Published online Apr 24, 2017. doi: 10.5500/wjt.v7.i2.117
Peer-review started: January 7, 2017
First decision: February 17, 2017
Revised: February 28, 2017
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: April 24, 2017
Processing time: 106 Days and 3.5 Hours
The intra-islet microvasculature is a critical interface between the blood and islet endocrine cells governing a number of cellular and pathophysiological processes associated with the pancreatic tissue. A growing body of evidence indicates a strong functional and physical interdependency of β-cells with endothelial cells (ECs), the building blocks of islet microvasculature. Intra-islet ECs, actively regulate vascular permeability and appear to play a role in fine-tuning blood glucose sensing and regulation. These cells also tend to behave as “guardians”, controlling the expression and movement of a number of important immune mediators, thereby strongly contributing to the physiology of islets. This review will focus on the molecular signalling and crosstalk between the intra-islet ECs and β-cells and how their relationship can be a potential target for intervention strategies in islet pathology and islet transplantation.
Core tip: This review article summarizes recent developments in the cross-talk relationship between intra-islet endothelial cells and beta cells. The molecules involved in the signalling pathways can be potential targets for therapeutic strategies and islet transplantation.
- Citation: Narayanan S, Loganathan G, Dhanasekaran M, Tucker W, Patel A, Subhashree V, Mokshagundam S, Hughes MG, Williams SK, Balamurugan AN. Intra-islet endothelial cell and β-cell crosstalk: Implication for islet cell transplantation. World J Transplant 2017; 7(2): 117-128
- URL: https://www.wjgnet.com/2220-3230/full/v7/i2/117.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i2.117
Pancreatic islets represent endocrine “island” cell clusters, embedded and scattered throughout the pancreas within large amounts of exocrine acinar tissue[1]. Islets are perfused by a dense, specialized microcirculation and receive 10% of the pancreatic blood flow despite comprising only 1%-2% of the overall tissue mass[2]. Most islets are irregularly shaped spheroids with a size distribution ranging from 50-500 μm, each composed of 800-3000 individual cells. The islet microcirculation is characterized by pre islet arterioles that rapidly arcade to a dense population of capillaries[3].
The cellular components of the islet include β-cells, other endocrine cells, as well as endothelium, perivascular, and support cells such as pericytes[4-9]. The cellular composition of islets is not uniform across species. Rodent and rabbit islets are primarily composed of a β-cell core with other cell types in the periphery whereas human and primate islets exhibit endocrine cell types intermingled with each other[4,10,11]. Beta cells, the central regulator of glucose homeostasis, are the largest cellular component of islets in most species[9,10].
Studies using vascular corrosion casts have demonstrated that 1-3 arterioles feed larger islets[12]. The capillary network within islets is about five times denser in comparison with exocrine tissue[3]. The capillary wall is composed of a permeable layer of ECs and contains ten times more fenestrae than ECs present in the exocrine pancreas[13,14]. The islet endothelial fenestra are highly specialized and contain a diaphragm that regulates solute transport[15,16]. Typically, a microvessel consists of ECs arranged into a tube formation wrapped by one or more layers of perivascular cells. Vascular ECs represent a major cell type present in islets and these cells are organized into a highly regulated and morphologically unique microcirculation. In culture, islet ECs express the classic endothelial markers such as von Willebrand factor, CD31, CD105, CD146, uptake of acetylated LDL, expression of leucocyte adhesion molecules, contain Weibel-Palade bodies in the cytoplasm, and form tight junctions[17,18]. Other markers expressed within islet ECs include α-1 antitrypsin, a major proteinase inhibitor[17,19,20]; nephrin, a highly specific barrier protein[16]; platelet-activating factor receptor[21], and genes expressing angiogenic (vascular endothelial growth factor, VEGF) and angiostatic (endostatin, pigment epithelial-derived factor) molecules[22].
Islet ECs have a significant relationship with islet function. For example, islets grafts, when co-transplanted[23] with ECs in diabetes induced rats or coated[24] with ECs in diabetes induced mice, have better engraftment capacity and improved islet function. Donor islet ECs, immediately after transplantation, participate in neovascularization by increasing β-cell survival[25] and promote both pancreatic stem cell proliferation and islet regeneration after β-cell injury[26]. Research performed over the last two decades has evaluated the link between islets and the ECs, demonstrating how the molecular interplay between these two cell types can regulate many critical physiological processes associated with the islet.
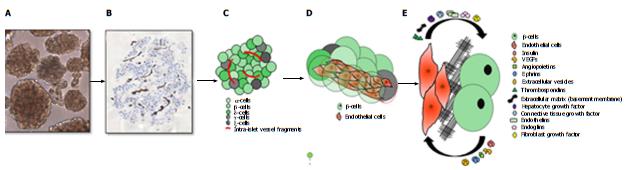
In vitro studies demonstrate that conditioned medium derived from cultured rat islets induces liver and islet-derived EC proliferation and migration[27], suggesting presence and secretion of paracrine pro-angiogenic factors (Figure 1) which promote islet vascularization[28]. As a major soluble β-cell secreted product, insulin promotes β-cell survival. In addition, insulin causes the upregulation of endothelial nitric oxide synthase in ECs promoting intra-islet blood flow[29]. Post-natal beta mass is dynamic and can increase in function and mass to compensate for additional physiological requirements[30].
The family of VEGF ligands and their receptors are critical as they regulate a number of developmental processes and play major roles in wound healing and vessel homeostasis in adult organisms[31,32]. VEGF secretion is stimulated by tumor, hypoxia, low pH and many other factors. Beta-cells secrete large amounts of VEGF-A early in development and throughout adult life[33]. The VEGF binds to its receptor (VEGFR) located on the blood vessel ECs, which activates multiple signalling cascades eventually resulting in the production of enzymes and other specific molecules required for EC growth and proliferation. Other activation effects include mobilization of endothelial progenitor cells from bone marrow, increased vascular permeability and tissue factor induction[34]. The VEGF family comprises seven secreted glycoproteins that are designated VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, placental growth factor and VEGF-F[35-37]. VEGF family members interact with three main receptors, VEGFR-1 (FLt-1), VEGFR-2 (KDR in humans and Flk-1 in mice) and VEGFR-3 (Flt4), all tyrosine kinase receptors and members of the PGDF receptor family. VEGFR-2 appears to be the main receptor responsible for mediating the proangiogenic effects of VEGF-A[35,38,39]. The consequence of this specific ligand-receptor interaction facilitates EC proliferation via the PKC-Ras pathway (by inducing MAPK/ERK pathways)[40,41]; promotes cytoskeletal reorganization and cell migration via p38 and focal adhesion kinase activation[42]; and supports EC cell survival and migration by activating the PI3K/Akt/PKB pathway[43,44].
VEGF-A is known to utilize the VEGFR-2 receptor on ECs[45], with the receptor highly expressed in intra-islet capillaries[46]. VEGF likely stimulates EC growth in neonatal pancreas; increased levels of VEGF-A correspond with islet growth in pregnant rats[47]. VEGF-A signaling is also essential in maintaining vascular beds in adult islets, this was validated using VEGF receptor antagonists[48]. VEGF-A expression is further upregulated in islets by hypoxia and glucose[49,50] and is important for the establishment of native intra-islet vasculature[51], maintenance of β-cell mass[52], and the revascularization of islets following transplantation[53].
Apart from VEGF-A, other known factors such as those within Ang/Tie family are known to contribute towards the survival and integrity of blood vessels[33,54,55]. These angiogenic factors consist of ligands Ang-1, Ang-2 and Ang-4 (its mouse orthologue, Ang-3) and the tyrosine kinase receptors Tie-1 and Tie-2. Ang-1 is expressed mainly by the perivascular cells and β-cells in mouse and human islets[33], and its agonist Tie-2 is expressed by the ECs. Ang-1 activates the pI3k/Akt pathway and prevents cytokine mediated apoptosis in ECs[56]. Moreover, β-cell specific overexpression of Ang-1 or Ang-2 only slightly impairs insulin secretion and glucose tolerance together with marginal altered vascularization, islet mass and morphology[57]. Reports also suggest that Ang-1/Tie-2 signaling promotes cell-cell contacts and contact to extracellular matrix (ECM)[58,59]. Ang-2 however, expressed by ECs, classically antagonizes Tie-2 signaling[60] and plays key roles in angiogenesis and inflammation.
Ephrin ligands and their tyrosine kinase receptors are involved in various aspects of cell communication[61,62]. Each ephrin ligand together with its specific receptor (Eph) is categorised either into the A or B subclass. Most EphA receptors bind to ephrin-A ligand, while most EphB receptors bind to ephrin-B ligands[63]. Transcriptome analyses suggest that Eph-ephrin interaction between exocrine and endocrine cells contributes to pancreatic function[64]. Ephrin-A and its receptor EphA play a role in β-cell to β-cell communication; specifically, ephrin subtype A5 is required for glucose stimulated insulin secretion and the EphA-ephrin-A mediated interaction between β-cells is bidirectional[65]. The blood vessel ECs within pancreatic islets express Eph subtype A4 receptors[66] but how these ligands and receptors play a role between EC and β-cell crosstalk is subject to investigation.
Recent reports establish extracellular vesicles (EVs) as a novel player in cell-to-cell communication[67,68] and have been characterized both in human islets[69] and in experimental models of human islet xenotransplantation in SCID mice[70]. Studies exploring the functional contribution of β-cell EVs on islet ECs demonstrate that islet-derived EVs have the capacity to affect the surrounding ECs, which are then able to internalize the islet EVs in a dose dependent manner[69]. Furthermore, internalization of islet EVs results in transfer of multiple RNAs, including insulin mRNA and various microRNAs. Uptake of islet EVs conferred endothelial cell resistance to apoptosis and up-regulated expression of numerous proangiogenic factors[69]. In a different study, endothelial progenitor cell EVs, when internalized by islet α-, β- and ECs resulted in improved glucose-stimulated proliferation and angiogenesis[70].
Islet ECs, apart from their pivotal role in angiogenesis, also possess endocrine function. They produce multiple factors (Figure 1) that govern proliferation, survival, and gene expression, which contribute to the physiology and function of the β-cell[71-75].
ECM proteins provide biochemical cues interpreted by cell surface receptors and initiate signalling cascades controlling morphogenesis, cell survival, proliferation, differentiation, and stem cell state[76-78]. Islets are surrounded by a peri-islet basement membrane (BM) and an associated interstitial matrix containing multiple components such as collagen, laminin, fibronectin, perlecans, nidogens, and heparin sulphate[79,80]. Beta-cells depend on intra-islet ECs to synthesize their ECM components[75]. It has been reported that collagen IV, secreted by islet endothelium, can potentiate insulin secretion via interaction with its receptor integrin α1β1 on β-cells[81] similar to other BM components such as laminins and fibronectin which have been reported to act as endothelial signals promoting insulin gene expression and proliferation in β-cells[75,82]. Interaction of collagen IV with its receptors also contributes to β-cell differentiation, maturation, and survival[83-85]. Other BM components such as fibronectin and heparin sulfate also play roles in β-cell migration, growth, differentiation and survival[1,86-88].
The β-cell proliferative factor, connective tissue growth factor (CTGF/CCN2), is a member of the CCN family of secreted ECM-associated proteins[89,90] and is expressed in ECs during development[90,91]. It induces expression of platelet derived growth factor B (PDGF-B) in ECs, required for pericyte recruitment and retention[91]. CTGF promotes β-cell regeneration[92], proliferation[93], and modulates the response to high glucose[94]. Its inactivation results in defects in islet cell lineage allocation and β-cell proliferation during embryogenesis[95].
Islet ECs release the hepatocyte growth factor (HGF)[13] which induces β-cell proliferation and differentiation in embryonic and postnatal pancreas[47,75,95-98]. HGF plays a positive role in β-cell mitogenesis, differentiation, glucose sensing, and transplant survival[99,100]. In vitro, VEGF-A and insulin are islet-derived factors that induce the HGF secretion within purified islet ECs. In vivo, utilizing of pregnant rat pancreas, where a high physiological proliferation of β cells occur, resulted in a prominent expression of HGF, coinciding with the peak of β-cell proliferation[74].
Thrombospondins are matricellular glycoproteins that participate in a regulating cell proliferation, migration, and apoptosis, and have been implicated in angiogenesis, tumour invasion, and metastasis[101,102]. Thrombospondin-1 (TSP-1) is almost exclusively expressed by the intra-islet endothelium[71,103,104] and is not downregulated by hypoxia[105]. TSP-1 is mainly known for its antiangiogenic properties[106] but also may alter the morphology of pancreatic islets and function as a major activator of transforming growth factor TGFβ-1[107]. Animals deficient of this glycoprotein are characterized by hypervascular islets[107] and the EC-derived TSP-1 is important to maintain β-cell function postnatally[71].
Endothelin is a vasoconstrictive protein. Endothelin-1 (ET-1) predominantly is found to have strong effects on native islet blood vessels[108] while ET-1 and ET-3 may directly stimulate β-cell insulin secretion and release[73,109]. The gene expression of ET-1 in both ECs and islet endocrine cells is regulated by hypoxia[110,111]. Insulin can also stimulate the expression and secretion of ET-1 from bovine ECs[112] and endogenous insulin can regulate circulating ET-1 concentrations in humans[113]. ET-1 also upregulates the expression of the FOXO1 gene (encoding a transcription factor) on ECs contributing to its survival[114].
Endoglin (Eng) is a homodimeric transmembrane glycol protein within the TGF-β superfamily and is expressed by vascular ECs[115-118]. Studies have identified two distinct Eng positive cell types within human and mouse islets: The ECs and the mesenchymal stromal cells[119]. EC-specific endoglin expression in islets is sensitive to VEGF playing partial roles in driving islet vascular development[120].
The human islet isolation technique completely severs the islet vasculature[121,122]. During the enzymatic digestion step, islets undergo a number of cellular assaults such as ischemia, mechanical stress, loss of basement proteins, and partial disruption of intra-islet ECs[123-125] resulting in a substantial loss of viability before transplantation. Other than being devoid of ECs to support rapid revascularization, cytotoxic damage and cell death account for a loss of up to 80% of transplanted islets[126,127]. Rapid and adequate revascularization is critical for survival and function of transplanted islets[121,128,129]. Transplanted islet grafts initially have a significant reduction in vascular supply and low oxygen tension in comparison to normal islets[130-132]. The return of islet function depends on re-establishment of new vessels within islet grafts to derive blood flow from the host vascular system[123,133]. Islet engraftment is a slow process, while the islet blood flow re-establishment requires about two weeks, vessel maturation is likely to take a much longer period. Using immunosuppressive drugs such as rapamycin further affect this process by exerting antiangiogenic activities on mouse and human islet endothelium[134].
Though transplanted islets are considered avascular, freshly isolated islets retain angiogenic capacity as they contain intra-islet ECs. These cells can be triggered by various inducers such as VEGF to form vessels via angiogenic sprouting[33,135,136]. Revascularization is an important process for adequate engraftment of islets. Prevascularizing islets prior to transplantation could potentially improve islet survivability and function by aiding islet-to-host inosculation[25]. The intra-islet vasculature can also act as a barrier against infiltrating insults of autoreactive cells in type 1 diabetes (T1D) thereby implicating ECs as an important target in type 2 diabetes (T2D)[137-139].
Studies involving cell and tissue engineering approaches have considered factors such as pancreatic islet size-dependency[140], use of stem cells[141-144], creating engineered vascular beds and hydrogels[145-147], endothelial progenitor cell derived microvesicles[70], and repurposed biological scaffolds[148] to improve islet revascularization potential. The angiogenic capacity of islet ECs has been previously determined[136]. A number of factors which may potentially improve islet transplantation involve ECs. For example, vascular ECs of the embryonic aorta induce the development of endocrine cells from pancreatic epithelium in mice[149,150] and the overexpression of VEGF-A in transplanted mouse islets improves insulin secretion and blood glucose regulation in recipient mice[33,53]. Identifying novel factors and understanding nature of mechanisms that underlie bidirectional communication between β-cells and ECs should be of immense relevance for improved human islet transplantation or preventing pancreas associated diseases such as pancreatitis and diabetes.
ECs and β-cell crosstalk: Islet pathophysiology, current perspectives and future directions
Evaluation of factors contributing to mechanisms responsible for regulating the interaction between β-cells and intra-islet ECs would broaden our understanding of pancreatic tissue function, growth, and disease. In this context, VEGF-A has been the most well studied molecule[51,53]; however, reports have suggested the detrimental effects of VEGF on islets. Continued β-cell overexpression of VEGF-A impairs islet morphology and function by eliciting an inflammatory response[57,151]. Elevated levels of serum VEGF, Ang-2, and soluble Tie-2 have also been associated with T2D and vascular dysfunction[152-154]. Achieving an optimal VEGF-A dose to potentiate islet vascularization is subject to further investigation. The HGF production is increased during pregnancy in adult rats[74] and helps balance high glucose levels in diabetes induced mice[155]. HGF gene therapy has been suggested as a potential approach for improving islet transplantation rates and treatment of diabetes[156,157].
The dense pancreatic vasculature along with its associated ECM plays a key role in the physiology and disease associated with pancreatic islets. The islet is an ideal “tissue” model because of its heterogeneous cell population embedded within the ECM. Understanding the nature of how these cells communicate with each other and with their underlying BM is crucial for normal islet physiology and pathology. The β-cells rely on intra-islet ECs to synthesise their ECM components[75]. This dependency may potentially be compromised in chronic inflammatory pancreatic diseases such as chronic pancreatitis which is characterized by a number of alterations within ECM formation and composition resulting in destruction of acinar and islet cells, and subsequent replacement by connective tissue[158,159]. This connective tissue appears to result from an increased deposition and disorganization of the ECM proteins including collagens, fibronectins, and laminins[160-163]. Moreover, reports also suggest that one of the most enriched groups of over-expressed proteins in pancreatitis (mild and severe) and pancreatic ductal adenocarcinoma include those involved in the ECM structure and organization[164,165]. In addition, glycoproteins, especially those with N-linked glycosylation sites, are significantly enriched among the over-expressed proteins in mild and chronic pancreatitis[164]. Collagen, proteoglycans, and other ECM specialized glycoproteins such as fibrillin, fibronectin, and laminin, all part of the peri-islet BM, contain various degrees of glycosylation[166].
The connection between ECs and β-cells has been previously evaluated[28,51,57,167,168], particularly where different approaches have been utilized to increase β-cell mass and thereby insulin production. New factors have also been identified which may potentially contribute in further understanding islet cell communication and function. For example, R-spondins-1, an intestinal growth factor containing a thrombospondin domain, has been identified as a novel β-cell growth factor and insulin secretagogue[169]. It has potential to enhance β-cell growth and function in patients with T2D, and enhance of β-cell mass[170]. Connexins, ephrins, and cadherins, members of the transmembrane family of proteins are expressed in pancreatic islets. The major β-cell connexin is Cx36[171], Cx43, and Cx45 are specifically expressed on intra-islet ECs[172] whereas Cx30.2, recently identified, is expressed at cell-cell junctions in both cell types[173].
A number of studies have demonstrated that ECs play a very critical role within the islet microenvironment. A dysfunctional intra-islet vascular endothelium may contribute to the severity or progression of pancreatic disease etiologies. A deeper knowledge of islet endothelial phenotype and function will help identify specific targets and strategies for T1D prevention and successful outcomes for islet transplantation. Identifying and validating the potential therapeutic benefits of novel factors which either maintain the integrity of EC and β-cell communication or reinstate and balance the disrupted crosstalk is likely to benefit patients with diabetes and other pancreatic disorders.
The authors thank the Jewish Heritage Fund for Excellence for providing generous support to our program. The authors sincerely thank Kentucky Organ Donor Affiliates (KODA) for the supply of human pancreases. Special thanks to Leigh Kleinert and Brian Gettler for their assistance.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fujino Y, Kruel CRP, Perse M, Salvadori M, Sumi S, Wang CX S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Chandra R, Liddle RA. Neural and hormonal regulation of pancreatic secretion. Curr Opin Gastroenterol. 2009;25:441-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 545] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Khandekar N, Berning BA, Sainsbury A, Lin S. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol Cell Endocrinol. 2015;418 Pt 1:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, Koizumi M, Sone H, Nakata M, Kakei M. Ghrelin signalling in β-cells regulates insulin secretion and blood glucose. Diabetes Obes Metab. 2014;16 Suppl 1:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | DiGruccio MR, Mawla AM, Donaldson CJ, Noguchi GM, Vaughan J, Cowing-Zitron C, van der Meulen T, Huising MO. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5:449-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Braun M. The somatostatin receptor in human pancreatic β-cells. Vitam Horm. 2014;95:165-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Brereton MF, Vergari E, Zhang Q, Clark A. Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J Histochem Cytochem. 2015;63:575-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 901] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 11. | Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 550] [Article Influence: 61.1] [Reference Citation Analysis (2)] |
| 12. | Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 2006;38:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Cao Z, Wang X. The endocrine role between β cells and intra-islet endothelial cells. Endocr J. 2014;61:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Hart TK, Pino RM. Pseudoislet vascularization. Induction of diaphragm-fenestrated endothelia from the hepatic sinusoids. Lab Invest. 1986;54:304-313. [PubMed] |
| 16. | Zanone MM, Favaro E, Doublier S, Lozanoska-Ochser B, Deregibus MC, Greening J, Huang GC, Klein N, Cavallo Perin P, Peakman M. Expression of nephrin by human pancreatic islet endothelial cells. Diabetologia. 2005;48:1789-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lou J, Triponez F, Oberholzer J, Wang H, Yu D, Buhler L, Cretin N, Mentha G, Wollheim CB, Morel P. Expression of alpha-1 proteinase inhibitor in human islet microvascular endothelial cells. Diabetes. 1999;48:1773-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Favaro E, Bottelli A, Lozanoska-Ochser B, Ferioli E, Huang GC, Klein N, Chiaravalli A, Perin PC, Camussi G, Peakman M. Primary and immortalised human pancreatic islet endothelial cells: phenotypic and immunological characterisation. Diabetologia. 2005;48:2552-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lewis EC. Expanding the clinical indications for α(1)-antitrypsin therapy. Mol Med. 2012;18:957-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Papaccio G, Pedullà M, Ammendola E, Todaro M. Cytokine regulatory effects on alpha-1 proteinase inhibitor expression in NOD mouse islet endothelial cells. J Cell Biochem. 2002;85:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Flickinger BD, Olson MS. Localization of the platelet-activating factor receptor to rat pancreatic microvascular endothelial cells. Am J Pathol. 1999;154:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mattsson G, Danielsson A, Kriz V, Carlsson PO, Jansson L. Endothelial cells in endogenous and transplanted pancreatic islets: differences in the expression of angiogenic peptides and receptors. Pancreatology. 2006;6:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Pan X, Xue W, Li Y, Feng X, Tian X, Ding C. Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Barba-Gutierrez DA, Daneri-Navarro A, Villagomez-Mendez JJ, Kanamune J, Robles-Murillo AK, Sanchez-Enriquez S, Villafan-Bernal JR, Rivas-Carrillo JD. Facilitated Engraftment of Isolated Islets Coated With Expanded Vascular Endothelial Cells for Islet Transplantation. Transplant Proc. 2016;48:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, Dicker A, Ilegems E, Zahr-Akrawi E, Molina J. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60:2571-2577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Rivas-Carrillo SD, Kanamune J, Iwanaga Y, Uemoto S, Daneri-Navarro A, Rivas-Carrillo JD. Endothelial cells promote pancreatic stem cell activation during islet regeneration in mice. Transplant Proc. 2011;43:3209-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Johansson A, Lau J, Sandberg M, Borg LA, Magnusson PU, Carlsson PO. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia. 2009;52:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Watada H. Role of VEGF-A in pancreatic beta cells. Endocr J. 2010;57:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 417] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 30. | Yuan Q, Chen L, Liu C, Xu K, Mao X, Liu C. Postnatal pancreatic islet β cell function and insulin sensitivity at different stages of lifetime in rats born with intrauterine growth retardation. PLoS One. 2011;6:e25167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1110] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 32. | Patel-Hett S, D’Amore PA. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol. 2011;55:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 34. | Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1424] [Cited by in RCA: 1362] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 35. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6952] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 36. | Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 894] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 37. | Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005;280:2126-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368-4380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1170] [Cited by in RCA: 1151] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 39. | Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 40. | Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322-3331. [PubMed] |
| 41. | Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162-15170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 666] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 43. | Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2015] [Cited by in RCA: 2066] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 44. | Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336-30343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 1509] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 45. | Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Kostromina E, Wang X, Han W. Altered islet morphology but normal islet secretory function in vitro in a mouse model with microvascular alterations in the pancreas. PLoS One. 2013;8:e71277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Johansson M, Andersson A, Carlsson PO, Jansson L. Perinatal development of the pancreatic islet microvasculature in rats. J Anat. 2006;208:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560-H576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 604] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 49. | Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC. Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes. 1998;47:1894-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1482] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 51. | Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154-4164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, Prasadan K, Shiota C, Gittes GK. Hypoglycemia reduces vascular endothelial growth factor A production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288:8636-8646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Jabs N, Franklin I, Brenner MB, Gromada J, Ferrara N, Wollheim CB, Lammert E. Reduced insulin secretion and content in VEGF-a deficient mouse pancreatic islets. Exp Clin Endocrinol Diabetes. 2008;116 Suppl 1:S46-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 296] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56:2274-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE. Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev Biol. 2012;367:40-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 58. | Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 59. | Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 60. | Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2548] [Cited by in RCA: 2531] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 61. | Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 838] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 63. | Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 682] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 64. | Dorrell C, Schug J, Lin CF, Canaday PS, Fox AJ, Smirnova O, Bonnah R, Streeter PR, Stoeckert CJ, Kaestner KH. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 65. | Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Yao VJ, Ozawa MG, Trepel M, Arap W, McDonald DM, Pasqualini R. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am J Pathol. 2005;166:625-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 907] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 68. | Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1048] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 69. | Figliolini F, Cantaluppi V, De Lena M, Beltramo S, Romagnoli R, Salizzoni M, Melzi R, Nano R, Piemonti L, Tetta C. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS One. 2014;9:e102521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21:1305-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 71. | Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for β-cell function. Diabetes. 2011;60:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Lin HM, Lee JH, Yadav H, Kamaraju AK, Liu E, Zhigang D, Vieira A, Kim SJ, Collins H, Matschinsky F. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem. 2009;284:12246-12257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 73. | Gregersen S, Thomsen JL, Brock B, Hermansen K. Endothelin-1 stimulates insulin secretion by direct action on the islets of Langerhans in mice. Diabetologia. 1996;39:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 76. | Loganathan R, Rongish BJ, Smith CM, Filla MB, Czirok A, Bénazéraf B, Little CD. Extracellular matrix motion and early morphogenesis. Development. 2016;143:2056-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 959] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 78. | Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196:671-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 79. | Myllyharju J. Extracellular matrix and developing growth plate. Curr Osteoporos Rep. 2014;12:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, Rodgers RJ. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51:1680-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279:53762-53769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Krishnamurthy M, Li J, Fellows GF, Rosenberg L, Goodyer CG, Wang R. Integrin {alpha}3, but not {beta}1, regulates islet cell survival and function via PI3K/Akt signaling pathways. Endocrinology. 2011;152:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Riopel M, Krishnamurthy M, Li J, Liu S, Leask A, Wang R. Conditional β1-integrin-deficient mice display impaired pancreatic β cell function. J Pathol. 2011;224:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Riopel MM, Li J, Liu S, Leask A, Wang R. β1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab Invest. 2013;93:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Choong FJ, Freeman C, Parish CR, Simeonovic CJ. Islet heparan sulfate but not heparan sulfate proteoglycan core protein is lost during islet isolation and undergoes recovery post-islet transplantation. Am J Transplant. 2015;15:2851-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 88. | Hamamoto Y, Fujimoto S, Inada A, Takehiro M, Nabe K, Shimono D, Kajikawa M, Fujita J, Yamada Y, Seino Y. Beneficial effect of pretreatment of islets with fibronectin on glucose tolerance after islet transplantation. Horm Metab Res. 2003;35:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ. 2009;51:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci. 2011;68:3209-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Hall-Glenn F, De Young RA, Huang BL, van Handel B, Hofmann JJ, Chen TT, Choi A, Ong JR, Benya PD, Mikkola H. CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS One. 2012;7:e30562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Riley KG, Pasek RC, Maulis MF, Peek J, Thorel F, Brigstock DR, Herrera PL, Gannon M. Connective tissue growth factor modulates adult β-cell maturity and proliferation to promote β-cell regeneration in mice. Diabetes. 2015;64:1284-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Guney MA, Petersen CP, Boustani A, Duncan MR, Gunasekaran U, Menon R, Warfield C, Grotendorst GR, Means AL, Economides AN. Connective tissue growth factor acts within both endothelial cells and beta cells to promote proliferation of developing beta cells. Proc Natl Acad Sci USA. 2011;108:15242-15247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | James LR, Le C, Doherty H, Kim HS, Maeda N. Connective tissue growth factor (CTGF) expression modulates response to high glucose. PLoS One. 2013;8:e70441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Jiang FX, Cram DS, DeAizpurua HJ, Harrison LC. Laminin-1 promotes differentiation of fetal mouse pancreatic beta-cells. Diabetes. 1999;48:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Golocheikine A, Tiriveedhi V, Angaswamy N, Benshoff N, Sabarinathan R, Mohanakumar T. Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation. 2010;90:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Lai Y, Schneider D, Kidszun A, Hauck-Schmalenberger I, Breier G, Brandhorst D, Brandhorst H, Iken M, Brendel MD, Bretzel RG. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79:1530-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | García-Ocaña A, Vasavada RC, Cebrian A, Reddy V, Takane KK, López-Talavera JC, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Qian X, Rothman VL, Nicosia RF, Tuszynski GP. Expression of thrombospondin-1 in human pancreatic adenocarcinomas: role in matrix metalloproteinase-9 production. Pathol Oncol Res. 2001;7:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 291] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 103. | Dubois S, Madec AM, Mesnier A, Armanet M, Chikh K, Berney T, Thivolet Ch. Glucose inhibits angiogenesis of isolated human pancreatic islets. J Mol Endocrinol. 2010;45:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes. 2008;57:1870-1877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Tillmar L, Welsh N. In vitro cultured rat islets express genes that both prevent and promote angiogenesis. JOP. 2004;5:81-91. [PubMed] |
| 106. | Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 760] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 107. | Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 902] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 108. | Lai EY, Persson AE, Bodin B, Källskog O, Andersson A, Pettersson U, Hansell P, Jansson L. Endothelin-1 and pancreatic islet vasculature: studies in vivo and on isolated, vascularly perfused pancreatic islets. Am J Physiol Endocrinol Metab. 2007;292:E1616-E1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | De Carlo E, Milanesi A, Martini C, Maffei P, Sicolo N, Scandellari C. Endothelin-1 and endothelin-3 stimulate insulin release by isolated rat pancreatic islets. J Endocrinol Invest. 2000;23:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Kugelmeier P, Nett PC, Züllig R, Lehmann R, Weber M, Moritz W. Expression and hypoxic regulation of the endothelin system in endocrine cells of human and rat pancreatic islets. JOP. 2008;9:133-149. [PubMed] |
| 111. | Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 513] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 112. | Hu RM, Levin ER, Pedram A, Frank HJ. Insulin stimulates production and secretion of endothelin from bovine endothelial cells. Diabetes. 1993;42:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Ferri C, Bellini C, Desideri G, De Mattia G, Santucci A. Endogenous insulin modulates circulating endothelin-1 concentrations in humans. Diabetes Care. 1996;19:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Cifarelli V, Lee S, Kim DH, Zhang T, Kamagate A, Slusher S, Bertera S, Luppi P, Trucco M, Dong HH. FOXO1 mediates the autocrine effect of endothelin-1 on endothelial cell survival. Mol Endocrinol. 2012;26:1213-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Cheifetz S, Bellón T, Calés C, Vera S, Bernabeu C, Massagué J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027-19030. [PubMed] |
| 116. | Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 416] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 117. | Fonsatti E, Sigalotti L, Arslan P, Altomonte M, Maio M. Emerging role of endoglin (CD105) as a marker of angiogenesis with clinical potential in human malignancies. Curr Cancer Drug Targets. 2003;3:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 118. | Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010;86:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 119. | Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 120. | Clarkin CE, Mahmoud M, Liu B, Sobamowo EO, King A, Arthur H, Jones PM, Wheeler-Jones CP. Modulation of endoglin expression in islets of langerhans by VEGF reveals a novel regulator of islet endothelial cell function. BMC Res Notes. 2016;9:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Bellacen K, Kalay N, Ozeri E, Shahaf G, Lewis EC. Revascularization of pancreatic islet allografts is enhanced by α-1-antitrypsin under anti-inflammatory conditions. Cell Transplant. 2013;22:2119-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 122. | Zhao M, Choudhary P, Srinivasan P, Tang H, Heaton N, Fung M, Barthel A, Bornstein SR, Amiel SA, Huang GC. Modification of human islet preparation: an effective approach to improve graft outcome after islet transplantation? Horm Metab Res. 2015;47:72-77. [PubMed] |
| 123. | Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 124. | Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004;26:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Lukinius A, Jansson L, Korsgren O. Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets. Am J Pathol. 1995;146:429-435. [PubMed] |
| 126. | Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 127. | Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 128. | Zeng W, Gouw AS, van den Heuvel MC, Zwiers PJ, Zondervan PE, Poppema S, Zhang N, Platteel I, de Jong KP, Molema G. The angiogenic makeup of human hepatocellular carcinoma does not favor vascular endothelial growth factor/angiopoietin-driven sprouting neovascularization. Hepatology. 2008;48:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 129. | Jiang X, Abiatari I, Kong B, Erkan M, De Oliveira T, Giese NA, Michalski CW, Friess H, Kleeff J. Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology. 2009;9:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 131. | Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 132. | Lau J, Carlsson PO. Low revascularization of human islets when experimentally transplanted into the liver. Transplantation. 2009;87:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 133. | Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M. Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant. 2006;6:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 135. | Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 136. | Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, Kiefer F, Bretzel RG. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 137. | Enghofer M, Bojunga J, Ludwig R, Oldenburg A, Bernd A, Usadel KH, Kusterer K. Lymphocyte transfer in streptozotocin-induced diabetes: adhesion of donor cells to islet endothelium. Am J Physiol. 1998;274:E928-E935. [PubMed] |
| 138. | Heller B, Wang ZQ, Wagner EF, Radons J, Bürkle A, Fehsel K, Burkart V, Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995;270:11176-11180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 139. | Steiner L, Kröncke K, Fehsel K, Kolb-Bachofen V. Endothelial cells as cytotoxic effector cells: cytokine-activated rat islet endothelial cells lyse syngeneic islet cells via nitric oxide. Diabetologia. 1997;40:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 140. | Kampf C, Mattsson G, Carlsson PO. Size-dependent revascularization of transplanted pancreatic islets. Cell Transplant. 2006;15:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 141. | Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes. 2008;57:2393-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 142. | Ilgun H, Kim JW, Luo L. Adult Stem Cells and Diabetes Therapy. J Stem Cell Res Transplant. 2015;2:pii: 1020. [PubMed] |
| 143. | Rackham CL, Dhadda PK, Le Lay AM, King AJ, Jones PM. Preculturing Islets With Adipose-Derived Mesenchymal Stromal Cells Is an Effective Strategy for Improving Transplantation Efficiency at the Clinically Preferred Intraportal Site. Cell Med. 2014;7:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 144. | Fransson M, Brännström J, Duprez I, Essand M, Le Blanc K, Korsgren O, Magnusson PU. Mesenchymal stromal cells support endothelial cell interactions in an intramuscular islet transplantation model. Regen Med Res. 2015;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Kaufman-Francis K, Koffler J, Weinberg N, Dor Y, Levenberg S. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS One. 2012;7:e40741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Phelps EA, Templeman KL, Thulé PM, García AJ. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res. 2015;5:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 147. | Amer LD, Mahoney MJ, Bryant SJ. Tissue engineering approaches to cell-based type 1 diabetes therapy. Tissue Eng Part B Rev. 2014;20:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Willenberg BJ, Oca-Cossio J, Cai Y, Brown AR, Clapp WL, Abrahamson DR, Terada N, Ellison GW, Mathews CE, Batich CD. Repurposed biological scaffolds: kidney to pancreas. Organogenesis. 2015;11:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 780] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 150. | Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 151. | Agudo J, Ayuso E, Jimenez V, Casellas A, Mallol C, Salavert A, Tafuro S, Obach M, Ruzo A, Moya M. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of β-cell mass. Diabetes. 2012;61:2851-2861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Rasul S, Reiter MH, Ilhan A, Lampichler K, Wagner L, Kautzky-Willer A. Circulating angiopoietin-2 and soluble Tie-2 in type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2011;10:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 153. | Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 154. | Campochiaro PA, Peters KG. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr Diab Rep. 2016;16:126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 155. | Nakano M, Yasunami Y, Maki T, Kodama S, Ikehara Y, Nakamura T, Tanaka M, Ikeda S. Hepatocyte growth factor is essential for amelioration of hyperglycemia in streptozotocin-induced diabetic mice receiving a marginal mass of intrahepatic islet grafts. Transplantation. 2000;69:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 156. | Lopez-Talavera JC, Garcia-Ocaña A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 157. | Rao P, Cozar-Castellano I, Roccisana J, Vasavada RC, Garcia-Ocaña A. Hepatocyte growth factor gene therapy for islet transplantation. Expert Opin Biol Ther. 2004;4:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 158. | Park WG. Clinical chronic pancreatitis. Curr Opin Gastroenterol. 2016; Jun 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2011;27:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 160. | Ellenrieder V, Schneiderhan W, Bachem M, Adler G. Fibrogenesis in the pancreas. Rocz Akad Med Bialymst. 2004;49:40-46. [PubMed] |
| 161. | Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, Suwa H, Ishigami S, Imamura M. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas. 1995;11:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 162. | Shields MA, Dangi-Garimella S, Redig AJ, Munshi HG. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem J. 2012;441:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 163. | Buchholz M, Biebl A, Neesse A, Wagner M, Iwamura T, Leder G, Adler G, Gress TM. SERPINE2 (protease nexin I) promotes extracellular matrix production and local invasion of pancreatic tumors in vivo. Cancer Res. 2003;63:4945-4951. [PubMed] |
| 164. | Pan S, Chen R, Stevens T, Bronner MP, May D, Tamura Y, McIntosh MW, Brentnall TA. Proteomics portrait of archival lesions of chronic pancreatitis. PLoS One. 2011;6:e27574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 165. | Paulo JA, Kadiyala V, Brizard S, Banks PA, Steen H, Conwell DL. A proteomic comparison of formalin-fixed paraffin-embedded pancreatic tissue from autoimmune pancreatitis, chronic pancreatitis, and pancreatic cancer. JOP. 2013;14:405-414. [PubMed] |
| 166. | Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 167. | Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 168. | Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Human Islets Have Fewer Blood Vessels than Mouse Islets and the Density of Islet Vascular Structures Is Increased in Type 2 Diabetes. J Histochem Cytochem. 2015;63:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 169. | Wong VS, Yeung A, Schultz W, Brubaker PL. R-spondin-1 is a novel beta-cell growth factor and insulin secretagogue. J Biol Chem. 2010;285:21292-21302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 170. | Chahal JK, Wong VS, Chaboissier MC, Brubaker PL. R-spondin1 deficiency enhances β-Cell neogenesis in a murine model of diabetes. Pancreas. 2014;43:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 171. | Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol. 2011;589:5453-5466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 172. | Theis M, Mas C, Döring B, Degen J, Brink C, Caille D, Charollais A, Krüger O, Plum A, Nepote V. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 173. | Coronel-Cruz C, Hernández-Tellez B, López-Vancell R, López-Vidal Y, Berumen J, Castell A, Pérez-Armendariz EM. Connexin 30.2 is expressed in mouse pancreatic beta cells. Biochem Biophys Res Commun. 2013;438:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |