BR, Barnes JF, Bauer SR, Spinner ML. Renal Transplant Acute Rejection with Lower Mycophenolate Mofetil Dosing and Proton Pump Inhibitors or Histamine-2 Receptor Antagonists. Pharmacotherapy. 2017;37:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Rouse GE, Hardinger K, Tsapepas D, Tichy EM. A Comparison of Histamine Receptor Antagonists Versus Proton Pump Inhibitor Gastrointestinal Ulcer Prophylaxis in Kidney Transplant Recipients. Prog Transplant. 2017;27:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Takahashi K, Yano I, Fukuhara Y, Katsura T, Takahashi T, Ito N, Yamamoto S, Ogawa O, Inui K. Distinct effects of omeprazole and rabeprazole on the tacrolimus blood concentration in a kidney transplant recipient. Drug Metab Pharmacokinet. 2007;22:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Miura M, Inoue K, Kagaya H, Satoh S, Tada H, Sagae Y, Habuchi T, Suzuki T. Influence of rabeprazole and lansoprazole on the pharmacokinetics of tacrolimus in relation to CYP2C19, CYP3A5 and MDR1 polymorphisms in renal transplant recipients. Biopharm Drug Dispos. 2007;28:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] |
| 28. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12634] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 29. | Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 962] [Cited by in RCA: 1143] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 30. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30400] [Article Influence: 779.5] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 32. | Patel SJ, Moten MA, Noell BC, Brann S, Sydnor C, DeVos JM, Knight RJ. Clinical significance of proton pump inhibitor effect on mycophenoloic acid exposure in kidney transplantation. Am J Transplant. 2012;12:27-542. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Van Ende C, Van Laecke S, Marechal C, Verbeke F, Kanaan N, Goffin E, Vanholder R, Jadoul M. Proton-pump inhibitors do not influence serum magnesium levels in renal transplant recipients. J Nephrol. 2014;27:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Alhosaini MN, Leehey DJ, Vellanki K. Use of proton pump inhibitors is associated with severe hypomagnesemia in kidney transplant recipients. Int J Nephrol Kidney Fail. 2015;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Sezer S, Gurlekdemirci B, Uyanik S, Erkmenuyar M, Haberal M, Sayin B. Impact of proton pump inhibitors on hypomagnesemia and arterial stiffness in renal transplant recipients: Bo150. Transplant Int. 2015;28:181. |
| 36. | Shabaka A, Vian J, López de la Manzanara V, Pérez Flores I, de los Angeles Moreno de la Higuera M, Sánchez-Fructuoso A. Risk factors and prevalence of hypomagnesemia in kidney transplantation. Nephrol Dial Transplant. 2017;32:iii395. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Uludag O, Mirioglu S, Dirim A, Akardere O, Akyildiz A, Sever M, Caliskan Y. Effects of proton pump inhibitors on kidney transplant recipients. Nephrol Dial Transplant. 2017;32 suppl 3:iii730. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Gomes-Neto A, Douwes R, Eisenga M, Berger S, Gans R, Berg E, Navis G, Blokzijl H, Bakker S. Use of proton-pump inhibitors is associated with lower magnesium and iron status and excess mortality in renal transplant recipients. Am J Transplant. 2018;18:300-301. |
| 39. | Kipp G, Dancsecs K, Lapping A. Proton- pump inhibitor utilization is associated with higher rates of Clostridium difficile infection and hypomagnesemia after kidney transplant. Am J Transplant. 2017;17 Suppl 3:5-815. |
| 40. | Douwes R, Neto GA, Eisenga M, Gans RO, van den Berg E, Navis G, Blokzijl H, Bakker SJ. Chronic use of proton-pump inhibitors is associated with lower magnesium and iron status and mortality in renal transplant recipients. Ann Nutr Metab. 2017;71:979. |
| 41. | Rupprecht K, Schmidt C, Raspé A, Schweda F, Shipkova M, Fischer W, Bucher M, Kees F, Faerber L. Bioavailability of mycophenolate mofetil and enteric-coated mycophenolate sodium is differentially affected by pantoprazole in healthy volunteers. J Clin Pharmacol. 2009;49:1196-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit. 2001;23:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 43. | Kuypers DR, Ekberg H, Grinyó J, Nashan B, Vincenti F, Snell P, Mamelok RD, Bouw RM. Mycophenolic acid exposure after administration of mycophenolate mofetil in the presence and absence of cyclosporin in renal transplant recipients. Clin Pharmacokinet. 2009;48:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Xu L, Cai M, Shi BY, Li ZL, Li X, Jin HL. A prospective analysis of the effects of enteric-coated mycophenolate sodium and mycophenolate mofetil co-medicated with a proton pump inhibitor in kidney transplant recipients at a single institute in China. Transplant Proc. 2014;46:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol. 2016;27:3153-3163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 46. | William JH, Nelson R, Hayman N, Mukamal KJ, Danziger J. Proton-pump inhibitor use is associated with lower urinary magnesium excretion. Nephrology (Carlton). 2014;19:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J Nephrol. 2016;5:152-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 48. | Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf). 2008;69:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Garnier AS, Duveau A, Planchais M, Subra JF, Sayegh J, Augusto JF. Serum Magnesium after Kidney Transplantation: A Systematic Review. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Holzmacher R, Kendziorski C, Michael Hofman R, Jaffery J, Becker B, Djamali A. Low serum magnesium is associated with decreased graft survival in patients with chronic cyclosporin nephrotoxicity. Nephrol Dial Transplant. 2005;20:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Miura K, Nakatani T, Asai T, Yamanaka S, Tamada S, Tashiro K, Kim S, Okamura M, Iwao H. Role of hypomagnesemia in chronic cyclosporine nephropathy. Transplantation. 2002;73:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Yuan J, Zhou J, Chen BC, Zhang X, Zhou HM, Du DF, Chang S, Chen ZK. Magnesium supplementation prevents chronic cyclosporine nephrotoxicity via adjusting nitric oxide synthase activity. Transplant Proc. 2005;37:1892-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Cheungpasitporn W, Thongprayoon C, Harindhanavudhi T, Edmonds PJ, Erickson SB. Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. Endocr Res. 2016;41:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |