Published online Nov 30, 2018. doi: 10.5500/wjt.v8.i7.252
Peer-review started: May 25, 2018
First decision: June 9, 2018
Revised: September 7, 2018
Accepted: November 3, 2018
Article in press: November 3, 2018
Published online: November 30, 2018
Processing time: 205 Days and 10.6 Hours
To examine the outcome and prognostic factors for high risk patients with acute lymphoblastic leukemia/lymphoma (ALL/LBL) who underwent allogeneic hematopoietic stem cell transplantation (HCT) at our center during the period of 2010-2017
After due institutional review board approval, patients with high risk ALL/LBL post HCT were identified and included. All records were retrospectively collected. Time to event analysis was calculated from the date of HCT until event of interest or last follow up with Kaplan-Meir means. Cox regression model was used for multivariable analysis calculation.
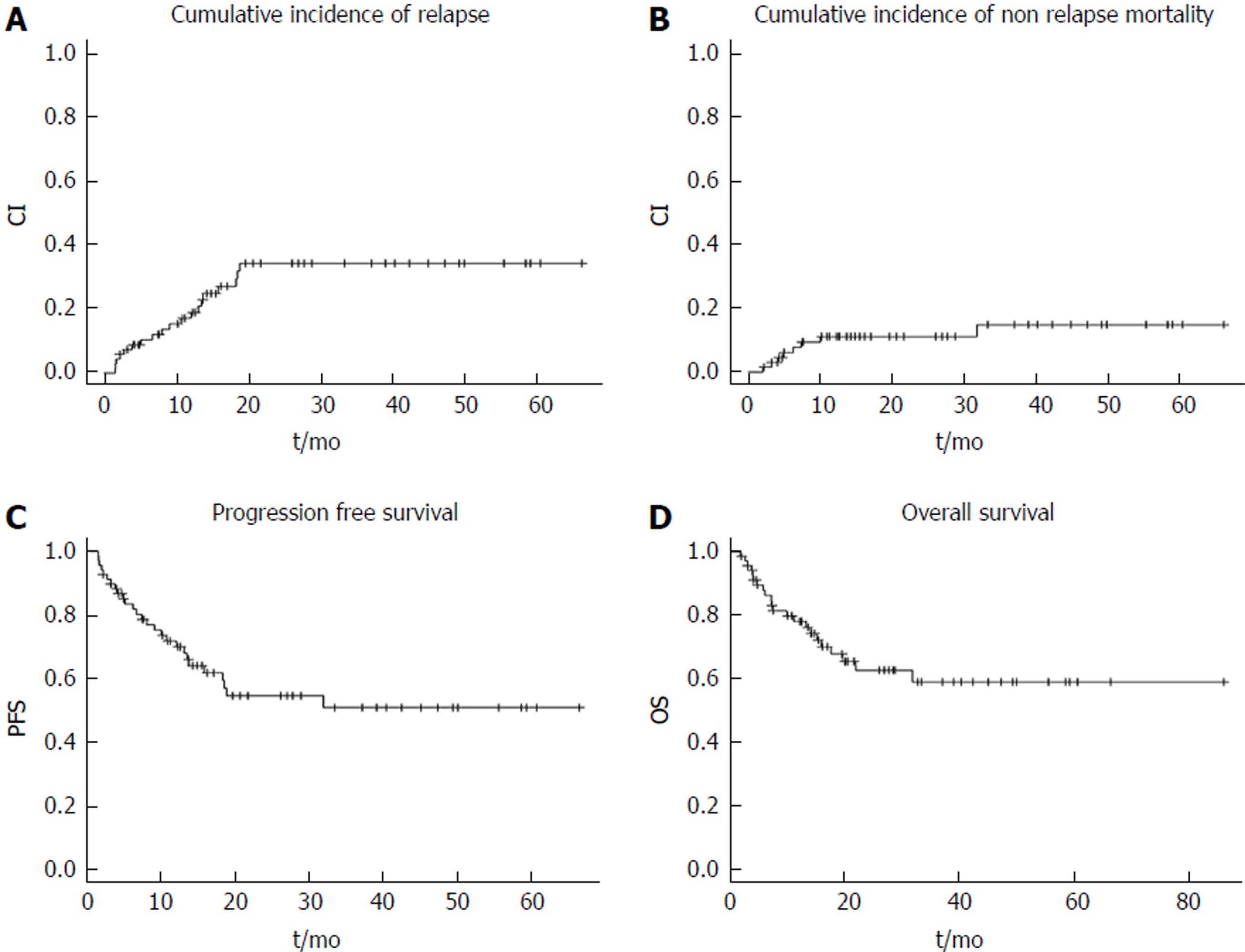
A total of 69 patients were enrolled and examined with a median age of 21 (14-61). After a median follow up of 15 mo (2-87.3), the 2-year cumulative incidence of relapse, cumulative incidence of non-relapse mortality, progression free survival and overall survival (OS) were 34.1%, 10.9%, 54.9% and 62.8%, respectively. In a multivariable analysis for OS; acute graft vs host disease (GVHD) and chronic GVHD were significant with corresponding hazard ratio 4.9 (1.99-12; P = 0.0007) and 0.29 (0.1-0.67; P = 0.0044), respectively.
Allogeneic-HCT for high risk ALL/LBL resulted in promising remissions particularly for patients with cGVHD.
Core tip: Allogeneic hematopoietic stem cell transplantation (HCT) is a potentially curative therapy for acute lymphoblastic leukemia/lymphoma (ALL/LBL) patients. We examined the outcome and prognostic factors of HCT for high risk ALL/LBL at our center. After due institutional review board approval, 69 patients were enrolled. After a median follow up of 15 mo (2-87.3), the 2-year overall survival (OS) was 62.8%. In a multivariable analysis; acute graft vs host disease (GVHD) and chronic GVHD predicted OS. In conclusion, allogeneic-HCT for ALL/LBL results in promising remissions in high risk disease and early referral for HCT to be considered for young and fit patients.
- Citation: Damlaj M, Snnallah M, Alhejazi A, Ghazi S, Alahmari B, Alaskar A, Al-Zahrani M. Graft vs host disease impacts overall survival post allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia/lymphoma. World J Transplantation 2018; 8(7): 252-261
- URL: https://www.wjgnet.com/2220-3230/full/v8/i7/252.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i7.252
Acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) constitute around 5% of all adult lymphoid malignancies and is typically diagnosed in the second to third decade of life. Complete morphologic remission, evident by presence of less than 5% clonal blasts in the bone marrow, following induction therapy can be achieved in the majority of patients. Incidence of relapse (IR) remains high; therefore, optimization of post remission therapy is vital. Furthermore, outcome of patients post relapse is dismal[1].
The role of allogeneic hematopoietic stem cell transplantation (HCT) in adult ALL/LBL in first complete remission (CR1) is debated. This is in part due to conflicting evidence with regards to the utility of this therapy due to on-going developments in the field. Typically accepted indications for allogeneic HCT in CR1 include elevated white blood count (WBC) > 30 × 109/L in B-cell disease and > 100 × 109/L in T-cell disease, age > 35 years, CD20 expression in B-cell disease, high risk cytogenetics including Philadelphia chromosome (Ph +ve), among others[2,3].
A number of prospective studies have examined the role of allogeneic HCT in CR1 spanning an enrolment period of almost two decades (1986-2005). The French Leucemie Aigue Lymphobalstique del’Adulte (LALA) group reported outcomes on over 400 patients from two studies (LAL-87 and LALA-94) and found that allogeneic HCT in CR1 resulted in improved survival in high risk patients[4,5]. Similar conclusions were drawn from the Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang (GOELAL02) clinical trial[6]. Conversely, the Eastern Cooperative Oncology Group/Medical Research Council (ECOG/MRC) and the Haemato-Oncology Foundation for adults in the Netherlands (HOVON) clinical trials demonstrated that this survival advantage is restricted to patients with standard risk disease[7,8]. Collectively, these results created some controversy within the transplant community on the optimal indication for all-HCT in CR1. The American Society of Blood and Marrow Transplantation recently published recommendations for the indications of various diseases for HCT, and they endorsed transplant for ALL in high risk disease in CR1 or CR2; however, these recommendations were not consistent with their European counterparts[9,10].
Out our center, we reserve allogeneic HCT for patients exhibiting conventional high risk features or evidence of minimal residual disease (MRD) at end of induction. We also perform allogeneic HCT for patients in second or subsequent CR (≥ CR2) due to its curative potential, albeit lower, in these patients and lack of better therapeutic strategies in this setting. Our aim from this analysis is to examine the prognostic factors and outcome in these high risk patients.
The project was approved by the institutional review board (IRB) prior to commencing. We identified all patients ≥ 14 years of age at our institution that underwent HCT for ALL during the time period of 2010-2017. All clinical records with regards to patient, disease, therapy and outcome were collected retrospectively from electronic medical records at our institution. The inclusion criteria were; patients who received allogeneic HCT for ALL using different conditioning intensity from matched related donor (MRD), matched unrelated donor (MUD) or haploidentical donors. The intensity of the conditioning regimen was based on the criteria suggested by the Centre of International Blood and Marrow Transplant Research (CIBMTR)[11]. Choice of regimen was based on the Hematopoietic Stem Cell Co-morbidity index (HCT-CI); patients scoring < 3 were considered for a myeloablative (MAC) regimen while the remaining patients received reduced intensity conditioning (RIC) regimen. Patients preferentially received a total body irradiation (TBI) regimen if they were candidates for a MAC regimen. We excluded patients who received a cord blood or bone marrow graft, second transplant and any patient that underwent in vivo or in vitro T-cell depletion. All records were retrospectively collected. Cytogenetics with hypodiploid karyotype, translocations at (4;11), (11q23), (9;22) and (1;19) were classified as high risk while all others were deemed standard risk.
The majority of patients received hyper-fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone with high dose methotrexate and cytarabine (HyperCVAD) given in alternating cycles (A and B) with cycle A consisting of 300 mg/m2 of intravenous (IV) cyclophosphamide every 12 h on days 1-3 for a total of 6 doses with appropriate mesna dose for bladder protection; vincristine 1.4 mg/m2 (maximum dose 2 mg) IV for two days (day 1 and 11); doxorubicin 50 mg/m2 IV on day 4 followed by dexamethasone 40 mg IV on days 1-4 then 11-14. Cycle B contained of high dose methotrexate 1 g/m2 given over 24 h on day 1 with appropriate hydration with sodium bicarbonate, leucovorin and therapeutic drug monitoring; cytarabine 3000 mg/m2 IV over 2 h given every 12 h on days 2-3 for a total of 4 doses and methylprednisolone 50 mg IV every 12 h on days 1-3. Patients with CD20 expression were given the monoclonal antibody rituximab on days 1 and 8 at a dose of 375 mg/m2. Ph positive ALL patients were given tyrosine kinase inhibitor (TKI) dasatinib 140 mg daily days 1-14 of each cycle of therapy and reinitiated post HCT once immunosuppression is tapered. Central nervous system prophylaxis consisted of intrathecal (IT) methotrexate 12 mg and hydrocortisone 50 mg given on day 2 of cycles A and B, and cytarabine 50 mg on day 8 of cycle A only. Patients were given at least 6 doses of IT chemotherapy prior to HCT. Patients were given 4 cycles of therapy (until 2B) prior to proceeding to HCT.
Supportive care consisted of granulocyte colony stimulating factor (GCSF) 300 mcg given starting day 5 until neutrophil recovery; ciprofloxacin 500 mg orally or IV equivalent twice daily; acyclovir 200 mg orally or IV equivalent twice daily; fluconazole 200 mg orally or IV equivalent twice daily and prednisolone 1% eye drops in each eye four times daily 1 d prior to and continued for 3 d post completion of cytarabine.
Bone marrow aspirate and trephine biopsy was done on day 28 post cycle 1A induction to assess for remission status with morphologic remission defined as < 5% blasts in the bone marrow with complete count recovery. The following high risk features were considered as indications for allogeneic HCT in first remission; presenting WBC > 30 × 109/L or 100 × 109/L in B- vs T-cell ALL, respectively; high risk cytogenetics as indicated above or evidence of persistent MRD post induction with HyperCVAD. Patients with relapsed disease and successfully achieved CR2 following salvage chemotherapy proceeded to HCT.
The MAC preparative regimen for matched related or unrelated donors (MRD or MUD) consisted of cyclophosphamide 60 mg/kg IV for a total of two days then a total of 1200 cGy of TBI divided twice daily for three days. Mesna was given for bladder protection. The MAC preparative regimen for haploidentical HCT consisted of fludarabine 25 mg/m2 IV for 3 d and TBI 1200 cGy fractionated twice daily for 4 d as previously described[12]. For RIC regimens and MRD or MUD donors, patients received fludarabine 30 mg/m2 IV on a daily basis for a total of 5 d with melphalan 70 mg/m2 IV for 2 d. For those with RIC haploidentical HCT, the preparative regimen consisted of fludarabine 30 mg/m2 IV daily for 5 d, cyclophosphamide 14.5 mg/kg IV daily for 2 d and TBI 200 cGy in a single fraction[13].
Prophylaxis for graft vs host disease (GVHD) contained methotrexate and cyclosporine for MRD and MUD HCT. Methotrexate was administered at 15 mg/m2 on day +1 then at 10 mg/m2 on days +3, +6 and +11. GVHD prophylaxis for haploidentical HCT consisted of tacrolimus 0.1 mg/kg per day orally twice daily (or IV equivalent) starting on day +6 adjusted to trough level of 10-15 ng/mL, mycophenolate mofetil (MMF) 15 mg/kg/dose three times daily starting on day +6 until +36 and cyclophosphamide 50 mg/kg IV daily on days +3 and +5 with appropriate mesna dose for bladder protection.
We defined overall survival (OS) as the time from transplant until the time of death of any cause or last patient encounter while progression free survival (PFS) was defined as the time from transplant until death due to any cause or relapsed disease. Cumulative incidence of relapse (CIR) was defined as the time from transplant until evidence of disease relapse or last patient encounter. While cumulative incidence of non-relapse mortality (NRM) was defined as the time from transplant until death due to any cause without evidence of relapse. Absolute neutrophil count (ANC) of 0.5 × 109/L or for 3 d constituted neutrophil engraftment while platelet count greater than 20 × 109/L for 7 d without transfusion support constituted platelet engraftment.
All baseline variables relating to patient, disease or treatment characteristics were reported in a descriptive fashion. Pearson’s χ2 and Wilcoxon/Kruskal-Wallis tests were used to analyze categorical or continuous variables, respectively. The Kaplan-Meir method with log ranks was used to estimate the probability of OS and PFS. Grey’s model was used to estimate the incidence of events with competing nature, i.e., CIR and cumulative incidence of NRM (CI-NRM). Cox regression model was used for univariate and multivariate analysis with outcome expressed as a hazard ratio (HR) with 95% confidence interval (CI) and P value. Variables with a P ≤ 0.05 were inserted into the multivariate model. Analysis was performed using JMP and EZR[14].
During the study period, 69 patients were identified per our inclusion criteria and were further analyzed. The median (range) age was 21 (14-61) years with 41 (59%) being male. B-cell ALL was the most common pathology representing 50 (72%) of cases with the remaining being T-cell subtype. Ph-ALL was detected in 16/50 (32%) of B-cell ALL. LBL was seen in 17 (25%) of cases. 35 (51%) of patients had high risk cytogenetics. A total of 42 (61%) of patients received HCT in CR1 while the remaining patients were in second or subsequent CR. Indications for HCT in these patients were; 27 (64%) for high risk cytogenetics including Ph-ALL; 11 (26%) for high presenting WBC at diagnosis and 4 (10) for persistent MRD post induction. Matched sibling donor (MSD) was the most common donor type in 58 (84%) of cases and the majority of patients received MAC regimen (90%) containing TBI (87%). The baseline characteristics of the cohort are shown in Table 1.
| Characteristic | Entire cohort (n = 69) |
| Patient age in years, median (range) | 21 (14-61) |
| Recipient gender, male | 41 (59%) |
| Cell subtype | |
| B-cell | 50 (72) |
| T-cell | 19 (28) |
| Philadelphia chromosome (B-cell) | 16/50 (32) |
| Disease subtype | |
| Lymphoblastic leukemia | 52 (75) |
| Lymphoblastic lymphoma | 17 (25) |
| Cytogenetic status | |
| Standard | 30 (43) |
| High risk | 35 (51) |
| Missing | 4 (6) |
| ECOG, median (range) | 0 (0-2) |
| HCT-CI, median (range) | 0 (0-5) |
| Gender mismatch | 28 (41) |
| Female donor/male recipient | 11 (16) |
| Donor type | |
| MSD | 58 (84) |
| MORD | 2 (3) |
| MUD | 3 (4) |
| Haploidentical | 6 (9) |
| Status at HCT | |
| CR1 | 42 (61) |
| ≥ CR2 | 27 (39) |
| ABO matching | |
| Match | 50 (73) |
| Major/bidirectional | 10 (14) |
| Minor | 9 (13) |
| TBI containing regimen | 60 (87) |
| Conditioning intensity | |
| MAC | 62 (90) |
| RIC/NMA | 7 (10) |
The median total of CD34 cells infused was 6 × 106/kg of recipient weight (range; 8.9-2) and all collected cells were infused through a Hickman catheter or a peripherally inserted central catheter (PICC). Infusion was over one day for all patients. GCSF was used in 33 (47.8%) of patients at the discretion of the treating physician. Median time to ANC engraftment, defined as ANC ≥ 0.5 × 106/L sustained over three days was 17 d (range; 9-28). There was no significant difference between time to ANC engraftment between patients receiving GCSF and those who did not. On the other hand, the median time to platelet engraftment was 12 (range; 0-29).
Acute GVHD (aGVHD) developed in a total of 20 patients (29%), with grades II, III or IV with 8 (40%), 8 (40%) and 4 (20%), respectively. All of them required systemic corticosteroid therapy, 5/20 (25%) required second line immune-suppressants while 2/20 (10%) required third line immune-suppressants. A high incidence of mortality was noted within these patients with 8/20 (40%) dying due to organ toxicity or infectious etiology. On the other hand, chronic GVHD (cGVHD) developed in a total of 30 patients (43.5%) with mild, moderate or severe forms in 8 (26.7%), 15 (50%) and 7 (23.3%), respectively. A total of 9 patients had overlap GVHD syndrome.
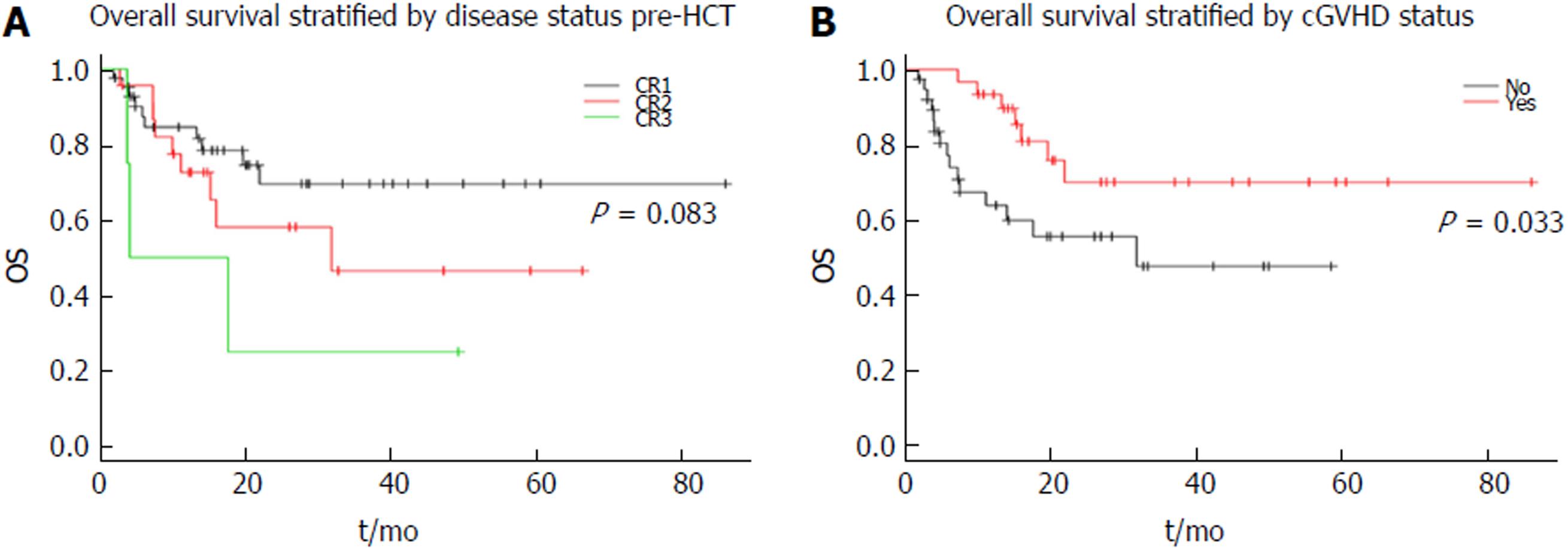
Overall cohort: The median follow up was 15 mo (2-87.3), following which the 2 year CIR, CI-NRM, PFS and OS were 34.1%, 10.9%, 54.9% and 62.8%, respectively as shown in Figure 1). Stratified by remission status at the time of HCT, patients in CR1 had an improved survival compared to those in CR2 or CR3 with 2-year OS of 69.5% vs 46.5% vs 25% with a trend towards significance (P = 0.083) as shown in Figure 2A. On the other hand, when stratified by presence of cGVHD post HCT, patients with evidence of cGVHD had a significantly improved outcome with a 2-year OS of 70% vs 47.6% (p = 0.033) as shown in Figure 2B.
Predictors of outcome: In multivariable analysis for PFS or OS as the outcome of interest, the following variables were included; age at HCT, cell subtype, ALL vs LBL, Ph-chromosome status, female donor to male recipient, donor gender mismatch, MSD vs other donor source, TBI containing regimen, MAC regimen vs other, CR1 vs other, acute or cGVHD. For PFS, aGVHD and cGVHD were significant for PFS with corresponding HR of 3.14 (1.36-7.1; P = 0.008) and HR 0.38 (0.15-0.89; P = 0.026), respectively. Whereas for OS aGVHD and cGVHD were significant at the multivariable analysis with HR 4.9 (1.99-12; P = 0.0007) and 0.29 (0.1-0.67; P = 0.0044), respectively. These results are shown in Table 2.
| Univariable HR (95%CI; P value) | Multivariable HR (95%CI; P value) | ||
| PFS | Age at HCT | 1.5 (0.27-6; P = 0.6) | |
| B-cell vs T-cell | 0.53 (0.25-1.17; P = 0.11) | ||
| ALL vs LBL | 0.6 (0.27-1.45; P = 0.24) | ||
| Female D → male R | 0.87 (0.25-2.26; P = 0.79) | ||
| Donorgender mismatch | 0.53 (0.22-1.17; P = 0.12) | ||
| MSD vs other | 0.5 (0.22-1.28; P = 0.14) | ||
| TBI regimen | 1.1 (0.41-3.67; P = 0.89) | ||
| MAC vs RIC/NMA | 1.37 (0.41-8.5; P = 0.65) | ||
| CR1 vs other | 0.59 (0.28-1.28; P = 0.18) | ||
| aGVHD | 2.1 (0.95-4.5; P = 0.066) | 3.14 (1.36-7.1; P = 0.008) | |
| cGVHD | 0.43 (0.18-0.94; P = 0.033) | 0.38 (0.15-0.89; P = 0.026) | |
| OS | Age at HCT | 1.02 (0.98-1.05; P = 0.28) | |
| B-cell vs T-cell | 0.57 (0.24-1.37; P = 0.2) | ||
| ALL vs LBL | 0.44 (0.19-1.11; P = 0.08) | ||
| Female D → male R | 1.15 (0.33-3.1; P = 0.8) | ||
| Donorgender mismatch | 0.62 (0.23-1.48; P = 0.29) | ||
| MSD vs other | 1.27 (0.43-5.4; P = 0.69) | ||
| TBI regimen | 1.99 (0.58-12.5; P = 0.31) | ||
| MAC vs RIC/NMA | 0.69 (0.23-2.92; P = 0.56) | ||
| CR1 vs other | 0.5 (0.21-1.17; P = 0.11) | ||
| aGVHD | 3.35 (1.42-7.9; P = 0.006) | 4.9 (1.99-12; P = 0.0007) | |
| cGVHD | 0.4 (0.15-0.97; P = 0.043) | 0.29 (0.1-0.67; P = 0.0044) | |
The optimal post remission therapy in ALL/LBL continues to be debated amongst experts given the ongoing developments in the field. On the one hand, allogeneic HCT offers good disease control relative to chemotherapy alone but the potential toxicity depending on prior therapy and hematopoietic stem cell transplant comorbidity index (HCT-CI) can be a hindering factor for some patients[15]. On the other hand, more refined methods of risk stratification specifically with the use of MRD and the utilization of a pediatric inspired regimens in eligible patients have significantly reduced relapse rates[16]. Importantly, optimal therapy should be delivered upfront as outcome of these patients post relapse are inferior. Oriol et al[17] reported on outcome of ALL patients with relapsed disease treated on one of four risk adapted trials by the PETHEMA study group. Only 10% of patients were alive at 5 years but more favorable outcomes were seen in younger patients and those relapsing late beyond 2 years.
A large comparative study examined 422 Ph negative ALL patients who underwent HCT in CR1 from the Center of International Blood and Marrow Transplantation Research (CIBMTR) to an age matched concurrent cohort of 108 patients treated with the Dana-Farber Consortium (DFC) Pediatric protocol found that while the relapse rate was similar among both approaches, patients fared significantly better with the DFC mainly due to a transplant related mortality (TRM) of 37%[18]. With regards to chemotherapy regimen comparison, the MD Anderson Cancer Center performed a comparative analysis between HyperCVAD, a common regimen for ALL used at their institution and the Augmented Berlin-Frankfurt-Munster (ABFM)[19]. Both regimens were associated with comparable overall outcomes, but with differing adverse event profile; ABFM resulting in higher hepatotoxicity, pancreatitis and osteonecrosis whereas HyperCVAD resulting in more bone marrow suppression related toxicity. Of note, the 5-year OS was 60% in both groups and around 10% of patients underwent HCT in CR1. Collectively, it remains unclear which treatment modality is preferred and further studies are needed to resolve this debate. The heterogeneity within the inclusion criteria among studies is the likely result in such discrepant outcomes.
Our aim with this analysis was to ascertain outcome of patients whom underwent HCT for ALL/LBL at our center. The patients presented herein were all those with high risk features, i.e., conventional risk factors, positive MRD or those with relapsed disease in second or subsequent remissions. We observed an OS of 62.8% at 2-year for the entire cohort which is quite promising. Furthermore, the CIR at 2-years was 34.1% for the entire cohort irrespective of the remission status at HCT. The majority of patients in this cohort underwent HCT utilizing MAC intensity conditioning and a MSD. Previously, the largest prospective trial in ALL, i.e., the ECOG/MRC trial cohort reported a 5-year OS of 41% for high risk patients undergoing HCT in CR1[7]. Interestingly, the relapse rate observed within this trial was 37% for the high risk group and 24% within the standard risk which was comparable to our cohort. However, the incidence of NRM within the high risk cohort was 35.8% at 2-years which is substantially higher than what we observed despite having similar HCT criteria. We have two plausible observations that could have resulted in such higher NRM; first, the median age within our cohort was younger, and as such the expected complications post HCT are likely to be lower. This was reported previously where younger patients were reported to fare better than their older counterparts which was largely driven by higher incidence of NRM, whereas disease control with HCT is the same[20]. Second, the changes in supportive care over the last 1-2 decades, particularly with the use of antimicrobials for prophylaxis and management could have led to a reduction in post HCT complications[21].
Subsequently, we analyzed the cohort to ascertain factors influencing outcome at the multivariable analysis stage. We included typical patient, disease and transplant variables that may impact outcome. We observed that acute and chronic GVHD predicted for OS. There was a trend towards significance for B-cell subtype and CR1 remission status for OS and perhaps a larger sample size could have identified such variables as significant as well. Interestingly in our cohort, presence of Ph chromosome did not portend a negative prognostic marker and is likely due to the use of dasatinib as targeted TKI therapy during induction and as post HCT maintenance.
Allogeneic HCT is favored as post remission therapy due to relatively potent graft vs leukemia effect. Although difficult to measure or quantify, it is felt that cGVHD is a surrogate for such GVL effect[22,23]. Such effect is felt to be mediated by a number of donor factors but perhaps largely T-lymphocytes that exhibit their role by targetting any residual leukemia cells and prolonging patient’s remission. However, this is a double edged sword as significant GVHD can augment the NRM effect and lead to more detrimental outcomes. Our patients experienced largely mild to moderate cGVHD, possibly due to majority of donors being MRD and we observed a favorable effect of such cGVHD on OS. aGVHD on the other hand had a detrimental impact on OS with a high case fatality ratio due to organ toxicity or infectious complications. Lastly, all B-ALL/LBL within this cohort received the monoclonal antibody rituximab, if CD20 positive, and it is possible that this has contributed to the trend of improved OS seen within our cohort. Previously, multiple studies reported on the favorable impact of rituximab on the outcome of ALL including Burkitt type ALL[24-26].
This analysis has some inherent limitations, particularly with its retrospective single center design and sample size. However, a number of important observations were noted; First, conventional high risk features of ALL/LBL can be overcome by the conditioning effect of the transplant coupled by the GVL effect. This is evident as the survival curve has plateaued indicating the curative potential of this therapy. Second, cGVHD leads to enhanced OS likely as it represents a surrogate for GVL. Third, aGVHD can be detrimental to outcome as it causes significant morbidity and mortality mainly due to infectious complications. In conclusion, allogeneic-HCT for high risk ALL/LBL results in promising remissions in high risk disease and early referral for HCT to be considered for young and fit patients.
Allogeneic hematopoietic stem cell transplantation (HCT) is a potentially curative therapy for patients with high risk acute lymphoblastic leukemia (ALL). The indications for HCT have evolved over time with the introduction of pediatric inspired protocols and minimal residual disease (MRD) monitoring. Our aim from this study is to examine the outcome and prognostic factors for high risk ALL patients at our center.
Identifying the prognostic factors that may facilitate patient selection and select the ideal candidate for transplantation.
Our aim from this study is to examine the outcome and prognostic factors for high risk ALL patients.
After due institutional review board approval, patients with high risk ALL/ lymphoblastic lymphoma (LBL) post HCT were identified and included. All records were retrospectively collected. Time to event analysis, was calculated from the date of HCT until event of interest or last follow up with KM means. Cox regression model was used for multivariable analysis calculation.
A total of 69 patients were enrolled and examined with a median age of 21 (14-61). After a median follow up of 15 mo (2-87.3), the 2-year cumulative incidence of relapse (CIR), cumulative incidence of non-relapse mortality (CI-NRM), progression free survival (PFS) and overall survival (OS) were 34.1%, 10.9%, 54.9% and 62.8%, respectively. In a multivariable analysis for OS; acute graft vs host disease (GVHD) and chronic GVHD were significant with corresponding HR 4.9 (1.99-12; P = 0.0007) and 0.29 (0.1-0.67; P = 0.0044), respectively.
Allogeneic-HCT for high risk ALL/LBL results in promising remissions and early referral for HCT is to be considered for young and fit patients.
We identified that acute and chronic graft vs host diseases were prognostic for overall survival. We also observed that patients with Philadelphia positive ALL whom were given tyrosine kinase inhibitor therapy fared better than expected. Post HCT outcome of patients with ALL is expected to improve over time with the changing therapeutic landscape. We wished to examine the outcome of ALL patients treated in a contemporary era and identify prognostic factors for outcome. Our findings warrant confirmation in a larger cohort of patients.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kita K, Kin T, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ, Luger SM, Marks DI, Franklin IM. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 642] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 2. | Thomas DA, O’Brien S, Jorgensen JL, Cortes J, Faderl S, Garcia-Manero G, Verstovsek S, Koller C, Pierce S, Huh Y. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood. 2009;113:6330-6337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Rowe JM. Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol. 2010;150:389-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Sebban C, Lepage E, Vernant JP, Gluckman E, Attal M, Reiffers J, Sutton L, Racadot E, Michallet M, Maraninchi D. Allogeneic bone marrow transplantation in adult acute lymphoblastic leukemia in first complete remission: a comparative study. French Group of Therapy of Adult Acute Lymphoblastic Leukemia. J Clin Oncol. 1994;12:2580-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, Kovacsovics T, Delannoy A, Fegueux N, Fenaux P. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075-4086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Hunault M, Harousseau JL, Delain M, Truchan-Graczyk M, Cahn JY, Witz F, Lamy T, Pignon B, Jouet JP, Garidi R, Caillot D, Berthou C, Guyotat D, Sadoun A, Sotto JJ, Lioure B, Casassus P, Solal-Celigny P, Stalnikiewicz L, Audhuy B, Blanchet O, Baranger L, Béné MC, Ifrah N; GOELAMS (Groupe Ouest-Est des Leucémies Airguës et Maladies du Sang) Group. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood. 2004;104:3028-3037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, Chopra R, Wiernik PH, Foroni L. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 8. | Cornelissen JJ, van der Holt B, Verhoef GE, van’t Veer MB, van Oers MH, Schouten HC, Ossenkoppele G, Sonneveld P, Maertens J, van Marwijk Kooy M. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2009;113:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 10. | Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, Falkenburg JH, Farge-Bancel D, Gennery A, Kröger N. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50:1037-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1436] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 12. | Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, Morris LE, Bashey A. Total Body Irradiation-Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol Blood Marrow Transplant. 2015;21:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1597] [Cited by in RCA: 1472] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 14. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13272] [Article Influence: 1106.0] [Reference Citation Analysis (0)] |
| 15. | Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 2340] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 16. | Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, Thomas X, Chevallier P, Nguyen S, Coiteux V. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125:2486-96; quiz 2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Oriol A, Vives S, Hernández-Rivas JM, Tormo M, Heras I, Rivas C, Bethencourt C, Moscardó F, Bueno J, Grande C. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 18. | Seftel MD, Neuberg D, Zhang MJ, Wang HL, Ballen KK, Bergeron J, Couban S, Freytes CO, Hamadani M, Kharfan-Dabaja MA. Pediatric-inspired therapy compared to allografting for Philadelphia chromosome-negative adult ALL in first complete remission. Am J Hematol. 2016;91:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM, Pemmaraju N, Daver NG, Ferrajoli A, Garcia-Manero G. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Burke MJ, Gossai N, Wagner JE, Smith AR, Bachanova V, Cao Q, MacMillan ML, Stefanski HS, Weisdorf DJ, Verneris MR. Survival differences between adolescents/young adults and children with B precursor acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Brissot E, Rialland F, Cahu X, Strullu M, Corradini N, Thomas C, Blin N, Rialland X, Thebaud E, Chevallier P. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone Marrow Transplant. 2016;51:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Appelbaum FR. Graft versus leukemia (GVL) in the therapy of acute lymphoblastic leukemia (ALL). Leukemia. 1997;11 Suppl 4:S15-S17. [PubMed] |
| 23. | Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371-4383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 24. | Maury S, Chevret S, Thomas X, Heim D, Leguay T, Huguet F, Chevallier P, Hunault M, Boissel N, Escoffre-Barbe M. Rituximab in B-Lineage Adult Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375:1044-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 25. | Levato L, Molica S. Rituximab in the management of acute lymphoblastic leukemia. Expert Opin Biol Ther. 2018;18:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Hoelzer D, Walewski J, Döhner H, Viardot A, Hiddemann W, Spiekermann K, Serve H, Dührsen U, Hüttmann A, Thiel E. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124:3870-3879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |