Published online Nov 18, 2022. doi: 10.5500/wjt.v12.i11.378
Peer-review started: July 24, 2022
First decision: August 22, 2022
Revised: September 5, 2022
Accepted: September 22, 2022
Article in press: September 22, 2022
Published online: November 18, 2022
Processing time: 115 Days and 9.5 Hours
Parvovirus B19 (B19V) is associated with a wide range of clinical manifestations. The major presentation is erythema infectiosum. However, a persistent infection may cause pure red cell aplasia and chronic anemia in immunocompromized patients. The B19V seroprevalence varies with age and geographical location.
To determine the B19V serological status and DNAemia in kidney, liver, and pancreas transplant candidates.
Patients who underwent kidney, liver, or simultaneous kidney and pancreas/liver transplantation between January 2021 and May 2022 were included in the study. The serum samples were collected before transplantation. For detection of B19V DNA, a LightMix Kit B19V EC (TIB MOLBIOL, Berlin, Germany) was used. B19V IgM and IgG antibodies were detected using a commercial ELISA test (Euroimmun, Lübeck, Germany).
One hundred and thirty-one transplant candidates were included in the study, 71.0% male, with an average age of 53.27 years ± 12.71 years. There were 68.7% liver, 27.5% kidney, 3.0% simul
The B19V seroprevalence is expectedly high among kidney, liver, and pancreas transplant candidates, but there are still 22.9% of seronegative individuals who remain at risk for primary disease and severe manifestations. Further research should elucidate the necessity of B19V screening in peri-transplant management.
Core Tip: Many liver, kidney, or pancreas transplant recipients are parvovirus B19 seronegative and at risk for primary disease and severe manifestations. Serological studies on pretransplant could simplify the diagnostic work-up of anemia after transplantation in these complex patients.
- Citation: Simunov B, Mrzljak A, Jurekovic Z, Zidovec Lepej S, Bainrauch A, Pavicic Saric J, Hruskar Z, Radmanic L, Vilibic-Cavlek T. Parvovirus B19 status in liver, kidney and pancreas transplant candidates: A single center experience. World J Transplant 2022; 12(11): 378-387
- URL: https://www.wjgnet.com/2220-3230/full/v12/i11/378.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i11.378
Parvovirus B19 (B19V) is a small non-enveloped single-stranded DNA virus of the family Parvoviridae, genus Erythroparvovirus[1]. It was first discovered in a healthy blood donor[2] and then linked to aplastic crises in children with sickle cell anemia[3]. Subsequently, the major presentation, erythema infectiosum (fifth disease), was described[4]. B19V mainly infects the human erythroid progenitor cells[5]. The cellular receptor is globoside (erythrocyte P antigen), found on erythroid cells, erythroid precursors and red cells of the placenta and fetal myocardium, fetal liver, and some megakaryocytes and endothelial cells[6]. Rarely, individuals may lack blood group P antigen, which confers resistance to B19V infection[7].
In healthy individuals, the disease is often asymptomatic or occurs as a two-phase illness: Fever and non-specific influenza-like symptoms during the early phase of viremia, followed by erythema, arthralgia, or both, at the time of appearance of specific antiviral antibodies[8,9]. The cutaneous manifestations of B19V infection vary. Four basic patterns have been reported: exanthema, gloves-and-socks, periflexural, and palpable purpura[10]. A robust humoral immune response is required to control B19V infection and clear DNAemia. Neutralizing antibodies to B19V structural proteins appear to confer life-long protective immunity[11]. Therefore, in immunocompromized patients unable to mount sufficient antibody response, the infection may persist and cause pure red cell aplasia and chronic anemia[12,13]. More recently, other disease manifestations have been reported, ranging from hepatitis and myocarditis to meningoencephalitis[14-17].
In the transplant setting, B19V is long known to cause persistent anemia and pure red cell aplasia due to the inability of the immunosuppressed host to clear the virus[18-20]. The epidemiology of B19V infection in solid organ transplant (SOT) recipients is unknown, with wide variances of rates reported in different studies, from 0% to 58%[21-24]. Some recent studies report a much lower rate, under 15%[23,25]. It is noteworthy that the immune response mediates non-hematological manifestations of B19V infection; thus immune-mediated symptoms may be absent or blunted in transplant recipients. Therefore, a high level of suspicion should be present to diagnose the infection.
Serology may not reliably establish the diagnosis in the transplant population due to the inability to produce a sufficient antibody response, and polymerase chain reaction (PCR) should be used to detect viral DNA in this population[11]. High-level viremia is more likely associated with symptomatic disease[11]. Conversely, if detected at low levels, persistent DNAemia after infection may not be clinically significant[11]. Despite the lack of robust data, intravenous administration of immunoglobulins (IVIg) and decrease of immunosuppression levels are the mainstay of treatment of SOT recipients with symptomatic B19V infection[11,19]. Although IVIg's optimal dosage and duration are unknown, most patients respond well to treatment. Unfortunately, recurrence of anemia is common[26-28]. There are preliminary reports of foscarnet being used for treatment[29]. Cidofovir has shown in vitro efficacy, but further research is needed[30]. Also, the conversion from calcineurin inhibitor-based immunosuppression to everolimus has been described[31].
Currently, routine screening of donor and recipient serostatus for B19V is not recommended; there have been research efforts[24,32]. There is also a lack of epidemiologic data, including the serop
This study aimed to determine the B19V serological status and active viral replication by B19V DNA quantification in kidney, liver, and pancreas transplant candidates at a large national transplant center.
Patients who were transplanted (kidney, liver, or simultaneous kidney and pancreas/liver) at Merkur University Hospital from January 2021 to May 2022 were included in the analysis. The hospital is a high-volume transplant center with approximately 110 liver and 50 kidney transplants performed yearly, representing over 90% of the liver transplantation program in the country and the only institution performing simultaneous transplantations. This was a single-center, prospective study.
The serum samples were collected before the transplantation. Data about the patients were collected prospectively using the hospital's electronic medical record.
Viral DNA was extracted from blood samples using a High Pure Viral Nucleic Acid Kit (Roche Applied Science, Penzberg, Germany). For quantification of B19V DNA in nucleic acid extracts, a LightMix Kit Parvovirus B19 EC (TIB MOLBIOL, Berlin, Germany) was used.
B19V IgG and IgM antibodies were detected using a commercial enzyme-linked immunosorbent assay (ELISA; Euroimmun, Lübeck, Germany). Results were interpreted according to the manufa
Statistical analysis was performed using SPSS version 25 (Armonk, NY, United States, IBM Corp). A P < 0.05 was considered to be significant. The data are expressed as the median and interquartile range (IQR), or mean ± SD, as appropriate. Categorical variables are presented as frequency counts and percentages. The normality of the data distribution was tested using the Shapiro-Wilks test. The categorical values were compared using the χ2 test. In cases with less than 5 outcomes, Fisher's exact test was used. For continuous variables, a parametric (Student’s t-test, ANOVA) or nonparametric test (Mann-Whitney U, Wilcoxon, Kruskal-Wallis) was used, depending on the distribution.
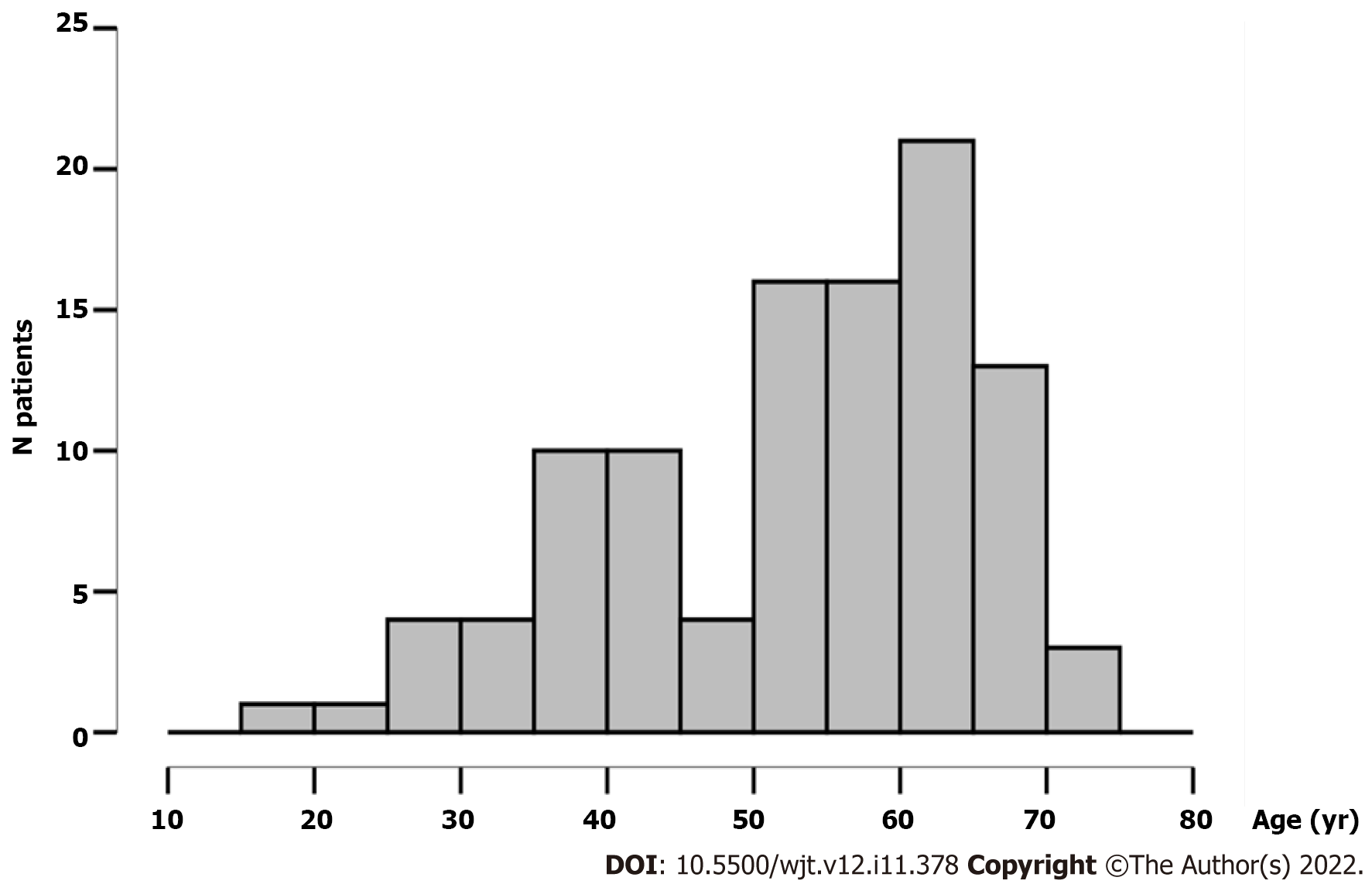
A total of 131 transplant candidates were included in the study, with 70.9% being male. The average age was 53.27 years ± 12.71 years. The median age was 57 years, IQR 43-63 years. The age distribution of patients is presented in Figure 1.
There were 68.7% liver, 27.5% kidney, 3.0% simultaneous pancreas-kidney transplant (SPKT) and 0.8% simultaneous liver-kidney transplant (SLKT) recipients (Table 1).
| Item | Value |
| Age, yr (mean ± SD) | 53.27 ± 12.71 |
| Gender | |
| Male | 93 (70.9%) |
| Female | 38 (29.1%) |
| Transplant type | |
| Liver | 90 (68.7%) |
| Kidney | 36 (27.5%) |
| SPKT | 4 (3.0%) |
| SLKT | 1 (0.8%) |
| Virology results | |
| B19V DNA positive | 0 (0%; one-sided 97.5% CI: 0-2.8) |
| B19V IgM positive | 0 (0%; one-sided 97.5% CI: 0-2.8) |
| IgG B19V positive | 101 (77.1%; 95% CI: 68.9-83.9) |
None of the tested patients had detectable B19V DNA. IgG seroprevalence was 77.1%. No recent infections (IgM antibodies) were detected.
There was no difference in the mean age of seronegative and seropositive patients (51.8 years ± 12.9 years vs 53.7 years ± 12.7 years, t = -0.603; P = 0.548). In addition, there was no difference in seropositivity between male and female transplant candidates, 76.3% vs 78.9%, respectively (χ2 = 0.104; P = 0.748). When divided into age groups, the seroprevalence was 66.7% in those under 30 years, 80.4% in those aged 30 to 59 years, and 78.1% in patients over 60 (χ2 = 0.619; P = 0.734) (Table 2).
| Characteristics | Tested, n (%) | IgG positive, n (%) | χ2 | P value |
| Gender | 0.104 | 0.748 | ||
| Male | 93 (71.0) | 71 (76.3) | ||
| Female | 38 (29.0) | 30 (78.9) | ||
| Age, yr | 0.619 | 0.734 | ||
| < 30 | 6 (5.8) | 4 (66.6) | ||
| 30-59 | 56 (54.4) | 45 (80.4) | ||
| > 60 | 41 (39.8) | 32 (78.1) | ||
| Transplant type | 5.297 | 0.151 | ||
| Liver | 90 (68.7) | 70 (77.8) | ||
| Kidney | 36 (27.5) | 29 (80.6) | ||
| SPKT | 4 (3.0) | 2 (50.0) | ||
| SLKT | 1 (0.8) | 0 (0) | ||
| IS before transplantation | 0.498 | 0.780 | ||
| Yes | 16 (18.2) | 13 (81.3) | ||
| No | 72 (81.8) | 55 (76.4) | ||
| Dialysis modality | 0.3721 | |||
| HD | 38 (95) | 27 (71.1) | ||
| PD | 2 (5) | 2 (100) |
The seroprevalence did not differ significantly among different organ recipients, with 77.8%, 80.6%, and 50% for liver, kidney, and SPKT, respectively, (χ2 = 5.297; P = 0.151). There was only one SLKT recipient who was seronegative. The recipients of SPKT were significantly younger than kidney or liver recipients (36.0 years ± 6.8 years, 52.6 years ± 11.6 years and 54.8 years ± 12.9 years, respectively, P = 0.014).
There was no association between immunosuppression prior to transplantation and seropositivity. B19V seroprevalence was 81.3% in the subgroup which received immunosuppression prior to transplantation and 76.4% in the subgroup that did not (χ2 = 0.176; P = 0.675).
No significant difference was found in the seroprevalence in kidney transplant candidates according to the dialysis modality. Seroprevalence was 71.1% in hemodialysis patients, and 100% in peritoneal dialysis patients (χ2 = 0.799; P = 0.372). In addition, there was no association with dialysis duration (40.1 mo ± 25.4 mo in seropositive vs 37.4 mo ± 17.6 mo in seronegative, t = -0.288, P = 0.775).
Our results show a high seroprevalence of B19V among transplant candidates. The seroprevalence of 77.1% was higher compared to a large previous study in the general Croatian population, where a seroprevalence of 64.1% was found[34]. Surprisingly, the seroprevalence did not differ with age, which is commonly reported. However, although not significantly, seropositivity was lower in patients aged less than 30 years (66.6%) compared to patients aged 30-59 and 60 years (80.4% and 78.1%, respectively). The transplant population tested in this study was skewed to slightly older recipients, as shown in the age distribution. This could partly explain the inability to detect the expected difference in seroprevalence with age. In the Croatian general population, seroprevalence in the matching age group 50-59 years was 69.1%[34], which is concordant to our findings. However, it is important to note that the seroprevalence in transplant patients younger than 30 years was higher (66.6%) compared to the same age group in the general population (53.2%)[34].
Additionally, it is important to emphasize that our study investigated transplant candidates, not recipients. The candidates, contrary to the recipients, have not yet received immunosuppression. The data on transplant candidates is even scarcer in literature than on SOT recipients[11]. A German study reported a similar seroprevalence rate of 82% in transplant candidates (kidney, liver, heart, and bone marrow)[35]. Moreover, no difference was found in seroprevalence between various organ recipients, but with a trend toward lower seroprevalence among simultaneous kidney and pancreas candidates. All kidney transplant candidates in our study were patients on dialysis. Few studies analyzed the B19V seroprevalence in hemodialysis or peritoneal dialysis patients. Prevalence rates of 67.5% and 54% were reported from Brazil and Iran, respectively[36,37], which is similar to our result of 71.1% in hemodialysis patients. In our study, we found no association of seroprevalence with the duration of hemodialysis (40.1 mo ± 25.4 mo in seropositive vs 37.4 mo ± 17.6 mo in seronegative, t = -0.288, P = 0.775). Due to better treatment of anemia today, most dialysis patients do not receive transfusions. Therefore, the duration of dialysis does not appear to be a risk factor. The lower prevalence in SPKT candidates was not statistically significant. The SPKT candidates were significantly younger than other transplant candidates, which could explain the trend. Moreover, although there is a paucity of data in the literature on B19V infection in SPKT recipients, the cases presented[38-40] imply a more severe course. We hypothesize that pancreas candidates may be at higher risk for infection given a larger proportion of seronegative recipients due to the immunosuppressive nature of diabetes[41] and the younger age of the recipients. The possible difference among various organ type recipients includes not only age as seen in SPKT recipients but also different numbers of blood transfusions due to bleeding events in cirrhotic patients. Interestingly there was no association between immunosuppression prior to transplantation (e.g., for glomerulonephritis or autoimmune liver disease) and seropositivity.
Following acute infection in immunocompetent individuals, viral genomes may persist in various tissues for life. However, acute B19V infection can lead to severe complications in immunocompromized patients. In our study, no B19V DNA was found. In a German study, B19V DNA was detected in 4.0% of patients. Whereas DNAemia was found in 5.5%, 6.7%, and 5.7% of liver, heart, and bone marrow recipients, and viral genomes were found in only 1.4% of kidney recipients[35]. In a large recent Chinese study, a B19V DNA positive rate of 1.9% was reported in transplant candidates[25].
In addition, a large proportion of patients are still seronegative at the time of transplant and remain at risk for severe disease manifestations. Currently, there is no specific prevention of B19V disease. There is also no routine screening of donor and recipient serostatus for B19V. The true incidence of parvovirus infection in SOT recipients is unknown, with rates varying considerably across different studies[21-25]. There have been efforts in prospective routine monitoring of B19V in the first 6 mo after transplantation in seronegative SOT recipients. The findings showed low incidence rates (1.2% recipients per month) and even lower clinically significant events[24]. In another recent study, prospective monitoring revealed a higher incidence of B19V (10.17%), all infections occurred in seronegative recipients and were deemed clinically significant[42]. To conclude, large prospective data series on B19V disease in transplant recipients are lacking, but in our opinion, at the moment there is no rationale for routine B19V testing. However, pretransplant serostatus could be cost-efficient given the lower cost of a serological test than PCR testing and could potentially reveal patients at high risk. Post-transplant anemia is prevalent and often multifactorial. Serostatus could potentially hasten the diagnosis of B19V infection in selected patients and thus help avoid diagnostic delay and unnecessarily broad testing.
Moreover, B19V has also been implicated as a trigger for thrombotic microangiopathy[43,44], especially in the transplant setting[45-48]. These implications warrant additional research, but the information on serostatus could be beneficial during thrombotic microangiopathy workup, which is expensive and usually long-lasting. A large number of post-transplant thrombotic microangiopathies are regarded as secondary, either to immunosuppressive drugs or transplant itself; thus, B19V infection as a possible causative agent is probably underdiagnosed[49]. Identifying high-risk individuals pretransplant could be beneficial and help elucidate this pathophysiologically complex state[50].
Our study has limitations. Firstly, it is a single-center study with low numbers of rare transplantations, e.g., SPKT and SLKT. Secondly, the incidence of clinical B19V infection was not reported in the post-transplant follow-up of these patients, reflecting the clinical significance of the serological status detected pretransplant. We plan to prospectively evaluate DNAemia and serostatus post-transplant as well as clinical manifestations to establish the clinical significance and epidemiology of B19V disease post-transplant. In addition, blood samples from control subjects were unavailable; therefore, it was not possible to compare the prevalence of B19V DNA in healthy individuals.
The B19V seroprevalence is expectedly high among kidney, liver, and pancreas transplant candidates, but 22.9% of seronegative individuals remain at risk for primary disease and severe manifestations. Further research should elucidate the utility of B19V screening in peri-transplant management.
Parvovirus B19 (B19V) is an important pathogen in transplant settings. The epidemiology of B19V infection in solid organ transplant (SOT) recipients is not well studied, and reported prevalence rates vary greatly.
Data on B19V infection in transplant settings are scarce.
To analyze the prevalence of B19V antibodies and DNA in SOT candidates (kidney, liver, or simultaneous kidney and pancreas/liver) at a large national transplant center.
Serum samples collected before transplantation were tested for the presence of B19V IgM and IgG antibodies and B19V DNA. Patients' data were collected using the electronic medical record.
A total of 131 transplant candidates were included in the study, with 70.9% being male. The average age was 53.27 years ± 12.71 years. None of the tested patients had detectable B19V DNA and IgM, while IgG seroprevalence was 77.1%. There was no difference in seropositivity between males and females (76.3% vs 78.9%). According to age, the seroprevalence was 66.7% in those under 30 years, 80.4% in those aged 30-59 years, and 78.1% in patients over 60. The seroprevalence did not differ significantly among different organ recipients, with 77.8%, 80.6%, and 50% for liver, kidney, and simultaneous pancreas-kidney transplant, respectively. There was no association between immunosuppression prior to transplantation and B19V IgG seropositivity.
The B19V seroprevalence is high in transplant candidates, but 22.9% of seronegative individuals remain at risk for primary disease and severe manifestations.
Further studies on large samples as well the B19V prevalence during the post-transplant period are needed to determine the clinical significance of B19V infection in transplant patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Shuang W, China S-Editor: Chen YL L-Editor: Ma JY-MedE A P-Editor: Chen YL
| 1. | Rogo LD, Mokhtari-Azad T, Kabir MH, Rezaei F. Human parvovirus B19: a review. Acta Virol. 2014;58:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 571] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Pattison JR, Jones SE, Hodgson J, Davis LR, White JM, Stroud CE, Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981;1:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 360] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Anderson MJ, Lewis E, Kidd IM, Hall SM, Cohen BJ. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond). 1984;93:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 222] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Potter CG, Potter AC, Hatton CS, Chapel HM, Anderson MJ, Pattison JR, Tyrrell DA, Higgins PG, Willman JS, Parry HF. Variation of erythroid and myeloid precursors in the marrow and peripheral blood of volunteer subjects infected with human parvovirus (B19). J Clin Invest. 1987;79:1486-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 95] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 536] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Brown KE, Hibbs JR, Gallinella G, Anderson SM, Lehman ED, McCarthy P, Young NS. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med. 1994;330:1192-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 478] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 10. | Mage V, Lipsker D, Barbarot S, Bessis D, Chosidow O, Del Giudice P, Aractingi S, Avouac J, Bernier C, Descamps V, Dupin N. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol. 2014;71:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Eid AJ, Ardura MI; AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician. 2007;75:373-376. [PubMed] |
| 13. | Wolfromm A, Rodriguez C, Michel M, Habibi A, Audard V, Benayoun E, Rogier O, Challine D, Chosidow O, Lelièvre JD, Chevalier X, Le Bras F, Pautas C, Imbert M, Pawlotsky JM, Wagner-Ballon O. Spectrum of adult Parvovirus B19 infection according to the underlying predisposing condition and proposals for clinical practice. Br J Haematol. 2015;170:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Bonvicini F, Marinacci G, Pajno MC, Gallinella G, Musiani M, Zerbini M. Meningoencephalitis with persistent parvovirus B19 infection in an apparently healthy woman. Clin Infect Dis. 2008;47:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Zou Q, Chen P, Chen J, Chen D, Xia H, Chen L, Feng H, Feng L. Multisystem Involvement Induced by Human Parvovirus B19 Infection in a Non-immunosuppressed Adult: A Case Report. Front Med (Lausanne). 2022;9:808205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Krygier DS, Steinbrecher UP, Petric M, Erb SR, Chung SW, Scudamore CH, Buczkowski AK, Yoshida EM. Parvovirus B19 induced hepatic failure in an adult requiring liver transplantation. World J Gastroenterol. 2009;15:4067-4069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Bültmann BD, Klingel K, Sotlar K, Bock CT, Kandolf R. Parvovirus B19: a pathogen responsible for more than hematologic disorders. Virchows Arch. 2003;442:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Pamidi S, Friedman K, Kampalath B, Eshoa C, Hariharan S. Human parvovirus B19 infection presenting as persistent anemia in renal transplant recipients. Transplantation. 2000;69:2666-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Zhong Q, Zeng J, Lin T, Song T. The detection, treatment of parvovirus B19 infection induced anemia in solid organ transplants: A case series and literature review of 194 patients. Transfus Clin Biol. 2022;29:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Carraturo A, Catalani V, Ottaviani D, Menichelli P, Rossini M, Terella D, Biondi B. Parvovirus B19 infection and severe anemia in renal transplant recipients. ScientificWorldJournal. 2012;2012:102829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Park JB, Kim DJ, Woo SY, Choi GS, Chun JM, Jung GO, Kwon CH, Kim SJ, Joh JW, Lee SK. Clinical implications of quantitative real time-polymerase chain reaction of parvovirus B19 in kidney transplant recipients-a prospective study. Transpl Int. 2009;22:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ki CS, Kim IS, Kim JW, Lee NY, Kim SH, Lee KW, Kim SJ, Joh JW, Huh WS, Oh HY. Incidence and clinical significance of human parvovirus B19 infection in kidney transplant recipients. Clin Transplant. 2005;19:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Rezahosseini O, Ekenberg C, Møller DL, Sørensen SS, Wareham NE, Perch M, Gustafsson F, Rasmussen A, Kirkby N, Reekie J, Lundgren J, Nielsen SD. Incidence and Impact of Parvovirus B19 Infection in Seronegative Solid Organ Transplant Recipients. J Infect Dis. 2021;224:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yu Y, Wei C, Lyu J, Wu X, Wang R, Huang H, Wu J, Chen J, Peng W. Donor-Derived Human Parvovirus B19 Infection in Kidney Transplantation. Front Cell Infect Microbiol. 2021;11:753970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Gosset C, Viglietti D, Hue K, Antoine C, Glotz D, Pillebout E. How many times can parvovirus B19-related anemia recur in solid organ transplant recipients? Transpl Infect Dis. 2012;14:E64-E70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Renoult E, Bachelet C, Krier-Coudert MJ, Diarrassouba A, André JL, Kessler M. Recurrent anemia in kidney transplant recipients with parvovirus B19 infection. Transplant Proc. 2006;38:2321-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Crabol Y, Terrier B, Rozenberg F, Pestre V, Legendre C, Hermine O, Montagnier-Petrissans C, Guillevin L, Mouthon L; Groupe d'experts de l'Assistance Publique-Hôpitaux de Paris. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis. 2013;56:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Yu Y, Bao R, Lyu J, Wu J, Chen J, Peng W. Foscarnet Therapy for Pure Red Cell Aplasia Related to Human Parvovirus B19 Infection in Kidney Transplant Recipients: A Preliminary Exploration. Infect Drug Resist. 2021;14:2911-2923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Bonvicini F, Bua G, Manaresi E, Gallinella G. Enhanced inhibition of parvovirus B19 replication by cidofovir in extendedly exposed erythroid progenitor cells. Virus Res. 2016;220:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Rodríguez-Espinosa D, Esforzado N, Hermida E, Cuadrado E, Broseta JJ, Diekmann F, Revuelta I. A case of recurrent anemia due to chronic parvovirus B19 infection in a kidney transplant recipient. Can everolimus make a difference? CEN Case Rep. 2021;10:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Barzon L, Murer L, Pacenti M, Biasolo MA, Vella MD, Ghirardo G, Gamba PG, De Arias AE, Zanon GF, Palù G. Detection of viral DNA in kidney graft preservation and washing solutions is predictive of posttransplant infections in pediatric recipients. J Infect Dis. 2009;200:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J. A cluster of parvovirus B19 infections in renal transplant recipients: a prospective case series and review of the literature. Am J Transplant. 2006;6:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Vilibic-Cavlek T, Tabain I, Kolaric B, Mihulja K, Blazevic L, Bogdanic M, Navolan D, Beader N, Mrzljak A. Parvovirus B19 in Croatia: A Large-Scale Seroprevalence Study. Medicina (Kaunas). 2021;57:1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Plentz A, Würdinger M, Kudlich M, Modrow S. Low-level DNAemia of parvovirus B19 (genotypes 1-3) in adult transplant recipients is not associated with anaemia. J Clin Virol. 2013;58:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Alves MT, Vilaça SS, Godoi LC, Rezende Júnior L, Carvalho Md, Silva Fde S, Guimarães FL, Fernandes AP, Dusse LM, Gomes KB. Parvovirus B19 (B19) and cytomegalovirus (CMV) infections and anti-erythropoietin (anti-EPO) antibodies in patients on dialysis hyporesponsive to erythropoietin therapy. Clin Chim Acta. 2014;431:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Sharif A, Aghakhani A, Velayati AA, Banifazl M, Sharif MR, Razeghi E, Kheirkhah D, Kazemimanesh M, Bavand A, Ramezani A. Frequency and Genotype of Human Parvovirus B19 among Iranian Hemodialysis and Peritoneal Dialysis Patients. Intervirology. 2016;59:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Lindahl JP, Barlinn R, Abrahamsen IW, Spetalen S, Midtvedt K, Jenssen T. Case Report: Pure Red Cell Aplasia Caused by Refractory Parvovirus B19 Infection After Pancreas Transplantation Alone. Front Med (Lausanne). 2022;9:849783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Nowacka-Cieciura E, Karakulska-Prystupiuk E, Żuk-Wasek A, Lisik W, Basak GW, Durlik M. Pure Red Cell Aplasia Related to Parvovirus B19 Infection in Simultaneous Pancreas and Kidney Recipient: A Case Report. Transplant Proc. 2020;52:2539-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Wang H, Fu YX, Song WL, Wang Z, Feng G, Zhao J, Nian YQ, Cao Y. Human parvovirus B19-associated early postoperative acquired pure red cell aplasia in simultaneous pancreas-kidney transplantation: A case report. World J Clin Cases. 2021;9:1968-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 519] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 42. | Huang Q, Wang Y, Chen R, Zhao Y, Wang H, Ma X, Li D, Liu Q, Chen X, He L, Zhang M, Li M. Parvovirus B19 infection in kidney transplant recipients: A prospective study in a teaching hospital in Shanghai, China. Transpl Immunol. 2022;74:101667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 43. | Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2 Suppl 1:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Murer L, Zacchello G, Bianchi D, Dall'amico R, Montini G, Andreetta B, Perini M, Dossi EC, Zanon G, Zacchello F. Thrombotic microangiopathy associated with parvovirus B 19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Ardalan MR, Shoja MM, Tubbs RS, Esmaili H, Keyvani H. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant. 2008;8:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Ardalan MR, Shoja MM, Tubbs RS, Jayne D. Parvovirus B19 microepidemic in renal transplant recipients with thrombotic microangiopathy and allograft vasculitis. Exp Clin Transplant. 2008;6:137-143. [PubMed] |
| 47. | Prasad B, St Onge J. Parvovirus leading to thrombotic microangiopathy in a healthy adult. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Steffen CJ, Koch N, Eckardt KU, Amann K, Seelow E, Schreiber A. Hemophagocytic lymphohistiocytosis and thrombotic microangiopathy after parvovirus B19 infection and renal transplantation: a case report. BMC Nephrol. 2021;22:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 49. | Bayer G, von Tokarski F, Thoreau B, Bauvois A, Barbet C, Cloarec S, Mérieau E, Lachot S, Garot D, Bernard L, Gyan E, Perrotin F, Pouplard C, Maillot F, Gatault P, Sautenet B, Rusch E, Buchler M, Vigneau C, Fakhouri F, Halimi JM. Etiology and Outcomes of Thrombotic Microangiopathies. Clin J Am Soc Nephrol. 2019;14:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 50. | Schwarz C, Brehon A, Mousseaux C, Luque Y, Senet P, Mariani P, Mohamadou I, Zafrani L, Frémeaux-Bacchi V, Rondeau E, Buob D, Rafat C. Ockham's razor defeated: about two atypical cases of hemolytic uremic syndrome. BMC Nephrol. 2020;21:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |